Abstract
Decreased number and impaired functions of polymorphonuclear neutrophils (PMN) due to the presence of anti-PMN autoantibodies in the serum render patients with systemic lupus erythematosus (SLE) susceptible to bacterial infections. However, the cognate antigens and pathological mechanisms of anti-PMN autoantibodies in SLE are rarely reported in the literature. In this study, we found approximately 20% of SLE sera contained anti-PMN autoantibodies detected by human PMN-coated cellular ELISA. A membrane protein with molecular weight of 50 kDa was identified as the cognate antigen of anti-PMN in Western blot after membrane-biotinylation and streptavidin column elution. The 50 kDa molecule was proved to be SSB/La after immunoscreening, molecular cloning and nucleotide sequencing of the gene from the human leucocyte cDNA library. Human anti-SSB/La autoantibodies purified from active SLE sera passing through the recombinant SSB/La conjugated Sepharose 4B affinity column could bind and penetrate into normal human PMN. Functional analysis revealed that the anti-SSB/La autoantibodies exerted a number of potent effects on human PMN, including suppressed phagocytosis, accelerated apoptosis and enhanced IL-8 production. These in vitro results suggest that anti-SSB/La is one of the anti-PMN autoantibodies capable of penetrating into PMN and responsible for neutropenia and functional impairment of PMN in patients with SLE.
Keywords: antineutrophil autoantibodies, bacterial infection, neutropenia, systemic lupus erythematosus, SSB/La
Introduction
Systemic lupus erythematosus (SLE), the archetype of systemic autoimmune disorder, is characterized by the presence of a diverse spectrum of autoantibodies in the serum. Some of the autoantibodies bind to circulating antigens forming immune complexes and deposit in different tissues to elicit tissue inflammation [1,2]. Some of the autoantibodies target the surface expressed antigens on the tissues directly, causing cytotoxicity [3,4]. Neutropenia is found in about 50–60% of patients with active SLE in the long-term course of the disease [5]. Polymorphonuclear neutrophils (PMN) are regarded traditionally as the first-line cell component of the body defence mechanisms against bacterial pathogens. However, recent investigations demonstrated that PMN play a role in the afferent and efferent limbs of immune responses that react to and produce different cytokines, including IL-1, IL-6, IL-10, IL-12, TNF-α, TGF-β1, G-CSF, M-CSF, GM-CSF and chemokines such as IL-8, MIP-1α, MIP-1β and MCP-1 [6,7]. Clinically, increased susceptibility to infections is a major cause of morbidity and mortality in patients with SLE [8,9]. Treatment with adrenal corticosteroids and/or immunosuppressants is obviously responsible for the increased infections. However, decreased numbers [10] and/or impaired functions [11–14] of PMN per se are equally crucial for susceptible to bacterial infection in SLE. The documented functional defects of SLE-PMN included reduced phagocytosis [11], presence of a serum inhibitor for phagocytosis [12,13], decreased nitroblue tetrazolium dye reduction [14] and impaired cytokine/chemokine production [15]. However, the real causes of the neutropenia and functional defects in SLE-PMN have not yet been entirely elucidated.
Drew and Terasaki [16] reported that autoimmune cytotoxic antigranulocyte antibodies were found in approximately 50% of SLE patients detected by a panel of 70 different granulocytes from random normal individuals. Lalezari et al. [17] and Jiang et al. [18] demonstrated that granulocytes possess a set of alloantigenic specificities encoded by separate genes, loci NA, NB and NC, other than HLA antigens when detected by leuco-agglutination assays. Starkebaum et al. [19] found that increased IgG binding on the surface of PMN was the major cause of neutropenia in SLE. These bound monomeric IgG anti-PMN autoantibodies facilitated the opsonization of PMN engulfed by mononuclear phagocytes. The F(ab′)2 fragment of the IgG molecules was confirmed as the true antibody in nature [20]. Furthermore, Hardley et al. [21] noted that the PMN-bound antibodies from SLE were capable of penetrating into cytosol and nuclei. Reports also demonstrated that SLE sera were able to opsonize PMN and fix C3 that correlated inversely with neutrophil counts in SLE [22,23]. The binding specificity of anti-PMN antibodies in SLE was investigated further by Sipos et al. [24], who demonstrated that the antibodies reacted with two membrane antigens on PMN with molecular weights of 50–60 kDa and 30 kDa. Functional analysis revealed that the anti-PMN autoantibodies inhibited the binding of mouse monoclonal antibodies with CD15 (granulocyte antigen, 46 kDa) and CD16 (FcγRIIIb, 50–80 kDa) on normal human neutrophils. However, whether the cognate antigens of anti-PMN in SLE serum are identical to CD15 or CD16 were not confirmed in that study.
To explore the nature of the cognate antigen(s) and immunopathological roles of anti-PMN in SLE, immunoscreening, molecular cloning and Escherichia coli expression of the gene(s) encoding the anti-PMN cognate antigens from human leucocyte λ–phage expression library was conducted. We found that 50 kDa SSB/La is one of the cognate autoantigens expressed on human PMN surface. Anti-SSB/La autoantibodies purified from SLE sera could penetrate into PMN after binding with the PMN surface responsible for increased apoptosis and functional impairments of normal PMN in vitro. These results suggest that SSB/La autoantibody is one of the antineutrophil autoantibodies responsible for the neutropenia and functional defects of PMN in vivo in patients with SLE.
Materials and methods
Patients and controls
The sera were collected from normal individuals and the stocked SLE sera containing anti-dsDNA antibodies titre ≥ 100 IU/ml after routine serological measurements in a clinical immunological laboratory. These SLE sera were expected to be discarded after 3–6 months storage. The normal sera were confirmed negative for antinuclear antibody test and normal haemogram.
Preparation of polymorphonuclear neutrophils from normal human peripheral blood
Heparinized venous blood obtained from healthy volunteers was mixed with one-fourth volume of 2% dextran solution (mol. wt. 464 000) (Sigma Chemical Company, St Louis, MO, USA) and sedimented at room temperature for 30 min. Leucocyte-enriched supernatant was collected and layered over Ficoll-Hypaque (specific gravity 1·077) (Pharmacia Biotech, Uppsala, Sweden) density gradient solution. After centrifugation at 300 g for 25 min, PMN were obtained from the bottom. In some experiments, mononuclear cells (MNC) were aspirated concomitantly from the interface after centrifugation. The contaminating red blood cells in PMN suspension were lysed by suspending in chilled 0·83% ammonium chloride solution at 4°C for 10 min. Cells concentration was adjusted to 5 × 106/ml in 10% fetal bovine serum (FBS) in RPMI-1640 (10% FBS-RPMI). Both purity and viability of PMN and MNC were greater than 95%, as confirmed by Wright's stain and trypan blue dye exclusion, respectively.
Preparation of surrogate immune complexes
The method reported by Gamberale et al. [25] was adapted. Briefly, human γ-globulin (Sigma) was heat-aggregated at 63°C for 1 h at a concentration of 5 mg/ml. The aggregated human IgG (aggIgG) was centrifuged at 10 000 g for 10 min and the precipitates were discarded. The collected supernatant was stored at 4°C as surrogate immune complex. In all the following experiments (PMN-binding, phagocytosis, PMN penetration and IL-8 production), freshly prepared PMN were usually incubated with aggIgG (50 µg/ml) at 37°C for 1 h in advance for ligating the Fcγ receptors on the surface of PMN. After two washes with PBS, pH 7·2, the aggIgG-treated PMN were used for the following experiments. FcγR ligation by aggIgG (50 µg/ml) did not affect the viability (MTT test) and function (phagocytosis) of PMN (data not shown). In addition, ligation by aggIgG and then blockade by specific anti-FcγRIII antibody (anti-CD16) of FcγR on PMN did not derange the phagocytosis (data not shown).
Detection of PMN-binding capacity of different sera by cellular ELISA
The method of Doughty et al. [26] was used for detecting the amount of neutrophil-binding immunoglobulins in the sera. Briefly, 50 µl of aggIgG-pretreated neutrophil suspension (5 × 106 cells/ml) were placed in 96-well U-shaped flat-bottomed microtitre plates (Linbro/Titertek®, Flow Laboratories, Inc., McLean, VA, USA). The microtitre plate was then centrifuged at 150 g for 10 min to precipitate the cells at the bottom. After removing the supernatant carefully, the neutrophils were fixed by 0·24% glutaraldehyde solution at 4°C for 30 min followed by incubation with 1% bovine serum albumin (Sigma) for 2 h to reduce the non-specific binding with protein molecules. One hundred microlitres of SLE or control sera by 1 : 50 dilution was added to the wells and incubated for 1 h at room temperature. After several washes with PBS, pH 7·2, 100 µl of 1 : 5000 diluted HRP-conjugated goat antihuman IgG (Chemicon International Inc., Temecula, CA, USA) was added and incubated for 1 h at room temperature followed by reacting with 150 µl of substrate containing ortho-phenilen diamine (Sigma) for 10 min in the dark. The reaction was stopped by the addition of 50 µl 4 N H2SO4 solution. The amount of antibodies bound to PMN was measured by an ELISA reader (Dynex Technologist, Chantilly, VA, USA) at O.D.490nm absorbance. We defined anti-PMN(+) SLE sera as those exhibited PMN-binding capacity (O.D.490nm) ≥mean + 2 s.d. of normal sera. Only these anti-PMN(+) SLE sera were used in the following experiments. Because at least two confounding factors, hypergammaglobulinaemia and immune complexes, existed in SLE sera, the binding of SLE sera with PMN could be enhanced by false positivity. However, after 50 × dilution of sera and previous ligation of IgGFcR on PMN by heat-aggregated human IgG, these two confounding factors in SLE sera could be minimized. The anti-PMN(+) SLE serum was adequate for the detection of cognate antigen(s) and immunoscreening for the gene(s) encoding the cognate antigen(s) in the human leucocyte cDNA library.
Detection of cognate antigen(s) for antineutrophil antibodies on PMN surface by membrane biotinylation, streptavidin-column elution and Western immunoblot
PMN lysates were prepared according to the method reported by Garrels and Gibson [27]. One hundred microlitres of sample buffer (1 × = 125 nm Tris-HCl pH 6·8, 2% SDS, 5% glycerol, 0·03% bromophenol blue and 1% β-mercaptoethanol) were added to lyse 1 × 107 freshly prepared neutrophils in pellets followed by boiling the lysates for 7 min with frequent vigorous vortexing. The precipitates were removed after centrifugation at 11 800 g at 4°C for 15 min. The clear PMN lysates (15 µl/well) were electrophoresed in 10% SDS-PAGE. The dispersed proteins in gel were transferred to a nitrocellulose paper in a semidry transfer system. The membrane was immersed in the blocking buffer (5% non-fat milk in wash buffer containing 10 nm Tris pH 7·5, 100 nm NaCl, and 0·1% Tween-20) for 1 h at room temperature and then probed by either anti-PMN(+) SLE or normal serum at 1 : 4 diluted in 5% non-fat milk containing wash buffer. HRP-conjugated goat antihuman IgG antibodies (Chemicon) diluted at 1 : 5000 in wash buffer was used as the secondary antibodies. The antigen–antibody reaction was detected by the enhanced chemiluminescence (ECL) protein detection system (Amersham International, Amersham, UK). To detect the cognate antigen(s) of anti-PMN expressed on the cell surface of PMN, freshly isolated PMN (5 × 106 cells/ml) suspended in borate buffer (50 mm sodium borate in 150 mm NaCl, pH 8·0) were membrane biotinylated by incubation with 1 ml of biotin-labelling buffer (0·1 mg biotin-7-NHS/ml in borate buffer) (Boehringer-Mannheim Biochemicals, GmbH, Mannheim, Germany) at 4°C for 1 h with gentle shaking. After several washes with PBS, 1 ml of the extraction buffer containing 50 mm sodium borate, 150 mm NaCl, 1% NP-40, 0·5% sodium deoxycholate and 0·1 mg/ml PMSF, pH 8·0 were added to the pellet to lyse the membrane biotinylated PMN. The cell lysates were sonicated further in an ice-bath. After centrifugation at 5200 g for 10 min, the biotinylated membrane proteins were absorbed into the streptavidin beads packing column (ImmunoPure®, Pierce, Rockford, IL, USA) and eluted by glycine buffer, pH 2·5. The detailed procedures were described in the manufacturer's instruction sheets. The eluted biotinylated membrane proteins of PMN were then treated with 2 × sample buffer and the cognate antigens of anti-PMN in PMN membrane lysates were identified by Western immunoblot probed by anti-PMN(+) SLE serum.
Peptide mapping of surface membrane expressed cognate antigen(s) for anti-PMN(+) SLE sera
The surface membrane-expressed molecules on PMN reactive with anti-PMN(+) SLE sera in Western immunoblot gel were excised and eluted. After proteases (Lys C) cleavage and alkylation to prevent the linkage of multiple peptides through disulphide bonds, the digested peptides were separated further by reverse-phase HPLC on octadecyl silica gel. The peptide mapping was analysed using the ProFound computer program at the Beckman Center, Stanford University Medical Center.
Immunoscreening and molecular cloning of the genes encoding the cognate antigens of anti-PMN from human leucocyte cDNA library by anti-PMN(+) SLE sera
The screening and cloning of the genes encoding the cognate antigens of anti-PMN autoantibodies was carried out according to the method reported by Young and Davis, with some modifications [28,29]. A human leucocyte cDNA expression library (λTriplEx™ and λTriplEx2™) purchased from Clontech Laboratories Inc. (Palo Alto, CA, USA) was immunoscreened by SLE sera containing anti-PMN autoantibodies. Briefly, recombinant phages were plated on E. coli strain XL1-Blue at 1 × 105 plaques per 15-cm diameter dishes. β-galactosidase fusion protein expression was induced and transferred to nitrocellulose filters presoaked with 10 mm isopropyl thiogalactopyranoside (IPTG). The filters were screened with SLE sera containing high titre anti-PMN autoantibodies by Western immunoblot. The filters were then reacted with HRP-conjugated goat antihuman IgG (Chemicon) and peroxidase colour development reagent 4-chloro-1-naphthol (Sigma). Positive regions (discoloration) were excised, eluted, replated and rescreened until the plaques were uniformly positive. The insert sizes of positive clones were determined by PCR. The nucleotide sequence of the inserts was determined by a dsDNA cycle sequencing system (Gibco BRL Inc., Gaithersburg, MD, USA) and identified by the nucleotide blast program from the National Center for Biotechnology Information (NCBI). The detailed procedures were described in the manufacturer's instruction booklet.
Cloning and expression of human full-length SSB/La protein
Total cellular RNA was extracted from Jurkat cells (human T-lymphoma cell line) (1 × 107/ml) after 6 h incubation with PHA (100 ng/ml) (Sigma) at 37°C, 5% CO2−95% air using the RNeasy® Mini kit (Qiagen GmbH, Hilden, Germany). cDNA was synthesized with 1 µg of denatured total RNA at 42° for 1 h in a final volume of 25 µl containing 1 µg of SSB/La antisense primer, 200 nmole of each dNTP (Pharmacia Fine Chemicals, Piscataway, NJ, USA) and 200 U M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA). The cDNA was amplified by PCR using paired oligonucleotide primers specific for human SSB/La (5′SalI-SSB, ACGCGAGTCGACATGGCTGAAAATGGTG AT AATGAAAAG; 3′XhoI-SSB(H), CATTATCTCGAGCTG GTCTCCAGCACCATTT TCTG). A HYBAID OmniGene DNA thermal cycler (Teddington, Middlesex, UK) was used to run 35 cycles of denaturation at 95°C for 1 min, annealing at 56°C for 1 min and extension at 60°C for 2 min. The PCR product of 1227 bp SSB/La cDNA was purified by GFX™ PCR DNA and Gel Band purification kit (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA), was used later as the insert.
Two microlitres of E. coli XL1-Blue were incubated in 3 ml LB broth (1 g NaCl, 1 g tryptone peptone and 0·5 g yeast extract in 100 ml LB broth) with shaking at 37°C overnight. After refreshing in 40 ml LB for 1 h and on ice for 10 min, the competent cell XL1-Blue was prepared by adding 100 mm CaCl2 and incubating on ice for 20 min. Plasmid vector pET23b (Novagen Inc., Madison, WI, USA) transformed into competent XL1-Blue by heat shock at 42°C for 2 min was incubated in LB broth with shaking at 37°C overnight. The pET23b was extracted and purified by GFX™ Micro Plasmid Prep kit (Amersham Pharmacia).
Five hundred nanograms of purified SSB/La PCR product and vector pET23b, respectively, were digested at 37°C by restriction enzyme SalI (Promega) and XhoI (Promega) overnight, respectively. The same amount of 50 ng digested SSB/La and pET23b was incubated with 1 µl T4 DNA ligase (Epicentre Technologies, Madison, WI, USA) and ATP (1 mm) at 4°C overnight for ligation. After cleaning with the GFX™ PCR DNA purification kit, 10 ng of ligased pET23b vector was transformed into competent XL1-Blue E. coli by heat shock. The transformed XL1-Blue was refreshed in LB broth at 37°C for 1 h and cultured on LB agar plates containing ampicillin (75 µg/ml) at 37°C overnight. The success of plasmid transformation enables the growth of XL1-Blue in LB agar plates with ampicillin at 37°C overnight. The single colony of XL1-Blue was transferred to 1 ml LB broth with ampicillin and incubated at 37°C overnight. After centrifugation at 1300 g for 10 min, the plasmid pET23b was purified by use of the GFX™ Micro Plasmid Prep kit. The pET23b vector containing SSB/La insert was screened by PCR using oligonucleotide primers of SSB/La and confirmed by DNA sequencing.
Competent cells of BL21(DB3)pLysS (Promega) were prepared and the pET23b containing SSB/La was transformed into these competent cells, as described above. The transformed BL21(DB3)pLysS was cultured in LB agar plates with ampicillin (75 µg/ml) at 37°C overnight. After refreshing the cultured BL21(DB3)pLysS colony in LB broth by shaking at 37°C for 3 h (bacterial concentration O.D.600nm = 0·6–1·0), the induction of recombinant SSB/La was conducted by adding IPTG (0·4 mm) and incubation at 37°C for 3 h. After sonication of IPTG-induced BL21(DB3)pLysS in 150 µl PBS and centrifugation at 11800 g for 30 min, the pellet (insoluble form) and supernatant (soluble form) were prepared for Western blotting, as mentioned above.
The SSB/La protein from IPTG-induced transformed BL21(DB3)pLysS was purified by use of a HisTrap kit (Amersham Pharmacia). In brief, the induced BL21(DB3)pLysS was sonicated completely after incubation with imidazole (10 mm) containing binding buffer. The supernatant was collected and eluted through HisTrap Chelating HP columns with immobilized divalent cations (Ni2+). The target SSB/La expressed protein was recovered by elution with gradient imidazole concentrations from 10 mm, 20 mm and 40 mm to 500 mm. The eluted purified protein was dialysed in PBS buffer, pH 7·2 and then concentrated with disposable microconcentrator. The detailed procedures were described in the manufacturer's instruction booklet. The purity and identification of the recombinant SSB/La protein were detected by 10% SDS-PAGE and Western blot probed by anti-SSB/La autoantibodies purified from anti-PMN(+) SLE sera.
Purification of anti-SSB/La antibodies from anti-PMN(+) SLE sera by recombinant SSB/La affinity column
The recombinant SSB/La protein conjugated CNBr-activated Sepharose 4B gel packing column (Pharmacia Biotech, Wisdoms, Sweden) was prepared by the method reported elsewhere in the literatures. The anti-PMN(+) SLE sera were incubated with the SSB/La affinity column at 4°C overnight. After washing with Tris buffer, pH 8·0, the anti-SSB/La antibodies were eluted by glycine buffer, pH 2·5. The detailed procedures were described in the manufacturer's instruction sheets. The antigen-binding activity of the purified human anti-SSB/La antibodies was detected by Western immunoblot using the same recombinant SSB/La as substrate. In addition, we used a commercial immunoassay kit (line immunoassay) (anti-ENA) (SLR Research Corporation, Carlsbad, CA, USA) to detect the presence of anti-extractable nuclear autoantibodies, including anti-RNP, anti-Scl-70, anti-SSA/Ro, anti-SSB/La, anti-Jo-1 and anti-Sm specificities in these affinity column-purified anti-SSB/La autoantibodies. The detailed procedures were described in the manufacturer's instruction sheets. We found that different eluted samples contained different potencies of anti-SSB/La but seldom other anti-ENA activity (data not shown).
Measurement of PMN apoptosis by flow cytometry
PMN apoptosis was measured by flow cytometry according to the method of Nicoletti et al. [30]. Briefly, 400 µl of aggIgG-pretreated PMN (2 × 106 cells/ml) were incubated with 100 µl non-specific human IgG (0·5 mg/ml) or anti-SSB/La (0·5 mg/ml) purified from anti-PMN(+) SLE in a conical tube for 2 h at 37°C in 5% CO2−95% air. After three washes, the treated PMN were incubated in 1 ml of 10% FBS-RPMI for 24 h and 48 h at 37°C in 5% CO2−95% air. At the end of each culture period, the PMN was stained in 0·5 ml of hypotonic fluorochrome solution (50 µg/ml propidium iodide in 0·1% sodium citrate and 0·2% Triton X-100) at 4°C in the dark for 1 h to stain the nuclei. The fluorescence intensity of the cell nuclei was measured by FACSort flow cytometer (Becton-Dickinson, Mountain View, CA, USA) with 488 nm excitation. The percentage of hypodiploid DNA appeared in the left of the diploid DNA peak was regarded as percentage apoptotic PMN.
Measurement of PMN phagocytosis by flow cytometry
The method described by Shalaby et al. [31] was used with some modification for measuring PMN phagocytosis. Briefly, the fluoresbrit carboxylate microspheres (0·75 µm in diameter, Polyscience Inc., Washington, PA, USA) were opsonized with fresh normal human serum at 37°C for 1 h. After two washes, the opsonized microspheres were adjusted to 1 × 109 particles/ml in PBS, pH 7·2. Freshly prepared PMN (2 × 106/ml) were preincubated with culture medium, LPS (100 ng/ml), non-specific human IgG (0·5 mg/ml) or purified human anti-SSB/La (0·5 mg/ml) for 45 min, followed by incubation with 100 µl opsonized fluorescence microspheres for another 45 min. The treated PMN was then fixed with 2% paraformaldehyde to stop phagocytosis. After several washes, the percentage and mean fluorescence of PMN phagocytosis was determined by FACSort flow cytometer (Becton-Dickinson) at 488 nm excitation.
Detection of anti-SSB/La antibody penetration into PMN by flow cytometry
AggIgG-pretreated PMN (1 × 106/ml) were incubated with culture medium, purified human anti-SSB/La (0·5 mg/ml) or non-specific human IgG (0·5 mg/ml) in ice-bath for 30 min. After washing three times with PBS, pH 7·2, the PMN was fixed with 4% paraformaldehyde for 15 min, treated with 0·1% Triton X-100 for 5 min and stained with 1 : 100 diluted FITC-conjugated goat antihuman IgG antibodies (Jackson ImmuniResearch Laboratories Inc., West Grove, PA, USA) in an ice-bath for 30 min. To prevent the non-specific endocytosis of PMN, the cells were pretreated with 10 ng/ml of cytochalasin B (CyB, Sigma) for 45 min. The percentage of active penetration of anti-SSB/La or non-specific IgG was calculated by the following formula after flow cytometric determination:
ELISA for IL-8 concentration of PMN cultured supernatants
AggIgG-pretreated PMN (1 × 106/ml) were preincubated with 0·5 mg/ml of human IgG or anti-SSB/La antibodies in conical tubes for 2 h as mentioned above. After washing, the treated PMN were cultured continuously in 10% FBS-RPMI for 24 h at 37°C in 5% CO2−95% air. The culture supernatants were collected for IL-8 quantification using a commercially available human IL-8 ELISA kit (Endogen, Woburn, MA, USA). The detailed procedures were described in the manufacturer's instruction booklet. The minimal detectable concentration of IL-8 was 18·1 ρg/ml.
Statistical analysis
Results represent the mean ± s.d. in this study. Statistical significance was assessed by Student's t-test or non-parametric Wilcoxon rank sum test.
Results
PMN-binding activity of SLE sera
Seventy-one sera from patients with active SLE and an equal number of normal individuals were compared for PMN-binding capacity using the cellular ELISA method. A significant difference of PMN-binding activity was found between SLE sera (O.D.490nm = 1·683 ± 0·312) and normal sera (O.D.490nm = 1·337 ± 0·347) (data not shown). For further investigation, the anti-PMN(+) serum was defined as the serum with a PMN-binding capacity ≥mean + 2 s.d. of normal sera. We found that 16/71 (22·5%) of SLE sera were positive for anti-PMN, as shown in Fig. 1a. These anti-PMN(+) SLE sera were used for the detection and immunoscreening of the genes encoding the cognate antigen(s) of anti-PMN from human leucocyte cDNA library, as described below.
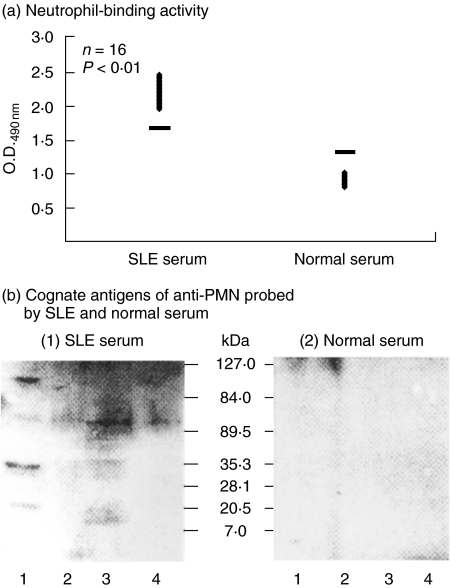
Fig 1.
Detection of cognate antigen(s) of anti-PMN autoantibodies on normal human neutrophil (PMN) surface (b) after screening the high titre of anti-PMN sera from patients with SLE (a). (a) Comparison of PMN-binding activity of SLE and normal sera by PMN cellular ELISA (please see Materials and Methods). (b) Identification of cognate antigen(s) of anti-PMN autoantibodies on PMN surface after membrane biotinylation, streptavidin-column purification and Western blot analysis of PMN lysates probed by (1) SLE serum, (2) normal serum. Lane 1: total human MNC lysate; lane 2: MNC lysate after membrane biotinylation and streptavidin column purification; lane 3: total PMN lysate; and lane 4: PMN lysate after membrane biotinylation and streptavidin column purification. A 50–60 kDa surface-expressed molecule on PMN was found reactive with anti-PMN autoantibodies in SLE serum [b-(1)], but not reactive with normal serum [b-(2)]. The same experiment has been conducted 10 times with a similar tendency.
Detection of cognate antigen(s) of anti-PMN autoantibodies on PMN by Western immunoblotting after membrane biotinylation and streptavidin-column elution
We used total cell lysates and the lysates after membrane biotinylation and streptavidin-column purification as substrates in Western blot that were probed by anti-PMN(+) SLE or normal serum. As demonstrated in Fig. 1b(1), several cognate antigens in total cell lysates of PMN (lane 3) and MNC (lane 1) were detected by anti-PMN(+) SLE serum, but not by normal serum [Fig. 1b(2)]. The molecular weights of the cognate antigens in total PMN lysates detected by anti-PMN autoantibodies were 15, 30, 50 and 100 kDa proteins (lane 3). However, when PMN membrane lysates were probed by anti-PMN(+) SLE in Western blot after membrane-biotinylation and streptavidin column purification, we found 30% of anti-PMN(+) SLE sera, but not normal sera, reactive with 50 kDa surface expressed molecules on PMN [Fig. 1b(1), lane 4]. This 50 kDa band was cut for peptide digestion and amino acid sequence identification. The preliminary result demonstrated that the protein is 46% homologous with SSB/La molecule (data not shown).
Immunoscreening, molecular cloning and nucleotide sequencing of the genes encoding cognate antigens of anti-PMN antibodies
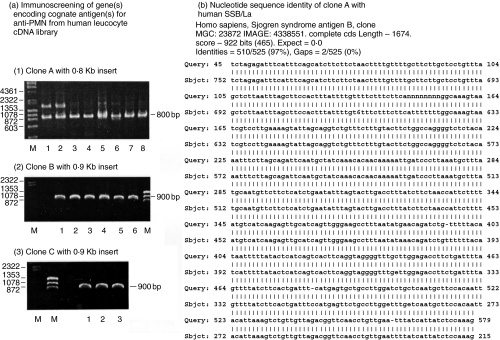
Immunoscreening of human leucocyte cDNA expression library by anti-PMN(+) SLE sera exhibited three positive clones (one 0·8 Kb and two 0·9 Kb) encoding the cognate antigens of anti-PMN autoantibodies [Fig. 2a(1), (2), (3)]. The nucleotide sequencing of the PCR amplified inserts revealed that the 0·8Kb clone A [Fig. 2a(1)] was 97% homologous with the gene encoding SSB/La protein (47·7 kDa) [Fig. 2b]. The 0·9Kb clone B was 99% homologous with human histone 3 (15·3 kDa), but the other 0·9Kb clone C was a hypothetical gene after computer analysis of nucleotide blast program provided by NCBI.
Fig 2.
Immunoscreening, molecular cloning, and nucleotide sequencing of the gene(s) encoding cognate autoantigen(s) of anti-PMN antibodies from human leucocyte cDNA expression library probed by anti-PMN(+) SLE sera. (a) Immunoscreen of a human leucocyte cDNA library, λTriplEx™ and λTriplEx2™, probed by high titres of anti-PMN(+) SLE serum. Three clones, clones A, B and C, were obtained. The DNA inserts in the positive clones were amplified by PCR and then electrophoresed in agarose gel. (1) Eight representative colonies were selected from clone A for PCR amplification and all exhibited 0·8Kb. (2) Six representative colonies are selected from clone B for PCR amplification and all exhibited 0·9Kb. (3) Three representative colonies were selected from clone C for PCR amplification and all of them exhibited 0·9Kb. M: DNA marker (λ/HindIII + φX174/HaeIII). (b) Nucleotide sequence of clone A (525 bp) showed 97% (510/525) homology with La/SSB (47·7 kDa) analysed by nucleotide blast program of NCBI.
Cloning and expression of full-length SSB/La DNA from Jurkat T cells
Full-length cDNA of SSB/La was prepared from PHA-stimulated Jurkat T cells by RT-PCR. After digestion by restriction enzyme, plasmid pET23b and SSB/La were ligated and transformed into E. coli XL1-Blue. The insertion of SSB/La in pET23b was amplified by PCR and confirmed further by direct DNA sequencing (data not shown). The SSB/La carried vector pET23b and was then transformed into the BL21(DB3)pLysS expression system. After induction with ITPG, the SSB/La protein was collected and conjugated with the HisTrap chelating HP column. The human recombinant SSB/La protein was eluted effectively by a high concentration (500 mm) of imidazole and the purity and molecular weight of the recombinant SSB/La were determined by 10% SDS-PAGE [Fig. 3a, lane 4].
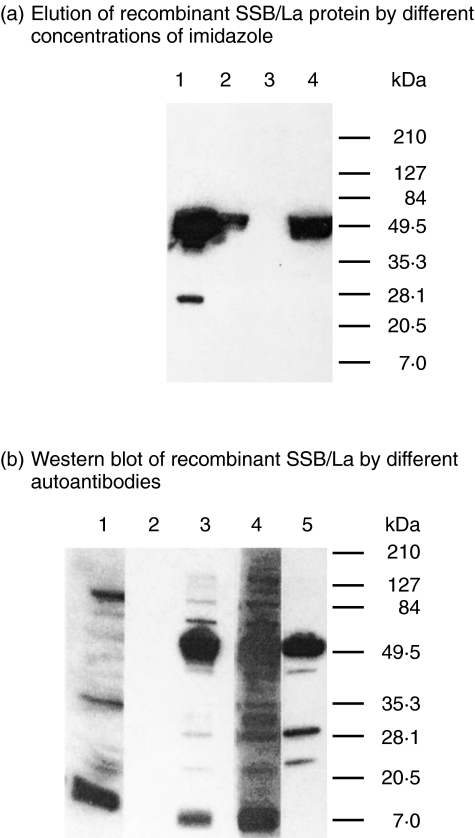
Fig 3.
Purification and identification of recombinant human SSB/La protein expressed in E. coli. (a) Detection of recombinant SSB/La protein by 10% SDS-PAGE after elution with different concentrations of imidazole through HisTrap chelating HP column. Lane 1: crude E. coli. extract; lane 2: elute with PB buffer pH 7·2; lane 3: elute with 10 mm imidazole in PB buffer; and lane 4: elute with 500 mm imidazole in PB buffer. (b) Western immunoblotting of the eluted SSB/La protein probed by different sera and antibodies; lane 1: 1 : 50 diluted normal human serum; lane 2: 1 : 20 diluted monoclonal mouse anti-dsDNA antibody; lane 3: 1 : 50 diluted human anti-SSB/La antibodies purified from anti-PMN(+) SLE sera; lane 4: 1 : 50 diluted SLE sera; and lane 5: 1 : 5000 diluted monoclonal anti6xHis C-terminal antibody.
Purification of anti-SSB/La antibodies from anti-PMN(+) SLE sera by affinity column
Anti-PMN(+) SLE serum was absorbed in a recombinant SSB/La conjugated Sepharose 4B gel column after incubation at room temperature for 2 h. After several washes, the anti-SSB/La antibodies were eluted by glycine buffer, pH 2·5 and the anti-SSB/La containing elute was neutralized immediately with PBS, pH 7·2. The antigen-binding activity of the purified anti-SSB/La antibodies was confirmed by Western blot using recombinant SSB/La as antigen (Fig. 3b, lane 3). Normal serum (lane 1) and mouse monoclonal anti-dsDNA (lane 2) did not react with 50 kDa SSB/La protein. Alternatively, monoclonal anti-dsDNA antibody did not cross-react with SSB/La but anti-PMN(+) SLE serum (lane 4), the purified anti-SSB/La (lane 3) and anti-His C-terminal antibodies (lane 5) reacted potently with SSB/La. In addition, a line immunoassay that is considered as the ‘gold standard’ in detecting anti-extractable nuclear protein autoantibodies used in the clinical immunological laboratory also confirmed that the purified anti-SSB/La autoantibodies reacted mainly with SSB/La (data not shown). These purified anti-SSB/La antibodies were tested for immunopathological effectiveness on normal PMN functions.
Impaired PMN phagocytosis by human anti-SSB/La autoantibodies
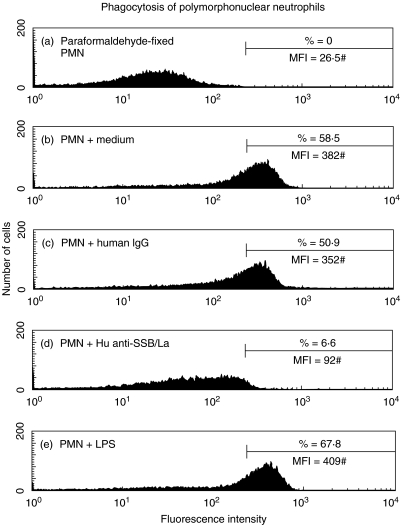
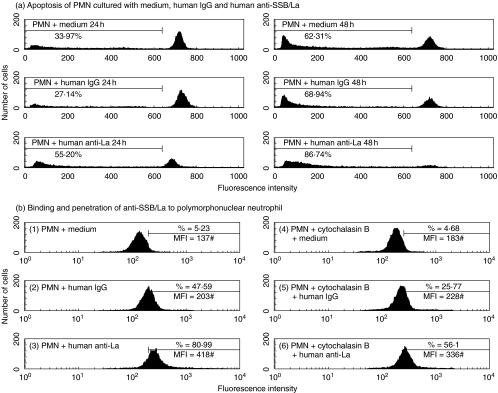
` neutrophils (1 × 106/ml) were incubated with human anti-SSB/La (0·5 mg/ml), non-specific human IgG (0·5 mg/ml), LPS (100 ng/ml) or culture medium for 1 h. One hundred microlitres of opsonized fluorescence microspheres (1 × 109 beads/ml) was added to PMN suspension and incubated for another 45 min for phagocytosis assay. A representative case demonstrating impaired PMN phagocytosis in both percentage (%) and mean fluorescence intensity (#MFI, denoted by mean fluorescence channel) by anti-SSB/La compared to non-specific human IgG is showed in Fig 4.. The same experiment was repeated three times with a similar tendency.
Fig 4.
A representative case demonstrates the effect of purified human anti-SSB/La on PMN phagocytosis. PMN was preincubated with anti-SSB/La (0·5 mg/ml) for 45 min, followed by reacting with opsonized fluorescence microspheres. The percentage (%) and mean fluorescence (MFI#) of PMN phagocytosis were detected by flow cytometry. The same experiment was repeated for three times with a similar result.
Increased neutrophil apoptosis by human purified anti-SSB/La autoantibodies
We preincubated normal human PMN with purified anti-SSB/La autoantibodies (0·5 mg/ml) or non-specific human IgG (0·5 mg/ml) for 24 h and 48 h. PMN apoptosis was determined by flow cytometry. The percentage of hypodiploid DNA in the pre-G0/G1 phase of cell cycle was regarded as the percentage PMN apoptosis. A representative case showed that the percentage apoptosis of PMN incubation with anti-SSB/La was higher than with non-specific human IgG after 24 h (Fig. 5a, left panel) and 48 h (Fig. 5a, right panel) incubation. The same experiment was repeated four times with a similar tendency.
Fig 5.
The effect of purified human anti-SSB/La autoantibodies on aggIgG-treated PMN apoptosis and the penetrating activity of the autoantibodies into human neutrophils.(a) The effect of purified human anti-SSB/La (0·5 mg/ml), and human IgG (0·5 mg/ml) on PMN apoptosis was determined by flow cytometry. PMN was incubated with medium, human IgG and anti-SSB/La for 24 h and 48 h. The percentage of hypodiploid DNA appeared before the G0/G1 phase of cell cycle was regarded as percentage apoptosis. The same experiment was repeated for four times with a similar tendency on each occasion. (b) A representative case demonstrates the binding and penetrating activities of the purified anti-SSB/La autoantibodies into PMN. PMN was preincubated with/without cytochalasin B (10 ng/ml) and then reacted with anti-SSB/La (0·5 mg/ml) or non-specific human IgG (0·5 mg/ml) for 30 min in an ice-bath. The percentage of intracellular IgG was determined by flow cytometry after fixed with 4% paraformaldehyde and permealization with 0·1% Triton X-100. The same experiment was repeated three times with a similar tendency.
Binding and penetration ability of anti-SSB/La autoantibodies toward PMN
For evaluating the penetrating activity of anti-SSB/La autoantibodies into PMN cells, we preincubated normal human PMN ± cytochalasin B (10 ng/ml) for 45 min and then reacted with anti-SSB/La or non-specific human IgG in an ice-bath for 30 min. After fixation and permeabization, the cells were stained with FITC-conjugated goat antihuman IgG. A representative case is presented in Fig. 5b in that the percentage of active penetration of anti-SSB/La (51·24%) was much higher than IgG (21·09%) by calculation. The same experiment was repeated six times with a similar tendency.
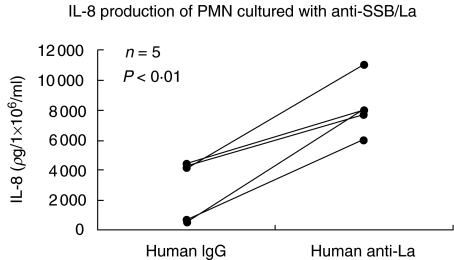
Increased neutrophil interleukin 8 production by anti-SSB/La
As demonstrated in Fig. 6, anti-SSB/La antibodies stimulated IL-8 production of human PMN more than non-specific human IgG. Combining the results of Figs 5 and 6, it is possible that anti-PMN autoantibodies activated simultaneously the genes mediating IL-8 synthesis and apoptosis that seems to be compatible with the phenomenon of activation-induced cell death (AICD).
Fig 6.
Comparison of IL-8 production by normal human PMN after incubation with purified anti-SSB/La (0·5 mg/ml) and human IgG (0·5 mg/ml) for 24 h detected by commercial ELISA kit.
Discussion
Polymorphonuclear neutrophils are regarded traditionally as terminally differentiated cells incapable of protein synthesis and are committed to death via apoptosis within 72 h [32–34]. However, recent studies indicate that neutrophils are not only capable of receiving signals from different proinflammatory cytokines, bacterial lipopolysaccharide and glucocorticosteroids to suppress apoptosis [35–38], but also synthesize many important proinflammatory cytokines, chemokines and haematopoietic growth factors after stimulation to modulate immune responses [6,7]. Neutropenia, one of the common haematological abnormalities in systemic lupus erythematosus, correlates with disease activity and becomes a major cause of morbidity/mortality in patients [19, 23, 39]. It is conceivable that both accelerated apoptosis and impaired functions of the neutrophils render SLE patients susceptible to bacterial infection [11, 12, 13, ]. Many authors have demonstrated that increased neutrophil apoptosis in SLE is owing to the cytotoxic effect of anti-PMN autoantibodies in the circulation [19–24,40]. However, the real immunopathological roles and the cognate antigen(s) of the anti-PMN autoantibodies were not elucidated in the literature.
Four original findings were derived from the present study: (a) approximate 20% active SLE sera contained PMN binding autoantibodies as demonstrated in Fig. 1a. (b) The cognate antigens of anti-PMN autoantibodies including 15, 30, 40, 50 and 80 kDa molecules (Fig. 1b, lane 3) were present in total PMN lysates, but only 50 kDa molecule (Fig. 1b(1), lane 4) is expressed on the surface of PMN. (c) Increased PMN apoptosis (Fig. 5a) and decreased PMN phagocytosis (Fig. 4) were exerted by anti-SSB/La autoantibodies. (d) Anti-SSB/La autoantibodies activated PMN to increase IL-8 production (Fig. 6). Biologically, apoptosis is linked intimately to cell stimulation and proliferation [41,42]. Accordingly, cell activation and cell death are inseparable once the cells are activated. The activation-induced cell death (AICD) phenomenon is found commonly in antigen- or mitogen-activated immune-related cells [43,44]. The immunopathological effects of anti-SSB/La on PMN were seem through the AICD mechanism, because both IL-8 and apoptotic genes in PMN are elicited simultaneously by these antibodies. Interestingly, our results are similar to those in a number of reports. Muhl et al. [45] reported the expression and release of chemokines associated with apoptotic cell death in human promonocytic U937 cells and peripheral blood mononuclear cells. Hagimoto et al. [46] demonstrated that Fas receptor ligation induced IL-8 secretion and apoptosis in bronchiolar epithelial cells. Lai et al. [47] demonstrated that polyclonal antidsDNA autoantibodies enhanced the gene expression of IL-8, TGF-β and nitric oxide synthase in cultured umbilical vein endothelial cells in parallel with the degree of cell apoptosis. However, whether these immunopathological effects of anti-SSB/La autoantibodies on PMN are compatible with AICD needs further investigation.
Because many IgG Fc receptors are expressed on the PMN surface, it is possible that the immunopathological effects of anti-SSB/La autoantibodies on PMN were through the interactions of Fc portion of antibody and Fcγ receptors on PMN to transduce certain signals. To avoid these non-specific effects of Fcγ receptor, we used heat-aggregated human γ-globulins as surrogate immune complexes to ligate FcγR on PMN in advance in some of our experiments. Our results indicate that the immunopathological effects of anti-SSB/La autoantibodies on PMN including cytotoxicity and decreased neutrophil phagocytosis were mediated by specific anti-PMN activity per se, but not by the non-specific interactions of IgG Fc portion and Fcγ receptors on PMN (data not shown). Our preliminary results also confirmed that anti-SSB/La F(ab′)2 fragments as well as intact IgG molecules could impose the above-mentioned immunopathological effects on human PMN (data not shown).
Interleukin 8 (IL-8) can stimulate many neutrophil functions, such as chemotaxis, release of lysosomal enzymes and generation of reactive oxygen metabolites [48,49]. In addition, IL-8 acts as an autocrine stimulator to enhance IL-8 production of neutrophils. A growing body of evidence suggests that IL-8 prolongs the life span of neutrophils by suppressing the apoptotic process [35]. Thus, PMN per se could amplify the recruitment of PMN, rescue the short life span and enhance inflammatory strength in the inflammatory sites by autocrinic IL-8 synthesis. In our previous report, we demonstrated that the spontaneous and bacterial lipopolysaccharide-stimulated production of IL-8 in vitro were impaired in neutrophils of patients with SLE [13]. Furthermore, our preliminary data suggested that the expression of CXCR2, a specific ligand for IL-8, on SLE-PMN was significantly decreased compared to normal PMN. This result suggests that intrinsic defects of PMN per se or the presence of certain undiscovered suppressor factors in the blood cause hyporesponsiveness of PMN to external stimulation in SLE patients. Accordingly, it is possible that anti-PMN autoantibody is one of the suppressor factors in SLE serum to derange the PMN functions via their cytotoxic effects. In the present study, we have successfully immunoscreened and cloned the gene-encoding SSB/La protein by anti-PMN(+) SLE sera and proved that anti-SSB/La antibody is one of the anti-PMN autoantibodies mediating immunopathological effects on PMN.
Several unique immunopathological activities of anti-SSB/La autoantibodies on PMN in SLE serum were noted: (a) anti-SSB/La is one of the anti-PMN autoantibodies in SLE serum. (b) Anti-SSB/La bind not only to the membrane expressed autoantigen SSB/La on PMN, but also penetrate into PMN. (c) The binding/penetration of anti-SSB/La enhances IL-8 production, but impairs the phagocytosis and accelerates the apoptosis of normal human PMN. SSB/La, a 43–52-kDa ribonucleoprotein, participates in the transcriptional regulation of RNA polymerase III as well as some messenger RNAs. Anti-SSB/La appears in as many as 87% of Sjögren's syndrome in association with extraglandular manifestations such as neurological involvement, vasculitis, purpura, leucopenia and lymphopenia [50]. Approximately 10–15% of lupus patients produce anti-SSB/La autoantibodies, especially in association with late-onset SLE, secondary Sjögren's syndrome and the neonatal lupus syndrome [50]. However, reports on the immunopathological roles and clinical relevance of anti-SSB/La in lupus pathogenesis are limited. It has been recognized that many cytoplasmic and nuclear proteins such as SSA/Ro, Sm, proteinase 3 and ribosomal phosphoproteins can translocate to the cell surface after stimulation or in apoptosis. These surface-translocated molecules then become the major autoantigens to induce autoantibody production in certain autoimmune diseases [51–53]. On the other hand, these induced autoantibodies against nuclear/cytoplasmic autoantigens exert their adverse effects on normal tissues via direct cytotoxicity/apoptosis, formation of immune complexes or penetration into cell interior [21, 54, 55]. In the present study, we have demonstrated that SSB/La autoantigen translocates to the cell surface of PMN (data not shown) and anti-SSB/La autoantibodies impose a pathological effect on PMN via cellular surface binding and then penetration into the cells to induce apoptosis. This may lead to neutropenia and functional impairment of PMN in patients with SLE. However, other autoantigen/autoantibody systems such as antibodies against dsDNA (56), ribosomal P proteins or HLA molecules should be considered as other potential contributory factors for neutropenia in patients with SLE.
In conclusion, we are the first authors to find that anti-SSB/La is one of the antineutrophil autoantibodies binding to the surface-expressed SSB/La autoantigens on PMN in vitro and is probably responsible for neutropenia and impaired neutrophil functions in vivo in patients with SLE.
Acknowledgments
This study was supported by grants from the National Science Council (NSC90-2314-B-002–271) and Yeng Tjing Ning Medical Research foundation (CI-90-3-3), Taiwan.
References
- 1.Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967;126:607–24. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levinsky RJ, Camerson JS, Soothill JF. Serum immune complexes and disease activity in lupus nephritis. Lancet. 1977;1:564–7. doi: 10.1016/s0140-6736(77)91998-5. [DOI] [PubMed] [Google Scholar]
- 3.Jacob L, Tron F, Bach J-F, Louvard D. A monocloncal anti-DNA antibody also binds to cell-surface proteins. Proc Natl Acad Sci USA. 1984;81:3843–5. doi: 10.1073/pnas.81.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raz E, Ben-Bassat H, Davidi T, Shlomai Z, Eilat D. Cross-reactions of anti-DNA autoantibodies with cell surface proteins. Eur J Immunol. 1993;23:383–90. doi: 10.1002/eji.1830230213. [DOI] [PubMed] [Google Scholar]
- 5.Budman DR, Steinberg AD. Hematologic aspects of systemic lupus erythematosus. Current concepts. Ann Intern Med. 1977;86:220–9. doi: 10.7326/0003-4819-86-2-220. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophils in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 7.Cassatella MA. Production of cytokines by polymorphonuclear neutrophils. In: Gabrilovich DI, editor. The neutrophils: new outlook for old cells. London: Imperial College Press; 1999. pp. 151–229. [Google Scholar]
- 8.Staples PJ, Gerding DN, Decker JL, Gordon RS., Jr Incidence of infection in systemic lupus erythematosus. Arthritis Rheum. 1974;17:1–10. doi: 10.1002/art.1780170102. [DOI] [PubMed] [Google Scholar]
- 9.Ginzler E, Diamond H, Kaplan D, Weiner M, Schlesinger M, Seleznick M. Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus. Arthritis Rheum. 1979;21:37–44. doi: 10.1002/art.1780210107. [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Urowitz MB, Bookman AA, et al. Systemic lupus erythematosus. A review of 110 cases with reference to nephritis, the nervous system, infections, aseptic necrosis and prognosis. Q J Med. 1977;46:1–32. [PubMed] [Google Scholar]
- 11.Zurier RB. Reduction of phagocytosis and lysosomal enzyme release from human leukocytes by serum from patients with systemic lupus erythematosus. Arthritis Rheum. 1976;19:73–8. doi: 10.1002/art.1780190112. [DOI] [PubMed] [Google Scholar]
- 12.Landry M. Phagocyte function and cell-mediated immunity in systemic lupus erythematosus. Arch Dermatol. 1977;113:147–54. [PubMed] [Google Scholar]
- 13.Yu C-L, Chang K-L, Chiu C-C, Chiang BN, Han S-H, Wang S-R. Defective phagocytosis, decreased tumor necrosis factor-α production, and lymphocyte hyporesponsiveness predispose patients with systemic lupus erythematosus to infection. Scand J Rheumatol. 1989;18:97–105. doi: 10.3109/03009748909099924. [DOI] [PubMed] [Google Scholar]
- 14.Wenger ME, Bole GG. Nitroblue tetrazolium dye reduction by peripheral leukocytes from patients with rheumatoid arthritis and systemic lupus erythematosus measured by a histochemical and spectrophotometric method. J Lab Clin Med. 1973;82:513–21. [PubMed] [Google Scholar]
- 15.Hsieh S-C, Tsai C-Y, Sun K-H, et al. Decreased spontaneous and lipopolysaccharide stimulated production of interleukin 8 by polymorphonuclear neutrophils of patients with active systemic lupus erythematosus. Clin Exp Rheumatol. 1994;12:627–33. [PubMed] [Google Scholar]
- 16.Drew SI, Terasaki PI. Autoimmune cytotoxic granulocyte antibodies in normal persons and various disease. Blood. 1978;52:941–52. [PubMed] [Google Scholar]
- 17.Lalezari P, Radel E. Neutrophil-specific antigens. immunology and clinical significance. Sem Haematol. 1974;11:281–90. [PubMed] [Google Scholar]
- 18.Jiang AF, Lalezari P. A micro-technique for detection of leukocyte agglutinins. J Immunol Meth. 1975;7:103–8. doi: 10.1016/0022-1759(75)90135-0. [DOI] [PubMed] [Google Scholar]
- 19.Starkebaum G, Price TH, Lee PM, Arend WP. Autoimmune neutropenia in systemic lupus erythematosus. Arthritis Rheum. 1978;21:504–12. doi: 10.1002/art.1780210503. [DOI] [PubMed] [Google Scholar]
- 20.Starkebaum G, Arend WP. Neutrophil-binding immunoglobulin G in systemic lupus erythematosus. J Clin Invest. 1979;64:902–12. doi: 10.1172/JCI109556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardley AG, Byron MA, Chapel HM, Bunch C, Holburn AM. Anti-granulocyte opsonic activity in sera from patients with systemic lupus erythematosus. Br J Haematol. 1987;65:61–5. doi: 10.1111/j.1365-2141.1987.tb06136.x. [DOI] [PubMed] [Google Scholar]
- 22.Rustagi PK, Currie MS, Logue GL. Complement-activating anti-neutrophil antibody in systemic lupus erythematosus. Am J Med. 1985;78:971–7. doi: 10.1016/0002-9343(85)90220-7. [DOI] [PubMed] [Google Scholar]
- 23.Logue GL, Shimm DS. Autoimmune granulocytopenia. Annu Rev Med. 1980;31:191–200. doi: 10.1146/annurev.me.31.020180.001203. [DOI] [PubMed] [Google Scholar]
- 24.Sipos A, Caortos C, Sipka A, Gergely P, Sonkoly I, Szegedi G. The antigen/ receptor specificity of anti-granulocyte antibodies in patients with SLE. Immunol Lett. 1988;19:329–34. doi: 10.1016/0165-2478(88)90163-0. [DOI] [PubMed] [Google Scholar]
- 25.Gamberale R, Giordano M, Trevani AS, Andonegui G, Geffner JR. Modulation of human neutrophil apoptosis by immune complexes. J Immunol. 1998;161:3666–74. [PubMed] [Google Scholar]
- 26.Doughty R, James V, Magee JJ. An enzyme linked immunosorbent assay for leukocyte and platelet antibodies. J Immunol Meth. 1981;47:161–9. doi: 10.1016/0022-1759(81)90116-2. [DOI] [PubMed] [Google Scholar]
- 27.Garrels JI, Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976;9:793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- 28.Young RA, Davis RW. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983;222:778–82. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- 29.Young RA, Davis RW. Efficient isolation of gene using antibody probes. Proc Natl Acad Sci USA. 1983;80:1194–8. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 31.Shalaby MR, Aggarwal BB, Rinderknecht E, Svedersky LP, Finkle BS, Palladino MA., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985;135:2069–73. [PubMed] [Google Scholar]
- 32.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci. 1992;83:639–48. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- 34.Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–34. [PubMed] [Google Scholar]
- 35.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–8. [PubMed] [Google Scholar]
- 36.Dunican AL, Leuenorth SJ, Ayala A, Simms HH. CXC chemokine suppression of polymorphonuclear leukocytes apoptosis and preservation of function in oxidative stress. Shock. 2000;13:244–50. doi: 10.1097/00024382-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–8. [PubMed] [Google Scholar]
- 38.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. J Immunol. 1995;154:4719–25. [PubMed] [Google Scholar]
- 39.Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999;58:309–14. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonsson H, Sturfelt G. A novel assay for neutrophil clustering activity of human sera: relation to disease activity and neutropenia in systemic lupus erythematosus. Ann Rheum Dis. 1990;49:46–50. doi: 10.1136/ard.49.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–31. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 42.Mountz JD, Wu J, Cheng J, Zhou T. Autoimmune disease. a problem of defective apoptosis. Arthritis Rheum. 1994;37:1415–20. doi: 10.1002/art.1780371002. [DOI] [PubMed] [Google Scholar]
- 43.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–51. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 44.Nagata S, Goldstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 45.Muhl H, Nold M, Chang JH, Frank S, Eberhardt W, Pfeilschifter J. Expression and release of chemokines associated with apoptotic cell death in human promonocytic U937 cells and peripheral blood mononuclear cells. Eur J Immunol. 1999;29:3225–35. doi: 10.1002/(SICI)1521-4141(199910)29:10<3225::AID-IMMU3225>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Hagimoto N, Kuwano K, Kawasaki M, et al. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am J Respir Cell Mol Biol. 1999;21:436–45. doi: 10.1165/ajrcmb.21.3.3397. [DOI] [PubMed] [Google Scholar]
- 47.Lai KN, Leung JK, Lai CKW. Effect of anti-DNA autoantibodies on the gene expression of interleukin 8, transforming growth factor-β, and nitric oxide synthase in cultured endothelial cells. Scand J Rheumatol. 1997;26:461–7. doi: 10.3109/03009749709065720. [DOI] [PubMed] [Google Scholar]
- 48.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/ interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willems J, Joniau M, Cinque S, Van Damme J. Human granulocyte chemotactic peptide (IL-8) as a specific neutrophil degranulator: comparison with other monokines. Immunology. 1989;67:140–2. [PMC free article] [PubMed] [Google Scholar]
- 50.Peng SL, Graft J. Antinuclear antibodies. In: Ruddy S, editor. Kelley's textbook of rheumatology. Philadelphia: WB Saunders Co.; 2001. pp. 167–71. [Google Scholar]
- 51.Baboonian C, Venables PJ, Booth J, Willianms DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin Exp Immunol. 1989;78:454–9. [PMC free article] [PubMed] [Google Scholar]
- 52.Golan TD, Elkon KB, Gharavi AE, Krueger JG. Enhanced membrane binding of autoantibodies to cultured keratinocytes of systemic lupus erythematosus patients after ultraviolet B/ultraviolet A irradiation. J Clin Invest. 1992;90:1067–76. doi: 10.1172/JCI115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorner T, Hucko M, Mayet WJ, Trefzer U, Burmester GR, Hiepe F. Enhanced membrane expression of the 52kDa Ro (SS-A) and La (SS-B) antigens by human keratinocytes induced by TNF alpha. Ann Rheum Dis. 1995;54:904–9. doi: 10.1136/ard.54.11.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol. 1992;2:1345–54. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- 55.Yanase K, Smith RM, Cizman B, et al. A subgroup of murine monoclonal anti-deoxyribonucleic acid antibodies transverse the cytoplasm and enter the nucleus in a time- and temperature-dependent manner. Laboratory Invest. 1994;71:52–60. [PubMed] [Google Scholar]
- 56.Hsieh S-C, Sun K-H, Tsai C-Y, et al. Monoclonal anti-double stranded DNA antibody is a leukocyte-binding protein to up-regulate interleukin-8 gene expression and elicit apoptosis of normal human polymorphonuclear neutrophils. Rheumatology (Oxford) 2001;40:851–8. doi: 10.1093/rheumatology/40.8.851. [DOI] [PubMed] [Google Scholar]