Abstract
Our objective was to characterize T-cell responses to Phleum pratense in grass pollen allergic individuals and healthy controls using the fluorescent dye PKH26. Peripheral blood mononuclear cells were stimulated with P. pratense, or with recall antigens, and CD3+/CD4+ and CD3+/CD8+ T-cells that had proliferated were analysed by flow cytometry. In the presence of P. pratense CD4+/CD3+ T-cells proliferated more in grass pollen sensitive atopic patients than in nonallergic controls or in nongrass pollen sensitive atopic subjects. PPD and TT recall antigens elicited uniformly high proliferation in all T-cell subsets. Only half of pollen sensitive patients also had an increased proliferation of CD3+/CD8+ T-cells in response to P. pratense. We determined precursor frequency of CD4+ T cells in the original population that responded to P. pratense and found values ranging from 1 × 10−3 to 0·6 × 10−1, in the same range as those measured for PPD and TT. In conclusion, grass pollen sensitive atopic patients show enhanced CD4+ T-cell reactivity to P. pratense, and this could be related to the presence of elevated numbers of circulating allergen-specific CD4+ T cells. This flow cytometric method should allow the identification of other phenotypic markers such as intracellular cytokines in allergen specific responding CD4+ T cells.
Keywords: allergen, fluorescent dye, T-lymphocyte, precursor frequency
Introduction
Allergen-specific T-cells in patients with allergic asthma or rhinitis are characterized by abnormal expression of the Th2-type cytokines interleukin-5 (IL-5) and IL-4 [1–3]. In these patients, IL-4 and IL-5 production by T-cells is thought to underlie B cell IgE synthesis [4] and mucosal eosinophil recruitment and activation, respectively. While evidence for imbalance of Th2 cytokine production in atopic disease is compelling, many features of the allergen-specific T-cell response remain unclear. For example, it is controversial whether there is enhanced T-cell reactivity to allergen reflecting increased numbers of allergen-specific T-cells in atopic patients compared to nonatopic subjects, as well as Th2 deviation. Additionally, though it is sometimes presumed that in vitro peripheral blood T-cell responses to allergen are the result of CD4+ T-cell activation, the effects of allergen on CD4+ and CD8+ T-cell activation and expansion have yet to be compared. Evaluation of peripheral blood T-cell responses to aeroallergens has been the subject of numerous investigations. The usual methodology employed for quantifying such responses is based on the incorporation into DNA of radiolabelled precursors in whole cultures of cells and provides a snapshot of the rate of total cell proliferation over a limited period of time. In the present study we set out to perform a basic characterization of T-cell responses to grass pollen allergen using a different approach – based on fluorescent dye binding to plasma membranes and the subsequent dilution of fluorescence intensity in proliferating cells [5]. In contrast to previous studies of allergen-T-cell responses this method permits quantification of accumulated proliferating cells over the entire culture period, the determination of precursor frequency, as well as phenotypic characterization of these cells. We have used this approach to characterize the CD4+ and CD8+ T-cell proliferative responses to grass pollen allergen in atopic and nonatopic subjects.
Materials and Methods
Patients
For the purposes of this study, atopic subjects were required to have a positive skin prick test to one or more of a range of common aeroallergens in the presence of negative diluent and positive histamine controls. Grass pollen sensitive atopic subjects were required to have a history of either seasonal rhinitis with seasonal symptoms of nasal discharge, sneeze and/or discharge or seasonal asthma (with recorded variability of ≥15% in the forced expiratory volume in one second (FEV1) or peak flow rate (PEFR) either spontaneously or in response to inhaled β2-agonist. Atopic nongrass pollen sensitive subjects were required to have a negative skin test and normal levels of serum specific IgE to grass pollen (Phleum pratense), but to be sensitized to one or more other allergens using the same tests. Normal nonatopic control subjects were required to be skin test negative with normal levels of serum specific IgE to a range of 12 common aeroallergens. In addition these subjects were all required to have a life long absence of symptoms suggestive of atopic disease. All subjects were required to be>18 year and <55 year of age. The study was performed with the approval of the local ethics committee and informed consent of all participants.
PBMC labelling with PKH26
Peripheral blood mononuclear cells (PBMC) from human atopic or nonatopic donors were isolated by Ficoll-hypaque density gradient centrifugation. PBMC were washed three times in PBS and resuspended in Diluent C (Sigma, St. Louis, MO) at 106 cells/ml. The cell suspension was then diluted in the same volume of 2·5 µm PKH26 dye stock (Sigma) (prepared in Diluent C), incubated for 3 min at room temperature and then added to an equal volume of heat-inactivated fetal bovine serum (FCS) and incubated at room temperature for a further 2 min to stop the labelling. Cells were then washed three times in RPMI containing 10% heat-inactivated FSC and resuspended in RPMI containing 1 mm HEPES (Life Technologies, Paisley, UK), 1% antibiotics (penicillin, streptomycin and neomycin, Life Technologies) and 10% human AB serum (Valbiotech AbCys, Paris, France) (culture medium).
Proliferation and quantification of proliferating T-cells by flow cytometry
PBMC were resuspended in 96-well round bottomed plates at 105 cells/well with medium alone (negative control) or P. pratense (20 µg/ml; kind gift of ALK-Abello, Horsholm, Denmark), 1 µg/ml of tuberculin purified protein derivative (PPD; Statens Seruminstitut, Copenhagen, Denmark), or 10 µg/ml for tetanus toxoid (TT, Biorad, Ivry-sur-Seine, France). All cultures were performed in 6-replicate (6 wells by stimulation) at 37°C in 5% CO2 for 11 days and medium was changed on day 5–7. At the end of the culture (day 11), cells from the 6 wells were harvested, pooled and washed in PBS. Cells were then incubated for 30 min at 4°C in culture medium to block nonspecific binding by Fc-receptors. Cells were then stained with anti-CD3 antibody (FITC conjugated: UCHT1, IgG1, Immunotech, Marseille, France) plus either phycoerythrin-cyanin 5 (PC5) conjugated anti-CD4 (13B8·2, IgG1, Immunotech) or PC5-conjugated anti-CD8 (B9·11, IgG1, Immunotech). After 30 min incubation at 4°C, cells were washed twice and fixed in PBS containing 2% formaldehyde. Flow cytometric measurements were performed using a Becton Dickinson FACSscan cytometer. PKH26 fluorescent signal was collected in the FL2 channel (560–579 nm). A minimum of 10 000 events was analysed in each sample. In preliminary experiments, proliferation was measured after 7 and 11 days of P. pratense- or recall antigen-stimulation. For all three antigens tested the numbers of cells that had undergone cell division were higher after 11 days than 7 days, though the difference was most marked for grass pollen allergen (data not shown). Therefore in all subsequent experiments, PKH26 fluorescence was performed at day 11
The absence of toxicity of PKH26 was confirmed as follows: PBMC were labelled or not with PKH26, and cultured in same conditions in the presence or absence of antigens or P. pratense. Overnight 3H-thymidine incorporations were performed after 7 or 11 days of culture. No differences (less than 10%) of proliferative response was observed between PKH26-labelled PBMC and unlabelled PBMC (data not shown).
Data analysis
In most cases results are expressed as stimulation index (SI). SI was calculated as the proportion of cells proliferating in response to antigen stimulation divided by the proportion of cells proliferating spontaneously in medium. SI values>2 were considered as proliferative response. Statistical comparison of antigen-dependent proliferation in atopic (grass pollen sensitive), other atopic (i.e. nongrass pollen sensitive) and normal nonatopic controls was performed with the Mann–Whitney U-test using a commercially available statistical package (Statview® 4·5 Software, Abacus Concepts Inc., Berkeley, CA). P-values less than 0·05 were considered significant.
For determining the precursor frequency (PF) of CD4+ cells, flow cytometric data files were analysed with the proliferation Wizard module in ModFit LT Macintosh software (Verity Software House, Topsham, Maine, USA). PF represents the frequency of CD3+/CD4+ gated cells in the original population (before culture) that had proliferated, as previously described [5]. Briefly, the Modfit software deconvolutes the fluorescence intensity histogram with Gausian distribution centred on peaks at 19·9 channel intervals. The proportion of cells having undergone 3–8 divisions can be thus determined, and this in turn permits calculation of the PF [5].
Results
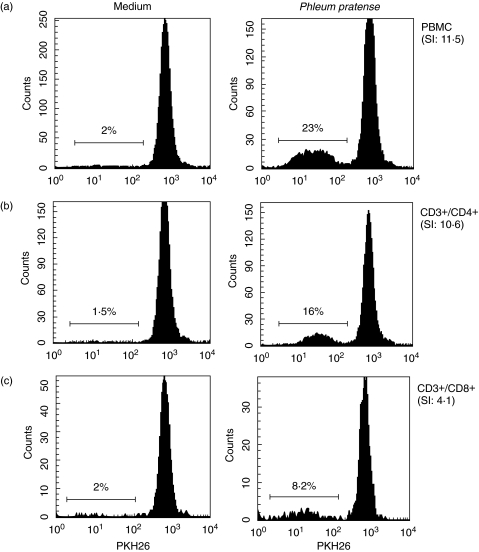
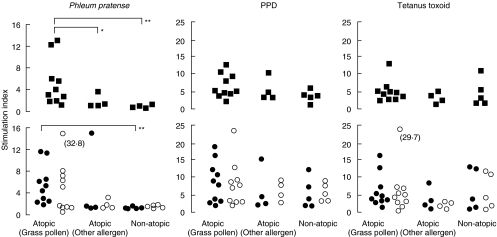
Proliferation of CD4+ and CD8+ T-cells in response to stimulation with grass pollen allergen (P. pratense), PPD or TT was evaluated in 10 grass pollen sensitive atopic subjects and 9 nongrass pollen sensitive control subjects (4 atopic subjects and 5 nonatopic subjects). In all cases, T-cells that had undergone proliferation were identified by flow cytometry as a result of diminution of plasma membrane fluorescence from PKH26 labelling. Figure 1 shows PHK26-fluorescence histograms obtained with 11-day stimulated PBMCs, from one representative grass pollen sensitive atopic subject. Cells proliferating in response to P. pratense can be clearly detectable, as their PKH26-fluorescence intensity was lower than the major population in the parental generation. At the end of the culture period, cells that had proliferated represented 23% of the total PBMC (Fig. 1a). Gating CD3+/CD4+ and CD3+/CD8+ cells separately, showed that among PBMCs, CD4+ T cells, and to a lesser extent, CD8+ T cells (Fig. 1b,c) responded to P. pratense, with stimulation indexes of 10·6 and 4·1, respectively. We performed similar analysis as the one shown in Fig. 1, on the 3 groups of subjects. As shown in Fig. 2, the proportion of PBMCs that had proliferated in response to allergen, but not to PPD or TT were higher in grass sensitive atopic patients than in either nongrass pollen sensitive atopic (P = 0·04) or nonatopic controls (P = 0·002). When cell division was examined in CD4+/CD3+ and CD8+/CD3+ T-cell subsets however, only CD4+/CD3+ T-cells in grass pollen sensitive atopics were characterized by a statistically significant response to grass pollen (P = 0·002) versus nonatopic subjects. When responses to PPD and TT recall antigens were analysed in both T-cell subsets, uniformly high proliferation was observed in all groups, in CD8+/CD3+ as well as CD4+/CD3+ T-cells (Fig. 2).
Fig. 1.
Flow cytometric analysis of (a) whole PBMC, and of (b) CD4+ and (c) CD8+ T-lymphocyte subsets proliferation in one grass pollen atopic subject, in response to grass pollen (P. pratense). Flow cytometry histograms show PKH26 fluorescence on (a) total cells (PBMC), (b) CD3+/CD4+ T-cells and (c) CD3+/CD8+ T-cells. Values represent percentages of cells that have proliferated as a proportion of all gated cells. Stimulation index (SI) represents the proportion of cells proliferating in response to P. pratense divided by the proportion of cells undergoing background proliferation (in medium only).
Fig. 2.
Proliferation of whole PBMCs (▪), CD3+/CD4+ (•) and CD3+/CD8+ (○) T-cells in response to grass pollen allergen (P. pratense), PPD and TT in grass pollen sensitive atopic donors with seasonal allergic symptoms (n = 10), atopic donors not sensitized to grass pollen (n = 4) or nonatopic normal control subjects (n = 5). *P < 0·05, **P < 0·01.
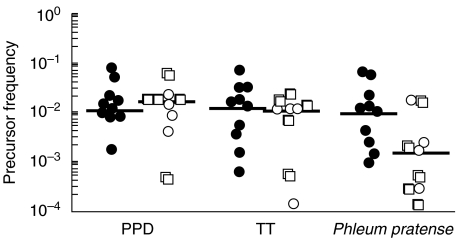
We next determined the precursor frequencies of grass pollen-specific CD4+ lymphocytes in grass pollen sensitive atopic subjects and compared these values with the precursor frequencies of PPD- and tetanus toxoid-specific CD4+ cells. Precursor frequency represents the proportion of CD4+ T cells present in the original population that had proliferated during the 11 days of culture in response to stimulation. Precursor frequency values (after subtracting the background) were between 1 × 10−3 and 0·6 × 10−1, with an average of 1·9 × 10−2 ± 0·7 × 10−2 in grass pollen atopic subjects (Fig. 3). PF of PPD and TT CD4+ T cells were in the same range (2·1 × 10−2 ± 0·7 × 10−2 and 1·9 × 10−2 ± 0·65 × 10−2, respectively). PF of grass pollen specific CD4+ cells in nongrass pollen sensitive subjects was lower than PF calculated in grass pollen atopic subjects (4·8 × 10−3 ± 2·4 × 10−3; mean ± sem; P = 0·04), with only 5 subjects with significant response (PF > 1 × 10−3), while PF of PPD- and TT- specific CD4+ T cells were in the same range as those measured in grass pollen atopic subjects (1·7 × 10−2 ± 0·4 × 10−2 and 1 × 10−2 ± 0·2 × 10−2, respectively). No significant differences in PF values were measured between nonatopics and atopic but nongrass pollen sensitive subjects (Fig. 3).
Fig. 3.
P. pratense, PPD and tetanus toxoid precursor frequencies of CD4+ T lymphocytes. Precursor frequency was determined in grass pollen sensitive atopic subjects (•) and in nongrass pollen sensitive subjects (atopics but nongrass pollen sensitive subjects (○), and nonatopics (□), from flow cytometric data files with the ModFit Software. Precursor frequencies of background proliferating cells (i.e. medium only without antigen) were subtracted from results. Bars represent the median.
Discussion
The data presented in this study confirmed our hypothesis that peripheral blood mononuclear cells from subjects with seasonal allergic disease show enhanced proliferation and cell division in response to grass pollen allergen. This altered reactivity was confined to allergen since responses to the recall antigens TT and PPD were similar in atopic and nonatopic groups. Moreover, it was not a feature of atopy or atopic disease per se, since grass pollen-dependent expansion was not observed in cells of patients sensitized to allergens other than P. pratense. Although the associations between allergic disease and aberrant Th2 cytokine expression by allergen-specific and allergen-stimulated T-cells are well established [1–3], the relationship between absolute numbers of allergen-specific T-cells in the peripheral circulation and allergic disease is more controversial. Some reports have described similar reactivity to pollens in normal and atopic subjects [6,7]. Other data generated in pollen or house dust mite allergic patients support the findings presented here that patients with atopic disease are indeed characterized by more vigorous peripheral blood T-cell response to specific allergens compared to controls and exhibited high frequency of allergen-specific T lymphocytes [3, 8, 9]. In contrast to previous studies based on the [3H]-thymidine incorporation into DNA in whole cell populations that provide a rate of total cell proliferation over a limited period of time, the technique used in this study allows phenotypic characterization of accumulating proliferating cells. We showed that when CD4+ and CD8+ subsets were analysed, statistically significant differences in T-cell responses were only observed in the CD4+ population. This is consistent with the dominance of CD4+ cells in allergen-specific T-cell clones expanded from allergic patients, and with the inhibition of peripheral blood mononuclear cell responses by anti-CD4 antibodies or CD4+ T-cell depletion [2]. We found precursor frequency values of grass pollen CD4+ responding cells between 1 × 10−3 and 0·6 × 10−1, in the same range as those determined for PPD and TT recall antigens. These values are also equivalent to those measured by Givan et al. [5] using the same method with TT-stimulated PBMC. Precursor frequency of grass pollen specific CD4+ cells in nongrass pollen sensitive subjects was very low (under the limit of detection for 4 subjects, and ranging from 1·8 × 10−3 to 1·8 × 10−2 for the 5 other subjects). Precursor frequency represents the proportion of CD4+ T lymphocytes present in the original population that proliferated during the 11 days of stimulation. However, it must be noted that this calculation was performed on cells that survived after 11 days of culture, and may thus not be considered the true frequency of circulating allergen-specific CD4+ cells. However, by comparing precursor frequency values of grass pollen- and recall antigen-specific CD4+ cells in grass pollen sensitive and nongrass pollen sensitive subject, our data thus support the idea that subjects with seasonal allergic disease exhibited higher number of grass pollen-specific CD4+ T, than control subjects.
In addition, it is noteworthy that there was also a marked allergen-dependent proliferation of CD8+ cells in half of the grass pollen-sensitive atopic patients. Whether this represents an activation of allergen-specific CD8+ T-cells or a bystander expansion of CD8+ T-cells secondary to CD4+ T-cell stimulation and cytokine production is unknown. Interestingly, a role for CD8+ T cells in atopic diseases has been proposed by Cho et al. [10] who found an increased frequency of circulating IFN-producing CD8+ T cells in asthmatic patients compared with normal subjects. Characterization of the cytokine profile of these expanded peripheral CD8+ cells could indicate whether they may contribute to the allergic process.
In conclusion, using the fluorescent dye PKH26, we showed that grass pollen sensitive atopic patients show enhanced CD4+ T-cell reactivity to P. pratense compared with nongrass pollen control subjects and this could be related to the presence of elevated number of circulating allergen-specific CD4+ T cells. Phenotypic characterization of accumulated proliferating CD4+ cells, such as intracellular cytokine staining, or Th2 specific markers quantification could be performed in association with PKH26 labelling to characterize allergen-specific proliferating cells.
Acknowledgments
This study was supported by grants from Universite Paris Sud and the College des Professeurs de Pneumologie (France). Dr Till was supported by fellowships from the European Respiratory Society and University College London. P. pratense was a gift from ALK-Abello, Horsholm, Denmark. The authors thank JP. Abastado, K. Ballouard and C. Pohardy for their help.
References
- 1.Wierenga EA, Snoek M, Jansen HM, Bos JD, van Lier RA, Kapsenberg ML. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991;147:2942–9. [PubMed] [Google Scholar]
- 2.Imada M, Simons FE, Jay FT, Hayglass KT. Allergen-stimulated interleukin-4 and interferon-gamma production in primary culture: responses of subjects with allergic rhinitis and normal controls. Immunology. 1995;85:373–80. [PMC free article] [PubMed] [Google Scholar]
- 3.Till S, Dickason R, Huston D, et al. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture. relationship to expression of allergic disease. J Allergy Clin Immunol. 1997;99:563–9. doi: 10.1016/s0091-6749(97)70085-x. [DOI] [PubMed] [Google Scholar]
- 4.Durham SR, Gould HJ, Thienes CP, et al. Expression of epsilon germ-line gene transcripts and mRNA for the epsilon heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur J Immunol. 1997;27:2899–906. doi: 10.1002/eji.1830271123. [DOI] [PubMed] [Google Scholar]
- 5.Givan AL, Fisher JL, Waugh M, Ernstoff MS, Wallace PK. A flow cytometric method to estimate the precursor frequencies of cells proliferating in response to specific antigens. J Immunol Meth. 1999;230:99–112. doi: 10.1016/s0022-1759(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Quintieri F, Pugliese O, Reale G, Pini C, Di Felice G. T-cell and antibody response to Parietaria judaica allergenic fractions in atopic and nonatopic individuals. Allergy. 1993;48:37–44. doi: 10.1111/j.1398-9995.1993.tb02172.x. [DOI] [PubMed] [Google Scholar]
- 7.Till S, Durham S, Dickason R, et al. IL-13 production by allergen-stimulated T cells is increased in allergic disease and associated with IL-5 but not IFN-gamma expression. Immunology. 1997;91:53–7. doi: 10.1046/j.1365-2567.1997.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wurtzen PA, van Neerven RJ, Arnved J, Ipsen H, Sparholt SH. Dissection of the grass allergen-specific immune response in patients with allergies and control subjects: T-cell proliferation in patients does not correlate with specific serum IgE and skin reactivity. J Allergy Clin Immunol. 1998;101:241–9. doi: 10.1016/S0091-6749(98)70389-6. [DOI] [PubMed] [Google Scholar]
- 9.Burastero SE, Fenoglio D, Crimi E, Brusasco V, Rossi GA. Frequency of allergen-specific T lymphocytes in blood and bronchial response to allergen in asthma. J Allergy Clin Immunol. 1993;91:1075–81. doi: 10.1016/0091-6749(93)90222-2. [DOI] [PubMed] [Google Scholar]
- 10.Cho SH, Stanciu LA, Begishivili T, Bates PJ, Holgate ST, Johnston SL. Peripheral blood CD4+ and CD8+ T cell type 1 and type 2 cytokine production in atopic asthmatic and normal subjects. Clin Exp Allergy. 2002;32:427–33. doi: 10.1046/j.1365-2222.2002.01281.x. [DOI] [PubMed] [Google Scholar]