Abstract
Superantigens (SAgs) are potent stimulators of T cells bearing specific Vβ T cell receptors (TCR) and may play a role in the aetiopathogenesis of systemic vasculitis, although this remains contentious. To investigate the possible aetiological role of SAgs, this study examined peripheral blood T cell Vβ repertoires in children with systemic vasculitis. FACS analysis of 17 different peripheral blood T cell Vβ families was performed in 20 healthy control children, 27 disease control children with nonvasculitic inflammatory disease, 25 children with primary systemic vasculitis, six patients with Kawasaki disease (KD) and six patients with Henoch–Schönlein purpura (HSP). There was a significantly increased variance of CD4 Vβ12 and Vβ17, and CD8 Vβ1 in the primary systemic vasculitis group compared to control and disease controls. Moreover, 80% of the primary systemic vasculitis children had one or more CD4 Vβ expansions or deletions, compared with 30% of controls (P < 0·002), and 37% of the disease controls (P < 0·002). In the KD group, the mean percentage of CD4 Vβ2 T cells was higher than in controls or disease controls. In the HSP group, there was no consistent skewing of the T cell Vβ repertoire. We have observed changes in the T cell Vβ repertoire in children with vasculitis over and above those observed in disease controls. While these data provide impetus for further research into this contentious field, they do not resolve unequivocally the question of the role of SAgs in childhood vasculitic syndromes.
Keywords: child, Kawasaki disease, superantigens, vasculitis
Introduction
Systemic vasculitis is characterized by the presence of inflammation in and around a blood-vessel wall [1]. The cause of the majority of the childhood vasculitides is unknown, and it is likely that a complex interaction between inherited determinants and environmental factors such as infections trigger the disease [2]. In recent years, there has been considerable interest in the role of superantigens (SAgs) in the aetiopathogenesis of several autoimmune diseases, including the systemic vasculitides [2–6].
SAgs are peptides that differ in several ways from conventional peptide antigens, and characteristically activate a much higher proportion of T cells than conventional antigens [7,8]. SAgs all share a common ability to bind the class II major histocompatibility complex (MHC-II) molecules on antigen-presenting cells, and the variable region of the T cell receptor β-chain (TCR Vβ), resulting in massive stimulation of any T cell that expresses the relevant TCR Vβ element on its surface. Typically, SAgs will stimulate up to 20% of circulating T cells [7], whereas conventional antigens stimulate approximately 1 in every million T cells [9]. Every SAg has a different TCR Vβ profile, and the complete responding TCR Vβ repertoire has not been identified for many of the known SAgs [2]. Moreover, it is likely that many SAgs, with their own responding TCR Vβ profile, remain to be discovered [9].
Following SAg activation, T cells rapidly proliferate resulting in T cell Vβ ‘expansions’ [7,9–11]. This is followed by T cell Vβ-restricted deletion from the peripheral circulation − a process mediated by Fas–Fas ligand [7, 9, 12, 13]. Thus the characteristic ‘immunological footprint’ left by SAgs are the presence of T cell Vβ expansions and deletions in the peripheral circulation of subjects exposed to these toxins.
Abnormal expansions and deletions of T cells bearing particular Vβ gene products have been found in the peripheral blood of adults with primary systemic necrotizing vasculitis (microscopic polyangiitis, Wegener's granulomatosis, giant cell arteritis and polyarteritis nodosa) [2–4,14], providing indirect evidence of superantigenic involvement in the aetiopathogenesis of these vasculitides. Furthermore, an important observation in Wegener's granulomatosis in adults is that those with the disease who are nasal carriers of superantigen-producing staphylococci are significantly more likely to have relapses of vasculitis compared with non-carriers [2,15].
In some studies, children with KD (the second most common vasculitic disorder of childhood in some series) also show non-clonal expansion of peripheral T cells bearing Vβ2 [16–18], although other workers have not confirmed this observation and argue against a SAg-mediated pathogenesis [19–23]. Furthermore, T cells infiltrating the walls of coronary arterial aneurysms [24] and the intestinal mucosa of patients with KD show a skewed T cell Vβ profile, with increased numbers of cells expressing Vβ2 [25]. These results have led to speculation that SAgs play an important aetiopathogenic role in this vasculitic illness. Similar studies on vasculitis in children, apart from KD, are however, lacking.
To investigate the possible aetiological role of SAgs this study examined peripheral blood TCR Vβ repertoires in children with primary systemic vasculitis, Kawasaki disease and Henoch–Schönlein purpura.
Patients and methods
Vasculitis patients
Thirty-seven children with vasculitis at various stages of disease activity and treatment were studied. The diagnosis of vasculitis was established in all on the basis of clinical features of vasculitis in addition to suggestive selective visceral angiography and/or tissue biopsy. The vasculitis was classified subsequently using the American College of Rheumatology (ACR) 1990 classification criteria [26] and the Chapel Hill consensus [27]. Children were classified using both systems because there is controversy over the most reliable classification system for the childhood vasculitides [1,28–30], and there is a considerable degree of ‘polyangiitis overlap’ in the childhood vasculitides [31,32], such that neither system is absolutely sensitive or specific [33]. Neither system, however, has been validated formally in children [1].
Twenty-five children were classified as primary systemic vasculitis. The mean age was 10·1 years (range 2·5–15·6 years), and the male to female ratio was 2·1 : 1. Of this group, using the ACR classification criteria, 19 had polyarteritis nodosa (PAN), one had Wegener's granulomatosis (WG), two had hypersensitivity vasculitis and three had unclassifiable vasculitis. Using the Chapel Hill consensus 16 had PAN, six had microscopic polyangiitis (MPA) and three had unclassifiable vasculitis. Clinical details of these patients are summarized in Table 1. Additionally, longitudinal studies were performed on paired samples collected from patients before and after induction of remission of vasculitis (n = 6). Remission was defined as patients with a Birmingham vasculitis activity score of zero [34] and with normal C-reactive protein and erythrocyte sedimentation rate.
Table 1.
Clinical features of patients with primary systemic vasculitis
| Patient no. | Sex/age (years) | Clinical features | Selective visceral angiography | Biopsy | ANCA (IIF) | Active disease at time of sampling | Vasculitis classification, and system used for classification |
|---|---|---|---|---|---|---|---|
| 1 | M/11·4 | Fever, purpura, crescentic nephritis, arthritis, intestinal inflammation | Renal and hepatic perfusiondefects; aneurysms of mediumand small hepatic arteries | Skin biopsy negative forvasculitis; 33% crescenticnephritis, pauci-immune | neg | N | 1. PAN (*ACR) 2. Microscopic polyangiitis (Chapel Hill consensus) |
| 2 | M/2·7 | Periodic fever, failure to thrive, testicular infarction, pulmonaryvasculitis | Small aneurysms and calibre variation of †SMA and itsbranches | Lung: medium-sized artery perivascular and vascular monocytic and granulocytic infiltrate; spleen: non-specific inflammatory changes; testis: infarction only (no vasculitis demonstrated) | neg | Y | 2. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 3 | F/13·6 | Fever, arthralgia, orbital granuloma,microscopic haematuria | Not done | Orbital granulomatous inflammation compatiblewith WG | neg | Y | 1. WG (ACR) 2. Unclassified (Chapel Hill consensus) |
| 4 | M/8 | Fever, myalgia, weight loss | Renal perfusion defects; SMA aneurysms; ‘corkscrew’ changesof medium and small-sizedhepatic arteries | Not done | neg | Y | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 5 | F/7·5 | Fever, myalgia, livedo reticularis, brain stem infarction, hypertension, renal impairment | Large aneurysms affecting predominantly medium-sizedrenal arteries | Skin biopsy negative forvasculitis | negneg | YY | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 6 | F/3·8 | Failure to thrive, encephalopathy, livedo reticularis, MR brain consistentwith cerebral vasculitis | Not done | Brain biopsy: non-specificreactive changes only | Unclassified using either ACR or Chapel hill con sensus criteria | ||
| 7 | F/14·5 | Fever, weight loss, myalgia, intestinal inflammation, arthralgia | Small aneurysms and calibre variation of medium-sized renal arteries; pruning of small renal arteries; perfusion defects of kidneys, tortuous small hepatic arteries | Indeterminate upper and lower gastrointestinal inflammation | neg | Y | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 8 | M/6·2 | Fever, arthritis, livedo reticularis, myalgia | Normal | Skin biopsy: vasculitis affecting medium and small arteries | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 9 | M/15 | Fever, weight loss, livedo reticularis, testicular pain | Small aneurysms affecting medium and small renal and hepatic arteries | Not done | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 10 | M/9·8 | Fever, urticaria, palpable purpura, myalgia, Raynaud’s | Not doneSkin: leucocytoclastic vasculitis | neg | N | 1. Hypersensitivity vasculitis (ACR)2. Microscopic polyangiitis orcutaneous leucocytoclastic angiitis (Chapel Hill consensus) | |
| 11 | M/13·6 | Fever, myalgia, weight loss, livedo reticularis | Calibre change and beading of medium and small renal arteries | Not done | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 12 | M/14·1 | Fever, weight loss, myalgia | Small aneurysms and calibre variation of medium and small renal arteries and branches of SMA | Not done | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 13 | F/9·7 | Fever, anaemia, myalgia, arthritis, weight loss | Small aneurysms affecting SMAand branches; small aneurysmsand stenoses of medium to smallrenal arteries | Not done | neg | Y | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 14 | M/14 | Fever, weight loss, testicular pain, myalgia, intestinal inflammation, subcutaneous nodules | Small aneurysms of SMA branches | Skin: perivascular monocytic infiltrate in small arteries | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 15 | M/11·2 | Fever, weight loss, myalgia, deranged liver function, no urinary sediment | Not done | Liver: perivascular granulomatous inflammation | §cANCA | N | 1. Unclassified (ACR) 2. Unclassified (Chapel Hill consensus) |
| 16 | F/10·9 | Fever, arthralgia, macular rash, nephritic–nephrotic | Not done | > 50% crescentic glomerulonephritis | ¶pANCA | N | 1. Unclassified (ACR) 2. Microscopic polyangiitis (Chapel Hillconsensus) |
| 17 | M/2·9 | Fever, heavy proteinuria, myalgia, weight loss, urticaria, pericarditis, negative streptococcal serology | Small aneurysms and calibre variation of medium andsmall renal arteries and SMA | Renal biopsy normal | neg | Y | 1. PAN (ACR) 2. Microscopic polyangiitis (Chapel Hillconsensus) |
| 18 | M/2·9 | Fever, weight loss, intestinal inflammation, bullous rash, arthritis | Small aneurysms and calibrechange affecting SMA and ‡IMA branches, small aneurysmsand pruning of medium and smallrenal arteries | Skin: perivascular monocytic infiltration of small arteries | neg | Y | 1. PAN (ACR) 2. Microscopic polyangiitis (Chapel Hillconsensus) |
| 19 | M/6·2 | Fever, myalgia, abdominal pain, weight loss | Small aneurysms and calibre variation with cut-off of medium and small renal arteries and SMA | Upper GI biopsy normal | neg | Y | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 20 | F/2·5 | Fever, weight loss, myalgia, polymorphous rash, peeling of extremities, conjunctival injection: chronic symptoms persisting for > 12 months; coronary arterial aneurysms on echocardiography | Small aneurysms and calibre variation of medium and small renal and mesenteric arteries | Not done | neg | Y | 1. PAN (ACR): initial classification of KD changed to PAN due to multiple relapses andchronicity of symptoms 2. PAN (Chapel Hill consensus) |
| 21 | F/15·6 | Fever, weight loss, myalgia | Small aneurysms affecting medium and small renal and hepatic arteries | Not done | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 22 | M/8·2 | Fever, myalgia, weight loss, hypertension, livedo reticularis | Small aneurysms affecting medium and small renal and hepatic arteries | Not done Skin: leucocytoclastic vasculitis | neg | N | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 23 | M/12 | Fever, myalgia, polymoprphousrash, lymphadenopathy, diarrhoea, conjunctival injection, weight loss, abdominal pain | Not done | 1. Hypersensitivity vasculitis (ACR) 2. Microscopic polyangiitis (Chapel Hillconsensus) | |||
| 24 | M/13·9 | Fever, weight loss, oral ulceration, myalgia, abnormal liver function | Renal perfusion defects and renal arterial pruning; small aneurysms of medium-sized hepatic arteries | Liver: non-specificinflammatory changes | neg | Y | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
| 25 | M/12·5 | Fever, palpable purpura, myalgia | Small aneurysms and calibre variation affecting branches of SMA and medium and smallhepatic arteries | Skin: perivascularlymphocytic infiltrate insmall arteries, but not arterioles | neg | Y | 1. PAN (ACR) 2. PAN (Chapel Hill consensus) |
ACR: American College of Rheumatology;
SMA: superior mesenteric artery;
IMA: inferior mesenteric artery;
cANCA: cytoplasmic antineutrophil cytoplasmic antibody;
pANCA: perinuclear antineutrophil cytoplasmic antibody.
Six children (five boys) fulfilled completely the diagnostic criteria for KD [35]. The mean age was 1·7 years (range 0·25–5·5 years). Four had coronary arterial aneurysms, and one had axillary arterial aneurysm formation in addition to coronary arterial aneurysms. All patients were in the second week of the illness, and four had already received intravenous immunoglobulin (i.v. IG) at the time of blood sampling. The patients with KD were described separately from those with primary systemic vasculitis because KD differs from these latter vasculitides in that it has a distinct clinical phenotype with unique association with the mucocutaneous lymph node syndrome [31,36] (although there are overlapping clinical features with classical ‘infantile PAN’, perhaps now regarded by some as a severe form of KD), is usually self-limiting (unlike PAN, WG and MPA) and has epidemiological features suggestive of an infectious aetiology, unlike PAN, WG or MPA.
Six children (two boys) were classified as Henoch–Schönlein purpura (HSP) using both ACR [37] and Chapel Hill consensus criteria [27]. The mean age was 11·1 years (range 7–15 years). Four had renal biopsy evidence demonstrating typical glomerulonephritis with IgA-predominant immune deposits and one had a skin biopsy, again demonstrating typical IgA-dominant immune deposits in a small arterial wall.
Controls and disease controls
Blood samples were obtained from 20 control children undergoing venepuncture for preoperative assessment prior to minor orthopaedic procedures. Children with syndromic diagnoses, underlying inflammatory disease, on regular medication, or with intercurrent infection were excluded from this group. The mean age was 10·6 years (range 0·5–19 years), and the male to female ratio was 1·5 : 1.
Disease controls comprised 27 children with bacterial or viral sepsis (n = 5), autoimmune disease without vasculitis (n = 6: one ulcerative colitis, one juvenile dermatomyositis, one CINCA syndrome (chronic idiopathic neurological cutaneous and articular syndrome), one Sneddon syndrome, one transient postviral anti-phospholipid syndrome and one focal and segmental glomerulosclerosis); or recipients of renal allografts (n = 16, eight children with dysplastic kidneys; eight children with posterior urethral valves). These recipients of renal allografts were included because they were on a similar spectrum of immunosuppressive therapy (prednisolone, azathioprine, and cyclosporin) to the primary systemic vasculitis group, but did not have a prior history of vasculitis or other autoimmune disease and hence would control for any effect on the T cell repertoire mediated by immunosuppressive drugs. The mean age of this group was 11·3 years (range 4·5–17·2 years), with male to female ratio of 1·5 : 1.
Positive controls comprised two children with the toxic shock syndrome (TSS). The first was a 3-week-old baby who developed the staphylococcal TSS secondary to a postoperative wound infection following repair of gastroschisis. The organism responsible was Staphylococcus aureus producing the superantigens TSST1 and staphylococcal enterotoxins A, G, and I. Blood was obtained for T cell analysis from this patient approximately 10 days into the illness. The second patient was a 2-year-old boy who developed streptococcal TSS secondary to an infected gastrostomy site. The responsible organism was a Lancefield group A Streptococcus, although this was not sent for toxin typing. Blood was obtained from this patient 12 h into the illness.
Written, informed consent was obtained from all parents, and older children involved in the study. The study was approved by the local hospital research ethics committee.
Analysis of peripheral blood T cell Vβ repertoires
Blood (5–10 ml) was collected into sterile universal bottles containing 40 microlitres of preservative-free heparin (1000 U/ml). Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by Lymphoprep™ (Nycomed, Roskilde, Denmark) centrifugation, and either prepared directly for flow cytometry or frozen suspended in fetal calf serum with 10% dimethyl sulphoxide (Sigma, St Louis, MO, USA). PBMCs were plated onto U-bottomed 96-well plates at a concentration of 1–2 × 106 cells per ml, and incubated for 30 min at 4°C using fluorochrome-conjugated monoclonal antibodies to CD3 (phycoerythrin, mouse IgG1, 1 : 20 dilution, Becton Dickinson, San Jose, CA, USA), CD4 (Quantum Red™, mouse IgG1, 1 : 20 dilution, Sigma) or CD8 (Quantum Red™, mouse IgG2a, 1 : 20 dilution, Sigma), and the following 17 different T cell Vβ families (all FITC conjugated, 1 : 10 dilution, Immunotech Marseille, France): Vβ1 (rat IgG1; clone BL37·2), Vβ2 (mouse IgG1; clone MPB2D5), Vβ3 (mouse IgM; cloneCH92), Vβ5·1 (mouse IgG2a; clone IMMU157), Vβ5·2 (mouse IgG1; clone 36213), Vβ7 (mouse IgG2a; clone ZOE), Vβ8·1 and 8·2 (mouse IgG2a; clone 56C5), Vβ11 (mouse IgG2a; clone C21), Vβ12 (mouse IgG2a; clone VER2·32·1), Vβ13·1 (mouse IgG2b; clone IMMU222), Vβ13·6 (mouse IgG1; clone JU-74), Vβ14 (mouse IgG1; clone CAS 1·1.3), Vβ16 (mouse IgG1; clone TAMAYA 1·2), Vβ17 (mouse IgG1; clone E17·5F3), Vβ 20 (mouse IgG; clone ELL 1·4), Vβ21·3 (mouse IgG2a; clone IG125), Vβ22 (mouse IgG1; clone IMMU 546). All antibody dilutions were made with 0·01 m phosphate-buffered saline (PBS) with 0·1% sodium azide and checked by plotting a dilution curve, the relevant dilution for each antibody corresponding to the shoulder of the curve. Each antibody was checked against an appropriate isotype-control antibody, as per the manufacturer's recommendation. Following incubation for 30 min, PBMCs were washed three times with 0·01 m PBS with 0·1% sodium azide. Three-colour flow cytometry was performed on a FACScalibur flow cytometer (Becton Dickinson) with optimal compensation set for green, orange and far-red fluorescence. T cells were identified by double-gating for forward and light-scatter characteristics and CD3 positivity. The CD4+ or CD8+ populations, and the percentage of cells bearing different Vβ gene products were subsequently calculated using quadrants set on dot-plots, with markers for positivity defined using isotype-control antibodies. Twenty thousand gated events were stored for each Vβ family.
Statistics
Comparison of group demographics was performed using the Kruskall–Wallis test. Assessment of skewing of the T cell Vβ repertoire between the groups of children was performed by comparison of mean percentages for each Vβ family using anova, and all P-values adjusted for multiple between-group comparisons using the Bonferroni method when the groups had equal variance, or the Tamhane method when significant differences in the variance for individual Vβ families was found. In addition, to further protect from the effect of multiple comparisons, biological significance was inferred from the statistical results only when the difference in the vasculitis group was consistent for a particular Vβ family between both the control and disease control groups. Vβ skewing was also assessed by comparison of the variance of each Vβ family between the groups using the F-test, with significance adjusted for 17 comparisons (i.e. the number of Vβ families examined). To check the validity of the F-test, normality of the data was assessed using the Kolmogorov–Smirnov and the Shapiro–Wilks tests, and no evidence for non-normality was demonstrated in any Vβ family.
For comparison, T cell Vβ skewing was also assessed using previously published but arbitrarily defined definitions of T cell Vβ expansions and deletions, with the proportion of children in each group having an expansion or deletion compared using the two-sample test of proportion. Thus, a Vβ expansion was defined as a value more than the control group mean plus 2 standard deviations (s.d.) for an individual Vβ family, and a deletion was a value less than the control group mean minus 2 s.d. [14,38].
Paired data derived from the longitudinal study of six of the vasculitis patients before and after induction of remission were analysed using the Wilcoxon signed-rank test. Statistical significance was defined at P < 0·05 (adjusted for multiple comparisons), and is implied in the results wherever differences are highlighted. All statistics were performed using Microsoft Excel 97 and SPSS 8·0 for Windows.
Results
Patient demographics
Children with KD were younger than children with other vasculitides, controls, and disease controls (P = 0·001); otherwise, there were no significant age or sex differences between the groups of children. The median time of blood sampling from disease onset was 12 months (range: 0·6–72 months) in the primary systemic vasculitis group and 1·5 months (range 0·5–6 months) in the HSP group. All the patients in the KD group were sampled in the second week of the illness.
T cell Vβ repertoires: comparison of means
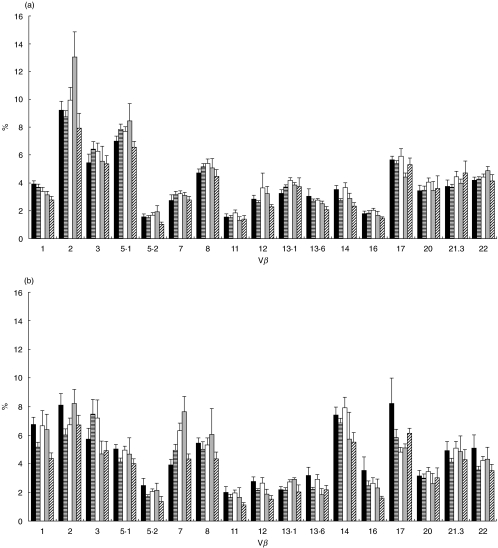
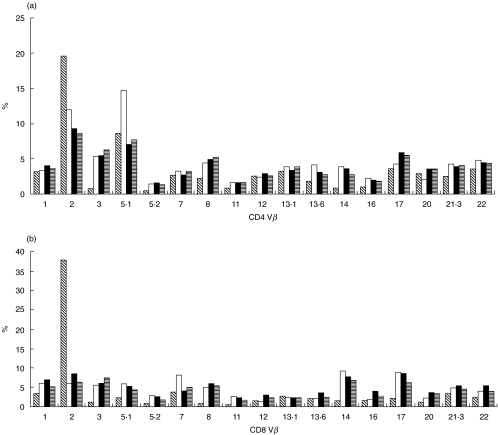
Figure 1a,b summarizes the mean percentage of CD4 and CD8 T cells expressing each of the individual Vβ gene products for controls, disease controls and children with vasculitis (PSV, KD and HSP). For comparison, the T cell Vβ repertoire from the two positive control children with TSS compared with controls and disease controls is shown in Fig. 2a,b.
Fig. 1.
(a, b) Mean (s.e.m.) CD4 and CD8 Vβ repertoire from healthy control children, disease control children and children with vasculitis (primary systemic vasculitis, Kawasaki disease and Henoch–Schönlein purpura). All comparisons were performed using anova, and all P-values adjusted to account for multiple comparisons (see text). ▪, Control mean;  , disease control mean; □, primary systemic vasculitis meran;
, disease control mean; □, primary systemic vasculitis meran;  , Kawasaki disease mean;
, Kawasaki disease mean;  , HSP mean.
, HSP mean.
Fig. 2.
(a, b) CD4 and CD8 Vβ repertoire from two children with toxic shock syndrome. The first patient with staphylococcal TSS shows an expansion of CD4 and CD8 Vβ2 T cells. The second patient with streptococcal TSS shows an expansion of CD4 Vβ5·1 T cells.  , Stapylococcal TSS; □, streptococcal TSS; ▪, control mean;
, Stapylococcal TSS; □, streptococcal TSS; ▪, control mean;  , disease control mean.
, disease control mean.
Primary systemic vasculitis
For CD4 T cells, the mean percentage of Vβ13·1 was higher in the primary systemic vasculitis group than controls (P = 0·02) but not disease controls (P = 0·2). For the CD8 T cells, the mean percentage of Vβ2 was lower in the disease controls versus controls (P = 0·03), although there was no difference between vasculitis and controls or disease controls. The mean percentage of CD8 Vβ7 T cells was higher in the primary systemic vasculitis group than the controls (P = 0·01) but not the disease controls (P = 0·1). The mean percentage of CD8 Vβ17 T cells was lower in the primary systemic vasculitis group than controls (P = 0·03) but not disease controls (P = 0·1).
Kawasaki disease
In the KD group the mean percentage of CD4 Vβ2 cells was higher than in both the control and disease control groups (P = 0·03, and P = 0·01, respectively). The mean percentage of CD8 Vβ2 cells was higher in the KD group than in the disease controls (P = 0·02) but not the controls (P = 0·9). The mean percentage of CD8 Vβ7 T cells was higher in the KD group than both the controls (P = 0·008) and disease controls (P = 0·03).
Henoch–Schönlein purpura
In the HSP group the mean percentage of CD4 Vβ1 T cells was lower than in both the control (P = 0·02) and disease control groups (P = 0·02). The mean percentage of CD4 Vβ14 was lower in the HSP group than in the controls (P = 0·01) but not the disease controls (P = 0·3). The mean percentage of CD8 Vβ1 was lower in the HSP group than in the controls (P = 0·007) but not the disease controls (P = 0·2).
T cell Vβ repertoires: comparison of variance
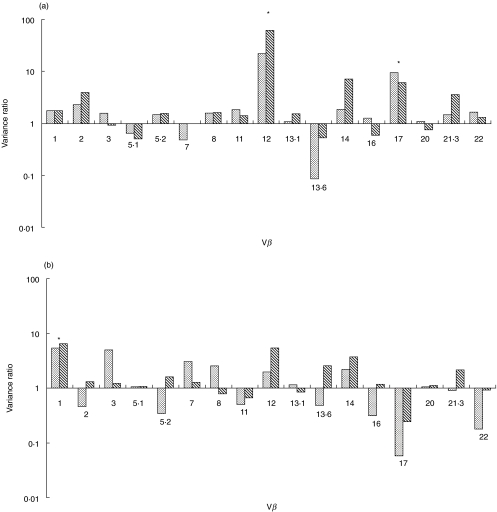
Figure 3a,b summarizes the ratios of the variance (F-ratio) of each Vβ family for CD4 and CD8 T cells from the primary systemic vasculitis group and the controls and disease controls. F-ratios were obtained by dividing the variance (‘spread’) for each Vβ family in the vasculitis group by the corresponding Vβ family variance in the control and disease control groups. If the variances are similar, the F-ratio is close to 1. If the variance of any Vβ family is greatly increased in the vasculitis group, the corresponding F-ratio is much greater than 1. P-values for the F-ratios thus obtained between the groups were then derived using the F-test, with alpha adjusted for 17 comparisons. Only when the variance ratio was statistically significantly increased for both vasculitis versus controls and vasculitis versus disease controls was it regarded as biologically significant.
Fig. 3.
(a,b) Variance ratios (F-ratios) of each Vβ family for CD4 and CD8 T cells. F ratios were obtained by dividing the variance (‘spread’) for each Vβ family in the vasculitis group by the corresponding Vβ family variance in the control, and disease control groups. If the variances are similar, the F-ratio is close to 1. If the variance of any Vβ family is greatly increased in the vasculitis group, the corresponding F-ratio is much greater than 1 (significant adjusted P-values asterisked). P < 0·05 (adjusted for 17 comparisons) for both vasculitis versus controls and vasculitis versus disease controls.  , Vasculitis versus controls;
, Vasculitis versus controls;  , vasculitis versus disease controls.
, vasculitis versus disease controls.
The variance was increased in the vasculitis group compared with both the controls and disease controls for CD4 Vβ12 (vasculitis versus controls, adjusted P < 0·00001; vasculitis versus disease controls, adjusted P < 0·00001); CD4 Vβ 17 (vasculitis versus controls, adjusted P = 0·005; vasculitis versus disease controlsn adjusted P = 0·0005); and CD8 Vβ1 (vasculitis versus controls, adjusted P = 0·005; vasculitis versus disease controls, adjusted P = 0·0001).
To minimize the effect of patient heterogeneity within the primary systemic vasculitis group the data were reanalysed comparing only the 18 patients with classical PAN, as defined by the ACR criteria, and who had diagnostic visceral angiography for PAN (one patient satisfying criteria for PAN with positive skin biopsy had a normal visceral angiogram, and so was excluded from this analysis). Again, the variance was increased in the PAN group compared with both control and disease control groups for CD4 Vβ12 (vasculitis versus controls, adjusted P < 0·00001; vasculitis versus disease controls, adjusted P < 0·00001); CD4 Vβ17 (vasculitis versus controls, adjusted P = 0·001; vasculitis versus disease controls, adjusted P = 0·0001); and CD8 Vβ1 (vasculitis versus controls, adjusted P = 0·001; vasculitis versus disease controls, adjusted P < 0·00001).
In the KD and HSP groups, there were no consistent differences in the variance compared with controls and disease controls.
T cell Vβ expansions and deletions
Using the arbitrary definitions of Vβ expansion and deletion described previously, 80% of the primary systemic vasculitis children had one or more CD4 Vβ expansions or deletions compared with 30% of the controls (95% CI of the difference 21–79%, P < 0·002) and 37% of the disease controls (95% CI of the difference 16–70%, P < 0·002). Fifteen of 18 (83%) of the patients with classical PAN (ACR defined and confirmed on visceral angiography) had one or more CD4 Vβ expansions or deletions, this percentage being significantly higher than the controls (P < 0·002) and disease controls (P = 0·002), although there was no clear relationship between the duration of vasculitic prior to blood sampling and the presence of expansions or deletions. There were no differences in the number of expansions or deletions in the CD8 T cell population between the vasculitis and control/disease control groups.
In the KD group, 67% of the patients had one or more CD4 Vβ expansions or deletions, no different from the controls (95% CI of the difference from controls −7–81%, P = 0·1; 95% CI of the difference from disease controls −14–73%, P = 0·1). There were no differences in the number of expansions or deletions in the KD CD8 T cell population.
In the HSP group there were no differences in the number of CD4 or CD8 expansions or deletions compared with either the control or disease control group.
Longitudinal studies on primary systemic vasculitis children
Overall, there were no differences in the mean percentage of individual Vβ families in either the CD4 or CD8 population of T cells when paired samples from six primary systemic vasculitis pre- and postinduction of remission were compared. However, there was a greater variance of certain Vβ families prior to induction of remission: CD4 Vβ12 (F-ratio 190, adjusted P = 0·00001), CD4 Vβ14 (F-ratio 296, adjusted P = 0·00005) and CD8 Vβ1 (F-ratio 81, adjusted P = 0·001). Moreover, five of six of the active vasculitis group had one or more CD4 T cell Vβ expansions or deletions (four patients with one or more expansions, and one patient with one expansion and one deletion), compared with one of six in the remission group (95% CI of observed difference 4–100%, P = 0·02). No difference in the numbers of expansions or deletions in the CD8 Vβ repertoire was observed pre- and postinduction of remission.
Discussion
There are limited data suggesting that SAgs play an important role in the initiation and/or subsequent relapse of the systemic vasculitides in adults [2]. No such data exist for the primary systemic vasculitides (PAN, WG, MPA) in children, although a recent small series of six children with HSP nephritis in association with staphylococcus infection demonstrated increased percentages in the peripheral blood of T cells bearing Vβ5·2, Vβ5·3 and Vβ8, suggesting superantigenic stimulation of the immune system in these patients [39].
The response of a T cell to superantigenic stimulation is complex, and the observed ‘skewing’ of the Vβ repertoire, which typically follows superantigenic stimulation (Fig. 2a,b) is dependent on several factors. First, timing of the blood sample from the onset of superantigenic exposure is critical, as initially there is Vβ restricted T cell activation, followed by specific T cell Vβ proliferation, and finally Vβ deletion [7,18]. It is reported that this peripheral Vβ deletion following superantigenic stimulation is the result of apoptosis of activated T cells although other possibilities, including internalization of T cell receptors (resulting in a state of anergy) [9] or sequestration of specific activated Vβ families into sites of tissue inflammation [24,25], may also contribute to removal of certain Vβ families from the peripheral circulation. Other influences such as the HLA type of an individual [40] and the recent description of ‘partially Vβ-restricted’ SAgs [41] could also theoretically affect the ability to detect peripheral Vβ skewing following SAg exposure in some studies. It is feasible, therefore, that depending on the timing of blood sampling in relation to SAg exposure or other SAg or host determinants, peripheral T cell Vβ skewing may not be observed, perhaps explaining some of the conflicting data emerging from studies in KD.
Despite the inherent limitations of examining peripheral blood T cell Vβ repertoires for evidence of previous SAg exposure, we were able to demonstrate changes in the Vβ repertoire of certain vasculitis patients. Thus, in the primary systemic vasculitis group (PAN, WG, MPA and unclassifiable vasculitis), although there were minor deviations in the mean percentages of CD4 and CD8 Vβ families, no consistent difference in the mean Vβ percentages for vasculitis versus both controls and disease controls was observed. Comparison of the Vβ variance, however, revealed significantly increased variance in the primary systemic vasculitis patients for some Vβ families compared to controls and disease controls: CD4 Vβ12 and CD4 Vβ17 and CD8 Vβ1. Additionally, significantly more ‘expansions’ and ‘deletions’ were observed in the primary systemic vasculitis group than in the control and disease control groups.
As documented in Table 1, there was a degree of heterogeneity within the primary systemic vasculitis group. We analysed these patients as one group because evidence for superantigenic involvement has been suggested for adult patients with PAN, MPA and WG. Moreover, there is a greater degree of polyangiitis overlap in children, making classification problematic. None the less, we were able to demonstrate skewing of the Vβ repertoire when these patients were compared with controls and disease controls. Furthermore, when the 18 patients satisfying classification criteria for classical PAN inclusive of diagnostic angiography were analysed separately, the result was unchanged suggesting that the observed skewing of the Vβ repertoire is not a consequence of patient heterogeneity.
In the KD group, although patient numbers were small, in accordance with the findings of others [16,17] we observed a significantly increased percentage of CD4 Vβ2 in the second week of the illness compared to controls and disease controls. Interestingly, we also observed a significantly increased percentage in CD8 Vβ7 in the KD group. We suggest that this observation was unlikely to be the result of the presence of genetic polymorphisms, which have been shown to affect Vβ7·2 [42], as the antibody clone used in this study (ZOE) recognizes Vβ7·1. That said, some cross-reactivity between this antibody and Vβ7·2 was not excluded formally in this study. In the HSP group, again although patient numbers were small, CD4 Vβ1 T cells were found to be lower in the peripheral circulation than that observed in control and disease control children, but no differences in variance or numbers of Vβ expansions or deletions were observed.
Lastly, paired samples obtained from six vasculitis patients (all with classical PAN) before and after induction of remission of vasculitis showed ‘normalization’ of the Vβ repertoire following treatment. Thus, the variance of CD4 12, 14 and CD8 Vβ1 was significantly greater in the group prior to treatment compared to those following induction of remission. Also, there were significantly more Vβ expansions in the pretreatment vasculitis group than the post-treatment group.
While these observations could be compatible with an effect of SAgs on the immune system in patients with vasculitis, we cannot conclude from this study that SAgs are directly involved in the initiation or relapse of vasculitis. Indeed, the T cell Vβ skewing observed could be an epiphenomenon in these patients. Potential confounding factors include a non-specific skewing of the Vβ repertoire as a result of chronic inflammation, the use of glucocorticoids and immunosupressants and prolonged periods of in-patient care (potentially altering carriage of bacterial commensals) in the vasculitis group. We attempted to control for these factors, however, by the inclusion of disease controls who were renal allograft recipients with no prior history of vasculitis or other autoimmune disease, and who were treated with a similar (but not identical) range of glucocorticoid and immunosuppressive therapy as the vasculitis children. These children were treated for lengthy periods of time as in-patients at the same institution as the vasculitis group, and thus theoretically exposed to the same range of microbial commensals. Moreover, the disease control group also included children with miscellaneous nonvasculitic autoimmune disease and sepsis. Even with this (arguably) over-controlled disease control group, we were able to demonstrate CD4 Vβ skewing in the children with vasculitis that was not observed in the disease controls.
We were also interested in the lack of similarity between the CD4 and CD8 Vβ changes observed in the vasculitis patients, as SAgs are known to affect both T cell subsets (as in our patient with staphylococcal TSS). While this observation may mitigate against SAgs as a major influence in these disease states, it should be borne in mind that the signalling events governing the T cell response to SAgs (including activation, expansion, deletion, anergy or tissue sequestration) may be different for CD4 and CD8 T cells, and could vary with time within these T cell subsets.
In conclusion we have observed changes in the T cell Vβ repertoire in children with vasculitis over and above those observed in disease controls (with inflammatory and immune-mediated conditions). While these data provide impetus for further research into this contentious field, they do not resolve unequivocally the question of the role of SAgs in childhood vasculitic syndromes. Areas for future work will include examination for T cell Vβ restriction at the tissue level, and investigation of possible mechanisms of SAg-induced endothelial cell activation and its potential blockade [43].
Acknowledgments
This work was funded by the Charlotte Parkinson Research Fund. The authors wish to acknowledge Professor Tim Cole of the department of Paediatric Epidemiology and Biostatistics, Institute of Child Health London for his assistance with the statistical analysis, and Ms Carol Hutchinson for help in obtaining control samples.
References
- 1.Petty RE, Cassidy JT. Vasculitis and its classification. In: Cassidy JT, Petty RE, editors. Textbook of pediatric rheumatology. Philadelphia: WB Saunders; 2001. [Google Scholar]
- 2.Cohen Tervaert JW, Popa ER, Bos NA. The role of superantigens in vasculitis. Curr Opin Rheumatol. 1999;11:24–33. doi: 10.1097/00002281-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Giscombe R, Grunewald J, Nityanand S, Lefvert AK. T cell receptor (TCR) V gene usage in patients with systemic necrotizing vasculitis. Clin Exp Immunol. 1995;101:213–9. doi: 10.1111/j.1365-2249.1995.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson IJ, Skinner MA, Geursen A, et al. Peripheral blood T lymphocytes in systemic vasculitis: increased T cell receptor V beta 2 gene usage in microscopic polyarteritis. Clin Exp Immunol. 1995;101:220–6. doi: 10.1111/j.1365-2249.1995.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffenbauer J, Soos J, Johnson H. The possible role of bacterial superantigens in the pathogenesis of autoimmune disorders. Immunol Today. 1998;19:117–20. doi: 10.1016/s0167-5699(97)01199-7. [DOI] [PubMed] [Google Scholar]
- 6.Torres BA, Johnson HM. Modulation of disease by superantigens. Curr Opin Immunol. 1998;10:465–70. doi: 10.1016/s0952-7915(98)80122-2. [DOI] [PubMed] [Google Scholar]
- 7.Shoukry NH, Lavoie PM, Thibodeau J, D’Souza S, Sekaly RP. MHC class II-dependent peptide antigen versus superantigen presentation to T cells. Hum Immunol. 1997;54:194–201. doi: 10.1016/s0198-8859(97)00074-8. [DOI] [PubMed] [Google Scholar]
- 8.Choi Y, Lafferty JA, Clements JR, et al. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J Exp Med. 1990;172:981–4. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser J, Arcus V, Kong P, Baker E, Proft T. Superantigens; powerful modifiers of the immune system. Mol Med. 2000;6:125–32. doi: 10.1016/s1357-4310(99)01657-3. [DOI] [PubMed] [Google Scholar]
- 10.Kappler J, Kotzin B, Herron L, et al. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–3. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 11.Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin ‘superantigens’ with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–5. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingari MC, Cambiaggi A, Vitale C, Schiavetti F, Bellomo R, Poggi A. Effect of superantigens on human thymocytes: selective proliferation of V beta 2+ cells in response to toxic shock syndrome toxin × 1and their deletion upon secondary stimulation. Int Immunol. 1996;8:203–9. doi: 10.1093/intimm/8.2.203. [DOI] [PubMed] [Google Scholar]
- 13.Webb SR, Hutchinson J, Hayden K, Sprent J. Expansion/deletion of mature T cells exposed to endogenous superantigens in vivo. J Immunol. 1994;152:586–97. [PubMed] [Google Scholar]
- 14.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Cohen Tervaert JW. T cell activation and TCR vbeta skewing in Wegeners's granulomatosis; indicators for superantigenic activation? Clin Exp Immunol. 1998;112(1):16. [Google Scholar]
- 15.Cohen Tervaert JW, Stegeman CA, Manson WL, Kallenberg CG, Johnson WM. Staphylococcus aureus superantigens: a risk factor for disease reactivation in Wegener's granulomatosis. FASEB J. 1998;12:A488. [Google Scholar]
- 16.Abe J, Kotzin BL, Jujo K, et al. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–70. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis N, Zheng R, Lamb JR, Levin M. Evidence for a superantigen mediated process in Kawasaki disease. Arch Dis Child. 1995;72:308–11. doi: 10.1136/adc.72.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung DY, Meissner C, Schlievert PM. The etiology and pathogenesis of Kawasaki disease − how close are we to an answer? Current Opinion Infectious Dis. 1997;10:226–32. [Google Scholar]
- 19.Choi IH, Chwae YJ, Shim WS, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–6. [PubMed] [Google Scholar]
- 20.Morita A, Imada Y, Igarashi H, Yutsudo T. Serologic evidence that streptococcal superantigens are not involved in the pathogenesis of Kawasaki disease. Microbiol Immunol. 1997;41:895–900. doi: 10.1111/j.1348-0421.1997.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 21.Pietra BA, De Inocencio J, Giannini EH, Hirsch R. TCR V beta family repertoire and T cell activation markers in Kawasaki disease. J Immunol. 1994;153:1881–8. [PubMed] [Google Scholar]
- 22.Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166:1334–43. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 23.Terai M, Miwa K, Williams T, et al. The absence of evidence of staphylococcal toxin involvement in the pathogenesis of Kawasaki disease. J Infect Dis. 1995;172:558–61. doi: 10.1093/infdis/172.2.558. [DOI] [PubMed] [Google Scholar]
- 24.Leung DYM, Giorno RC, Kazemi LV, Flynn PA, Busse JB. Evidence for superantigen involvement in cardiovascular injury due to kawasaki syndrome. J Immunol. 1995;155:5018–21. [PubMed] [Google Scholar]
- 25.Yamashiro Y, Nagata S, Oguchi S, Shimizu T. Selective increase of Vbeta2+ T cells in the small intestinal mucosa in Kawasaki disease. Pediatr Res. 1996;39:264–6. doi: 10.1203/00006450-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Lightfoot RW, Jr, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33:1088–93. doi: 10.1002/art.1780330805. [DOI] [PubMed] [Google Scholar]
- 27.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 28.Ozen S, Besbas N, Saatci U, Bakkaloglu A. Diagnostic criteria for polyarteritis nodosa in childhood. J Pediatr. 1992;120:206–9. doi: 10.1016/s0022-3476(05)80428-7. [DOI] [PubMed] [Google Scholar]
- 29.Besbas N, Ozen S, Saatci U, Topaloglu R, Tinaztepe K, Bakkaloglu A. Renal involvement in polyarteritis nodosa: evaluation of 26 Turkish children. Pediatr Nephrol. 2000;14:325–7. doi: 10.1007/s004670050769. [DOI] [PubMed] [Google Scholar]
- 30.Hunder G. The use and misuse of classification and diagnostic criteria for complex diseases. Ann Intern Med. 1998;129:417–8. doi: 10.7326/0003-4819-129-5-199809010-00013. [DOI] [PubMed] [Google Scholar]
- 31.Brogan PA, Dillon MJ. Vasculitis from the pediatric perspective. Curr Rheumatol Report. 2000;2:411–6. doi: 10.1007/s11926-000-0041-7. [DOI] [PubMed] [Google Scholar]
- 32.Leavitt RY, Fauci AS. Polyangiitis overlap syndrome. Am J Med. 1986;81:79–85. doi: 10.1016/0002-9343(86)90186-5. [DOI] [PubMed] [Google Scholar]
- 33.Jennette JC, Falk RJ. Do vasculitis categorization systems really matter? Curr Rheumatol Report. 2000;2:430–8. doi: 10.1007/s11926-000-0044-4. [DOI] [PubMed] [Google Scholar]
- 34.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Quart J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 35.Petty RE, Cassidy JT. Kawasaki disease. In: Cassidy RE, Petty JT, editors. Textbook of pediatric rheumatology. Philadelphia: WB Saunders; 2001. [Google Scholar]
- 36.Brogan PA, Bose A, Burgner D, et al. Kawasaki disease: an evidence based approach to diagnosis, treatment, and proposals for future research. Arch Dis Child. 2002;86:286–90. doi: 10.1136/adc.86.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lightfoot RW, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch–Schönlein purpura. Arthritis Rheum. 1990;33:11114–21. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 38.Wedderburn LR, Maini MK, Patel A, Beverley PCL, Woo P. Molecular fingerprinting reveals non-overlapping T cell oligoclonality between an inflamed site and peripheral blood. Int Immunol. 1998;11:535–43. doi: 10.1093/intimm/11.4.535. [DOI] [PubMed] [Google Scholar]
- 39.Hirayama K, Kobayashi M, Muro K, Yoh K, Yamagata K, Koyama A. Specific T-cell receptor usage with cytokinemia in Henoch-Schonlein purpura nephritis associated with Staphylococcus aureus infection. J Intern Med. 2001;249:289–95. doi: 10.1046/j.1365-2796.2001.00815.x. [DOI] [PubMed] [Google Scholar]
- 40.Herman A, Croteau G, Sekaly RP, Kappler J, Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990;172:709–17. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodtsev AS, Choi Y, Spanopoulou E, Posnett DN. Mycoplasma superantigen is a CDR3-dependent ligand for the T cell antigen receptor. J Exp Med. 1998;187:319–27. doi: 10.1084/jem.187.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao TM, Whitaker SE, Robinson MA. A genetically determined insertion/deletion related polymorphism in human T cell receptor beta chain (TCRB) includes functional variable gene segments. J Exp Med. 1994;180:1405–14. doi: 10.1084/jem.180.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baum D, Yaron R, Yellin MJ. TNF-alpha, not CD154 (CD40L), plays a major role in SEB-dependent, CD4 (+) T cell-induced endothelial cell activation in vitro. Cellular Immunol. 1998;190:12–22. doi: 10.1006/cimm.1998.1380. [DOI] [PubMed] [Google Scholar]