Abstract
Activation of leucocytes during airway inflammatory reaction involves adhesion to bronchial epithelial cells (BEC), a process implicating specific interactions between glycoproteins with epithelial cell surface proteins, mainly intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). In this study, the effect of keratinocyte growth factor (KGF), a growth factor involved in pulmonary epithelium repair, was evaluated on adhesion molecule expression with BEAS-2B cells and BEC and granulocyte adherence to BEAS-2B. The modulation by KGF of membrane and mRNA expression of ICAM-1 and VCAM-1 was studied on confluent cells stimulated or not with tumour necrosis factor-α (TNF) (200 UI/ml) or TNF and interleukin (IL)-4 (50 UI/ml and 10 ng/ml). Levels of soluble-(s)ICAM-1 and sVCAM-1 were measured by ELISA. Although moderately, KGF significantly decreased membrane ICAM-1 expression in unstimulated BEAS-2B cells (24% inhibition at 100 ng/ml) or in TNF- or TNF + IL-4-stimulated cells (22·5 and 18·7% inhibition, respectively). Treatment with KGF tended to decrease VCAM-1 expression in TNF- and TNF + IL-4-stimulated BEAS-2B (P = n.s. and P < 0·05, 14 and 15% inhibition, respectively). In primary culture of BEC, adhesion molecule expression was also reduced. ICAM-1 and VCAM-1 mRNA expression were also inhibited by KGF. Levels of sICAM-1 and sVCAM-1 were not significantly increased in supernatants from KGF-treated cells (30% and 24% increase at 100 ng/ml, respectively) compared to controls. Moreover, KGF decreased by 31% the adherence of neutrophils to TNF-activated BEAS-2B. In conclusion, KGF decreases ICAM-1 and VCAM-1 expression and neutrophil adherence in BEC. These suggest its involvement in the resolution of the inflammatory reaction.
Keywords: bronchial epithelial cell, ICAM-1, inflammatory reaction, KGF, VCAM-1
Introduction
The bronchial epithelium is a target of the local inflammatory response [1,2]. In asthma, the inflammatory infiltrate observed in bronchial mucosa is characterized by influx of eosinophils, activated T lymphocytes and monocytes, whereas neutrophil infiltrate is present in chronic obstructive pulmonary disease (COPD), cystic fibrosis and some forms of severe asthma [3,4]. Activation of these leucocytes by their adhesion on bronchial epithelial cells may contribute to airway epithelial injury. This process requires specific interactions between glycoproteins on leucocyte cells with the bronchial epithelial cell (BEC) surface proteins [5,6]. Among adhesion molecules involved in this process, two are prominent: intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Both belong to the superfamily of immunoglobulins and their expression can be induced by proinflammatory cytokines [7,8]. Compared with those from normal patients, increased expression of ICAM-1 and VCAM-1 has been shown on BEC from patients with chronic inflammatory diseases such as asthma and of ICAM-1 in COPD [9–12].
In vitro model studies indicate that VCAM-1 is involved in adhesion of eosinophils, monocytes and T lymphocytes via their cell surface, very late antigen-4 (VLA-4). ICAM-1 is implicated in the adhesion of leucocytes via β2 integrins such as lymphocyte function-associated antigen (LFA-1). In a monkey model of asthma, Wegner desmonstrates that eosinophil infiltration and bronchial hyperreactivity decrease under anti-ICAM-1 antibody infusion [9]. The proteolytic cleavage of ICAM-1 and VCAM-1 membrane-bound forms result in the release of their respective soluble forms. Their functions are not clear; however, in vitro the circulating forms were potent inhibitors of cell–cell adhesion at least for ICAM-1 [13–15].
Fibroblast growth factors (FGF) may play an important role in preventing and repairing alterations of the bronchial epithelium induced by local inflammation. Among these FGF, the keratinocyte growth factor (KGF), a single polypeptide chain of 28 kDa produced and secreted by fibroblasts, is an epithelial cell-specific growth factor [16,17], although some endothelial effects have been described recently [18]. Recombinant human KGF induces in vitro and in vivo proliferation and differentiation of bronchial and alveolar type II epithelial cells [19,20]. Intratracheal instillation of KGF prevents lung injury in rats exposed to hyperoxia and also protects against cyclic mechanic strain-, radiation-, bleomycin-, hydrochloric acid- and α-naphtylthiourea-induced lung injury [21–26]. Proinflammatory cytokines, such as interleukin (IL)-1β, tumour necrosis factor-α (TNF) and IL-6, strongly induce both KGF mRNA and protein expression by fibroblasts [27]. Thus KGF appears to have an important role in pulmonary and bronchial epithelial repair after inflammation injury [28], and release of proinflammatory cytokines leads to an increase of KGF production.
However, the potential effect of KGF on inflammatory properties of bronchial epithelial cells remains unknown, particularly on the expression of adhesion molecules. In primary culture of BEC and BEAS-2B cells, in this study we evaluated the effect of KGF on the expression of ICAM-1 and VCAM-1 and in BEAS-2B cells, the modulation of mRNA levels and on the adherence of neutrophils. BEAS-2B are bronchial epithelial cells transformed by an adenovirus 12-SV40 hybrid virus in which ICAM-1 and VCAM-1 expression are up-regulated by cytokines such as TNF [29]. Our data show that KGF is able to decrease ICAM-1 and VCAM-1 expression on bronchial epithelial cells mainly by the modulation of mRNA expression.
Materials And Methods
Cytokines, antibodies, and other reagents
The following materials were purchased: Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Life Technologies, Gaithersburg, MD, USA), bronchial epithelial cell growth medium kit (Promocell, Heidelberg, Germany), penicillin/streptomycin solution (Life Technologies), collagen G (3 mg/ml in 12 mm HCl) (Biochrom KG, Berlin, Germany), fetal calf serum (FCS), trypsin (containing 1 mm of ethylenediaminetetraacetic acid (EDTA)); agarose, phosphate-buffered saline (PBS), TRIZOL (Life Technologies), chloroform (Merck, Fontenay sous Bois, France) and isopropanol (Carlo Erba, Milan, Italy), gel star (FMC bioproducts, Rockland, ME, USA); and recombinant human TNF, interleukin-4 (IL-4) (R&D systems, Abingdon, Oxon, UK), KGF (a generous gift of Amgen, Thousands Oaks, CA, USA). The following mouse monoclonal antibodies (MoAb) were used: anti-ICAM-1 MoAb (IgG1), clone B159; anti-VCAM-1 MoAb (IgG1), clone 450–11 A (Pharmingen, San Diego, CA, USA), anticytokeratin antibody (NeoMarkers, Fremont, CA, USA) and the isotype control (IgG1), clone MOPC 21, as well as the secondary antibodies fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-labelled goat antimouse IgG (Sigma Chemical Co., St Louis, MO, USA).
Cell culture
Human bronchial epithelial biopsies were obtained by fiberoptic bronchoscopy from patients who were being investigated for bronchopulmonary carcinoma. Biopsies were taken at a distance from the tumour. Histological features of the bronchial mucosa were normal in all specimens. All procedures were reviewed and approved by the Hospital Institutional Review Board and written informed consent was obtained from all subjects included in the study.
BECs were cultured as described previously [30]. Briefly, two or three explants (approximately 0·5 × 0·5 mm in size) were placed on sterile plastic dishes coated with collagen G matrix (types I and III collagen) (Biochrom KG, Berlin, Germany). The explants were covered with 600 µl of DMEM/F12 (1 : 1) medium (Life Technologies, Gaithersburg, MD, USA) and were incubated for 24 h. Two ml of culture medium were then added to each dish. Culture medium consisted of serum-free DMEM/F12 (1 : 1) supplemented with 2% Ultroser G, 1% antibiotic (penicillin G sodium: 10 000 units/ml; streptomycin sulphate: 10 000 pg/ml; and amphotericin B, 25 µg/ml); and 2 mm l-glutamine (Life Technologies). Explants were placed in a humidified incubator at 37°C under 5% CO2 in air. The culture medium was changed every 3–4 days. Explants were cultured for 2 weeks, until BECs were confluent. The same explants were transferred three times to new, sterile, coated plastic dishes to initiate new primary BEC cultures. The epithelial phenotype was confirmed by staining with anticytokeratin 5/6/18 antibody. More than 96% were labelled with this antibody (data not shown).
BEAS-2B cells were obtained from the American Tissue Culture Collection (ATCC). This cell line was derived from human bronchial epithelium transformed by an adenovirus SV-40 hybrid virus. BEAS-2B cells were cultured in 75-cm2 culture flasks and maintained in bronchial epithelial cell growth medium, a medium containing penicillin and streptomycin and growth factors (Promocell, Heidelberg, Germany). After seeding, 5·105 cells were plated on six-well cluster plates (Costar, Cambridge, MA, USA) with or without collagen G coating and cultured with supplemented bronchial epithelial cell medium until confluence. When confluence was obtained, cells were cultured in DMEM F12 with 0·1% FCS, 2 mm l-glutamine, streptomycin and penicillin for 12 h.
Flow cytometric analysis
To assess the modulation of the ICAM-1 and VCAM-1 expression on BEAS-2B cells by the KGF, cells were stimulated by either TNF (200 UI/ml) or TNF (50 UI/ml) + IL-4 (10 ng/ml) (R&D Systems) and incubated simultaneously with increasing concentrations of KGF (10, 30 and 100 ng/ml) (a generous gift of Amgen) for 24 h. Supernatants were collected and used for analysis of s-ICAM-1 and s-VCAM-1. Cells were then treated briefly with versene–trypsin solution and, after neutralization of proteinase activity, removed from plates by repeated pipetting. Murine anti-ICAM-1 MoAb (IgG1, clone B159); anti-VCAM-1 MoAb (IgG1, clone 450–11 A), the isotype control (Pharmingen) and anti-CD29 MoAb (clone 4H4, Beckman Coulter, Roissy, France) were used as primary antibodies. For each analysis, cells were incubated in phosphate-buffered saline with 1% of human serum containing saturating concentrations of each monoclonal antibody for 30 min. Cells were washed, and incubated in 1/100 dilution of FITC- or PE-conjugated goat F(ab′)2 antimouse IgG antibody (Sigma Chemical Co.) for another 30 min, and then washed twice. The cells were resuspended and fixed in PBS with 0·25% of paraformaldehyde. The fluorescence of 10 000 events was measured using a flow cytometer (FACScalibur; Becton-Dickinson). In preliminary experiments, we checked that treatment with versene–trypsin of BEC did not affect ICAM-1 and VCAM-1 expression compared to cells detached in PBS plus EDTA 2 mm.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of ICAM-1 and VCAM-1 mRNA
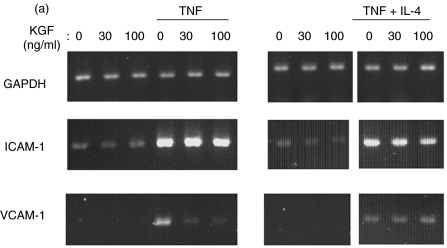
Total RNA was extracted from unstimulated and stimulated BEAS-2B cells, after 5 h with or without KGF, using the TRIZOL (Life Technologies) extraction technique as recommended by the manufacturer. Total RNA extracted from BEAS-2B was reverse transcripted with random oligo(dT) primers and Moloney murine leukaemia virus (MMLV) reverse transcriptase (Life Technologies). During PCR, the primers used were: ICAM-1: forward 5′-AGCTGGCACATTGGAGTCTG-3′ and reverse 5′-CCAACGTCTCGTCCTCTTC-3′; VCAM-1: forward 5′-CCAA GAATACAGTTATTTCGTG-3′ and reverse 5′-CTTAATTAA CAAGTTCGTAAGGGAT-3′; GAPDH primers were used as a control. The PCR reagents and Taq polymerase were purchased from Life Technologies. Thermocycler settings were an initial step at 94°C for 2 min to denature cDNA, followed by 27, 33 and 21 cycles, respectively, for ICAM-1, VCAM-1 and GAPDH of 94°C for 30 s to denature, 56°C for 30 s for annealing, and 72°C for 1 min for polymerization. The number of cycles was defined by a kinetic experiment and gave a linear dose–response curve. Amplified products were electrophoresed on a 1·5% agarose gel and stained with gel star (FMC products, Rockland, ME, USA). Intensity of each band of cDNA was quantified by densitometry. Results were expressed as a ratio of the signal obtained for GAPDH.
Measurement of soluble ICAM-1 and VCAM-1
Culture supernatants were analysed for the presence of soluble (s)ICAM-1 and VCAM-1 by specific enzyme-linked immunosorbent assay (ELISA) (Diaclone, Besançon, France) according to the manufacturer's instructions. The sensitivity is 0·1 ng/ml and 0·6 ng/ml for sICAM-1 and sVCAM-1, respectively.
Adherence of neutrophils
Neutrophils were purified from venous blood of healthy subjects by standard procedure and the adherence test was achieved as previously described [6,31] with minor modifications. Briefly, BEAS-2B were expanded and activated for 24 h, as described previously in 96-well plates. After one washing, 50 µl of fresh medium and 50 µl containing 0·5 × 106 neutrophils were added to each well. After 30 min incubation, non-adherent neutrophils were discarded by two washes with PBS. Adherent neutrophils were lysed by addition of 100 µl NH4Cl 0·1 m solution and numbered by myeloperoxidase assay. Ortho-phenylenediamine (OPD, Sigma Chemical Co.) in acetate buffer pH 5·5 was used as a substrate. Optical density (O.D.) was read with a multiwell spectrophotometer at 492 nm. All analyses were performed in triplicate and negative controls were performed with BEAS-2B alone (O.D. = 0·055 ± 0·01).
Statistical analysis
Data are expressed as means ± s.e.m. Results from different flow cytometer experiments are expressed as median fluorescence intensity (MFI) ± s.e.m. after subtracting the MFI of the isotype control. The percentage of variation from control value was calculated as follows: ((MFI of stimulated cells minus MFI of the relevant control) × (100)/MFI of the relevant control. Statistical analysis was performed with a Macintosh computer (Apple Company, Copernito, CA, USA) using Statview II software (Statview, Inc.). Statistical significance was assessed with the Wilcoxon test, and a value of P < 0·05 was considered significant.
Results
KGF decreases ICAM-1 expression by BEAS-2B cells
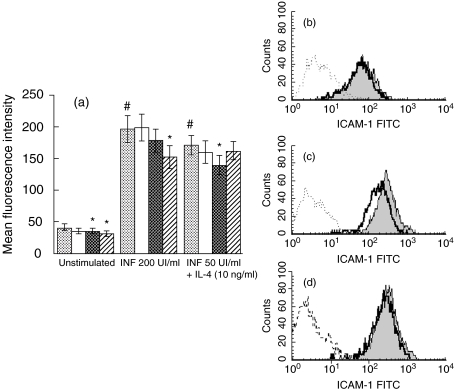
ICAM-1 was expressed constitutively in BEAS-2B (MFI = 40·7 ± 5) and was up-regulated in stimulated conditions. TNF (200 UI/ml) induced a marked increase in ICAM-1 expression, respectively, on BEAS-2B cultured on collagen (MFI = 196 ± 21) as well as TNF (50 UI/ml) + IL-4 (10 ng/ml) (MFI = 170 ± 15) (Fig. 1a). The level of adhesion molecule expression was similar in BEAS-2B cells expanded on plastic or on collagen G-coated wells (data not shown, P = n.s.).
Fig. 1.
Flow cytometry analysis of ICAM-1 expression on BEAS-2B incubated by KGF. (a) Cells were cultured on collagen-coated flasks with different concentrations of KGF for 24 h under basal conditions or stimulated with TNF (200 UI/ml) or the combination of TNF (50 UI/ml) and IL-4 (10 ng/ml). Results for six different experiments are expressed as mean fluorescence intensity (MFI) ± s.e.m. corrected by subtracting the mean fluorescence of the negative control. (b, c, d) FACS analysis was reported for one representative of the six experiments. The bold and shaded lines show the data obtained with anti-ICAM-1 antibody in BEAS-2B incubated with or without KGF, respectively, whereas the dotted line illustrated the results obtained with the isotype control. BEAS-2B incubated or without KGF (30 ng/ml) were either unstimulated ((b), MFI: 56 and 67, respectively), activated with TNF ((c), MFI: 178 and 286) or with TNF plus IL-4 ((d), MFI: 176 and 211). #P < 0·05: significantly different from unstimulated conditions; *P < 0·05, a significant decrease induced by KGF above the level in the corresponding control.  , Control; □, KGF 10 ng/ml;
, Control; □, KGF 10 ng/ml;  , KGF 30 ng/ml;
, KGF 30 ng/ml;  , KGF 100 ng/ml.
, KGF 100 ng/ml.
KGF did not modulate adhesion molecule expression on cells cultured on plastic (data not shown, P = n.s.). In contrast, when BEAS-2B were cultured on collagen G-coated flasks, KGF induced a significant decrease of ICAM-1 expression on unstimulated cells in a concentration-dependent manner (P < 0·05) (Fig. 1a). The maximal effect was observed for a dose of 100 ng/ml KGF (inhibition of 24%). KGF also inhibited cytokine-induced up-regulation of ICAM-1 expression on the BEAS-2B cells. The inhibitory effect was dose-dependent; maximal values were observed at 100 ng/ml of KGF under TNF (inhibition of 22·5%) (Fig. 1a,c) and at 30 ng/ml under TNF + IL-4 (inhibition of 18·7%) (Fig. 1a,d). In contrast, the level of CD29 expression was not affected by KGF (30 ng/ml) in unstimulated (MFI = 254·4 ± 81·9) or TNF-activated (259·4 ± 63·9) BEC compared with control (271·6 ± 80·5 and 227·4 ± 65·5, respectively) (P = n.s.).
Down-regulation of TNF-α + IL-4 induced VCAM-1 expression by KGF
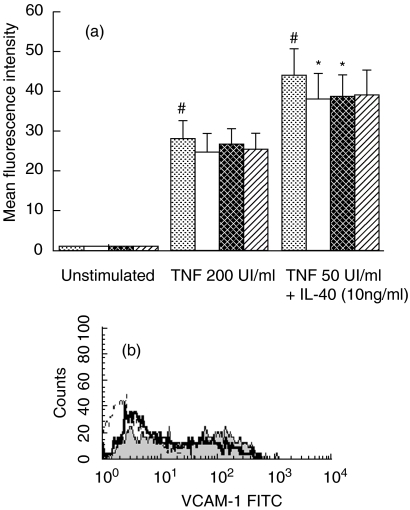
BEAS-2B did not express VCAM-1 either constitutively or with KGF alone (Fig. 2a). In accordance with previous studies, TNF (MFI = 28·12 ± 4) and TNF + IL-4 (MFI = 43·9 ± 7) induced VCAM-1 expression after an incubation period of 24 h on collagen-coated dishes at a level similar to cells in plastic wells. Under stimulated conditions, KGF decreased VCAM-1 expression in a concentration-dependent manner on BEAS-2B cultured on collagen G, whereas this growth factor had no effect on plastic cultivated cells. A non-significant decrease of 14% under TNF stimulation was obtained with 10 ng/ml of KGF (Fig. 2a), whereas 10 and 30 ng/ml of KGF decreased significantly TNF + IL-4-induced VCAM-1 expression (P < 0·05) (Fig. 2a,b).
Fig. 2.
Flow cytometry analysis of the VCAM-1 expression on BEAS-2B cells incubated with KGF. Cells were cultured in collagen-coated flasks with different concentrations of KGF for 24 h under basal conditions or stimulated with TNF (200 UI/ml) or the combination of TNF (50 UI/ml) and IL-4 (10 ng/ml). (a) Results for seven different experiments are expressed as mean fluorescence intensity (MFI) ± s.e.m. corrected by subtracting the mean fluorescence of the negative control. (b) One representative experiment, histograms obtained by FACS analysis with isotype control (dotted line) and anti-VCAM-1 antibody in BEAS-2B cells incubated with (bold line, MFI = 20·5) and without KGF at 30 ng/ml (shaded line, MFI = 30·2); the cells were stimulated with TNF plus IL-4. #P < 0·05: significantly different from unstimulated condition; *P < 0·05, a significant decrease induced by KGF above the level in the corresponding control.  , Control; □, KGF 10 ng/ml;
, Control; □, KGF 10 ng/ml;  , KGF 30 ng/ml;
, KGF 30 ng/ml;  , KGF 100 ng/ml.
, KGF 100 ng/ml.
Modulation by KGF of ICAM-1 and VCAM-1 expression in primary culture of bronchial epithelial cells
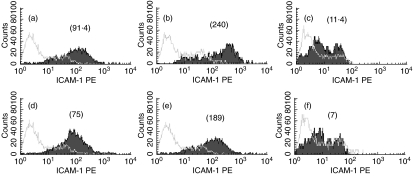
Primary cultures of BEC were used to confirm the data obtained with transformed BEC. FACS analysis showed a low level of ICAM-1 expression in unstimulated cells (Fig. 3a), whereas TNF (Fig. 3b) and TNF plus IL-4 (data not shown) strongly up-regulated its expression. KGF at 100 ng/ml significantly down-regulated ICAM-1 expression in unstimulated and TNF-activated BEC (Fig. 3d,e, respectively) (P < 0·05).
Fig. 3.
Flow cytometry analysis for the cell surface expression of ICAM-1 and VCAM-1 on unstimulated BEC from primary culture (BEC) (a–c) or cells incubated with KGF at 30 ng/ml (d–f). ICAM-1 expression was reported for unstimulated (a, d) and TNF-stimulated HBEC (b, e) whereas VCAM-1 expression was only shown for TNF + IL-4-stimulated BEC (c, f). Grey lines represent the histograms obtained in the presence of isotype control, whereas shaded lines showed data observed with anti-ICAM-1 or -VCAM-1 antibody. The values mentionned above the histograms represent the corresponding MFI. This is representative of four experiments.
Similarly to BEAS-2B, VCAM-1 expression was undetectable in unstimulated BEC whereas TNF (data not shown) and TNF plus IL-4 (Fig. 3c) induced its expression. In the presence of TNF plus IL-4, KGF significantly decreased VCAM-1 expression (Fig. 3f, P < 0·05), whereas it had no effect on unstimulated and TNF-stimulated cells (data not shown).
Effect of KGF on the release of soluble ICAM-1 and VCAM-1 by BEAS-2B cells
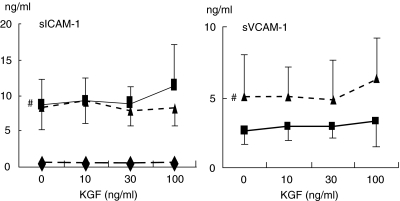
To determine whether the decrease of ICAM-1 and VCAM-1 expression by KGF was linked to an increased release of soluble forms, their concentrations were measured in BEAS-2B supernatants. As expected, stimulated BEAS-2B significantly released higher concentrations of sICAM-1 (from 0·6 ± 0·2 ng/ml in controls to 8·7 ± 3·4 ng/ml with TNF, P < 0·01, and to 8·5 ± 3·2 ng/ml with TNF + IL-4, P < 0·01) and sVCAM-1 than unstimulated cells (undetectable in controls (< 0·8 ng/ml) to 2·6 ± 0·9 ng/ml with TNF, and to 5·1 ± 2·9 ng/ml with TNF + IL-4) (Fig. 4). The cleavage of ICAM-1 and VCAM-1 was not affected by KGF at 10 and 30 ng/ml. KGF at 100 ng/ml tended to enhance the release of soluble ICAM-1 and VCAM- 1 only in TNF- and TNF + IL-4-stimulated cells (respectively, 30 and 24% increase; P = n.s.).
Fig. 4.
Effect of KGF on the release of the soluble forms of ICAM-1 and VCAM-1 by BEAS-2B under basal and stimulated conditions. Cells were cultured in collagen-coated flasks with different concentrations of KGF for 24 h, under basal or stimulated conditions with TNF (200 UI/ml) or the combination of TNF (50 UI/ml) and IL-4 (10 ng/ml). Culture supernatants were analysed by ELISA. Results for 10 and seven experiments for sICAM-1 and sVCAM-1, respectively, are reported as mean ± s.e.m. The level of sVCAM-1 was undetectable for unstimulated BEAS-2B cells. #P < 0·05: significantly different from unstimulated conditions. ⋄, Unstimulated; ▪, TNF; ▴, TNF + IL-4.
KGF down-regulated ICAM-1 and VCAM-1 mRNA expression in BEAS-2B
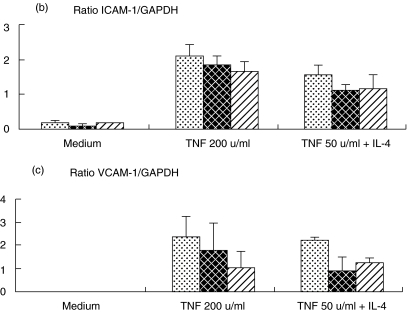
To determine at which level KGF acted, ICAM-1 and VCAM-1 mRNA expression was analysed in BEAS-2B cells by RT-PCR. The mRNA levels were normalized according to the level of GAPDH RNA, and the results for different culture conditions are shown in Fig. 5. According to the data concerning ICAM-1 expression on cell membrane, KGF decreased ICAM-1 mRNA expression up-regulated by TNF and by TNF + IL-4 (20 and 28% inhibition), whereas KGF had no effect on unstimulated cells (Fig. 5a,b). Concerning VCAM-1, specific mRNA was undetectable without stimulation (Fig. 5a,c). Treatment with KGF markedly inhibited VCAM-1 mRNA expression induced by TNF and by TNF + IL-4 (50 and 39% inhibition).
Fig. 5.
RT-PCR analysis of the mRNA expression on BEAS-2B incubated with and without KGF. Total RNA from BEAS-2B cultures were extracted after 5 h of culture, and then ICAM-1and VCAM-1 mRNA expression was analysed by semiquantitative RT-PCR. (a) A representative experiment was reported illustrating the bands obtained for GAPDH, ICAM-1 and VCAM-1 mRNA analysis. Quantification of mRNA was achieved by gel analysis and expressed as a ratio with GAPDH mRNA. (b) The mean ± SEM of four and three experiments were reported for ICAM-1 (b) and VCAM-1 (c), respectively.  , Control;
, Control;  , KGF 30 ng/ml;
, KGF 30 ng/ml;  , KGF 100 ng/ml.
, KGF 100 ng/ml.
Pretreatment with KGF decreased the adherence of neutrophils to TNF-α activated bronchial epithelial cells
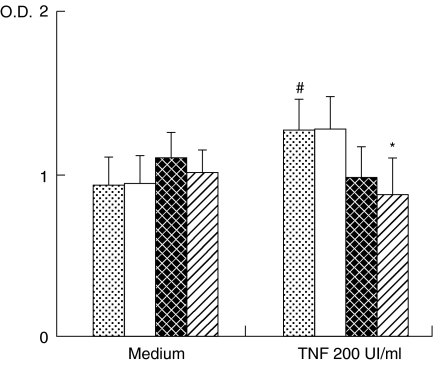
In order to define the biological relevance of the modulation of ICAM-1 expression in KGF-treated BEAS-2B cells, adherence of neutrophils was evaluated on TNF-stimulated and -unstimulated BEAS-2B cells treated or not with KGF. BEAS-2B cells preactivation with TNF significantly increased the adherence of neutrophils (40% increase; P < 0·05) (Fig. 6). Whereas KGF pretreatment of unstimulated cells had no effect on the adherence of neutrophils, addition of KGF to TNF-prestimulated BEAS-2B cells decreased the adherence of neutrophils significantly in a concentration-dependent manner (13 and 31% of inhibition, respectively, at 30 and 100 ng/ml, P < 0·05).
Fig. 6.
Pretreatment with KGF decreased the adherence of neutrophils to TNF-activated BEAS-2B cells but not to unstimulated cells. Adherent neutrophils were quantified by the measurement of myeloperoxydase in cell lysates. Results for seven experiments are expressed as mean optical density (O.D.) ± s.e.m. #P < 0·05: significantly different from unstimulated conditions; *P < 0·05, a significant decrease induced by KGF above the level in the corresponding control.  , Control; □, KGF 10 ng/ml;
, Control; □, KGF 10 ng/ml;  , KGF 30 ng/ml;
, KGF 30 ng/ml;  , KGF 100 ng/ml.
, KGF 100 ng/ml.
Discussion
The presence of neutrophils and eosinophils represents an important component of the host response to infection; however, their persistent presence and activation are thought to be largely responsible for the pathophysiology of several debilitating diseases, including COPD and asthma [2,3]. Adhesive interactions are thought to be important for both the localization and activation of leucocytes. Adhesion-dependent signalling has been reported to enhance both oxygen radical formation and degranulation responses in neutrophils and eosinophils [32,33]. Recent studies underlined the involvement of KGF as a protector and repair factor of the bronchial epithelium in various lung injuries (hyperoxia, cyclic mechanic stress, radiation, bleomycin, hydrochloric acid and naphtylthiourea) [21–26]. Morever, its production is increased during inflammatory reaction [28]. These data suggest that KGF may modulate bronchial inflammation.
Because adhesion molecules such as ICAM-1 and VCAM-1 are implicated in leucocyte recruitment toward target tissues, we investigated the potential modulation by KGF of their expression on bronchial epithelial cells. To evaluate this hypothesis, we used the bronchial epithelial cell line BEAS-2B, known to maintain a typical epithelial morphology and to express ICAM-1 and VCAM-1 in a manner similar to bronchial epithelial cells. As both adhesion molecules are up-regulated under proinflammatory cytokines, in the present study we tested TNF and IL-4 because the first is a major inflammatory cytokine produced in the lung by several cell types, including macrophages and mast cells, and the second, produced by Th2 lymphocytes, plays a central role in allergic disease such as asthma.
In our experiments, BEAS-2B expresses constitutively ICAM-1 and VCAM-1 and their expression is up-regulated by TNF or TNF + IL-4 after 24 h by incubation, as described previously by Atsuta and colleagues [29] with BEAS-2B cells cultured in uncoated flasks. Similar levels of expression are observed when BEAS-2B are maintained in collagen G-coated wells, whereas Bloemen and coworkers did not detect induction of VCAM-1 when cells were expanded on different extracellular matrix components such as vitrogen- or fibronectin-coated flasks [7].
Our results also showed that KGF only modulates ICAM-1 and VCAM-1 expression when BEAS-2B are cultured in collagen-coated flasks and not in uncoated flasks. The same effect was also obtained with human BEC. These observations suggest that the BEC have to be bound to extracellular matrix proteins, and particularly in our study with collagen G, to induce the control of ICAM-1 and VCAM-1 expression by KGF. In BEC, integrins, and especially β1-integrins, represent some of the collagen receptors and their mobilization activated different signalling pathways. The mitogen activated protein kinase (MAPK) pathway is a common pathway between β-integrins and FGF-receptor signalling [34,35]. As MAPK such as p38 regulate the expression of ICAM-1 in epithelial cells [36], we suppose that the modulation of ICAM-1 and VCAM-1 production by KGF in association with a co-signal induced by β1-integrin was mediated by their action on this signalling pathway.
Whereas KGF did not affect VCAM-1 expression in unstimulated BEC, we demonstrated that basal expression of ICAM-1 was significatively down-regulated in a concentration-dependent manner. In the presence of TNF or TNF + IL-4, KGF significantly decreased expression of both adhesion molecules. These results suggest that KGF effect require ICAM-1 and VCAM-1 turnover in BEC cells and in a manner independent of the stimulatory pathway.
Adhesion molecule mRNA expression was analysed by RT-PCR to evaluate a potential effect of KGF at this level. Our results showed that KGF decreased ICAM-1 and VCAM-1 mRNA on BEAS-2B, suggesting that this growth factor affects adhesion molecule gene transcription.
To progress in evaluation of KGF effects on ICAM-1 and VCAM-1 cell expression, measurements of the soluble forms of these adhesion molecules were performed by ELISA. Soluble adhesion molecules might interfere with the development of inflammatory reaction: sICAM-1 reduces leucocyte adhesion and induces cytokine release by lung macrophages [37,38]. In accordance with previous studies, TNF with or without IL-4 increased soluble ICAM-1 and VCAM-1 production by the BEAS-2B cell, whereas there was low production under basal condition. KGF, at a concentration of 100 ng/ml, enhanced the levels of sICAM-1 and sVCAM-1 in activated BEAS-2B cells; however, these differences are not statistically significant. Moreover, this effect was not observed at 30 ng/ml in contrast with the data on cell membrane expression. In summary, our results suggest that the effect of KGF on membrane expression was not the consequence of an increased rate of soluble form cleavage but rather a modulation of mRNA expression.
The biological relevance of KGF effects on adhesion molecules expression need to be defined. Under basal conditions, the protective effect of KGF may be mediated by a down-regulation of ICAM-1 cell expression, which probably induces a decrease of adhesion of polymorphonuclear leucocytes to airway epithelium. Whereas the proinflammatory cytokines increase ICAM-1 and VCAM-1 cell expression we find, in vitro, that the curative effect of KGF could be related to the decrease of both adhesion molecules. This mechanism might constitute a negative feed-back loop to control leucocyte migration because TNF also induces KGF production [27]. We also evaluated the modulation by KGF of neutrophil adherence to BEAS-2B cells. While KGF did not modulate the adherence to unstimulated BEAS-2B cells, it decreased by 31% the number of adherent neutrophils to TNF-activated BEAS-2B. As adherence of neutrophils to BEC is dependent mainly on ICAM-1 binding with β1 integrins [31], these data suggest that modulation by KGF of ICAM-1 expression in activated BEC might have some consequences on the development of the lung inflammatory reaction. In contrast, the neutrophil adherence to unstimulated BEAS-2B cells might implicate ICAM-1-independent mechanism. Nevertheless, these data have to be confirmed in vivo. Moreover, further studies are required to determine the consequence of VCAM-1 modulation on the adherence of eosinophils and T lymphocytes to BEC which might be altered by treatment with KGF.
In conclusion, this study demonstrates, in vitro, using BEAS-2B and primary culture of BEC in collagen-coated culture, an effect of KGF on the regulation of local inflammation. Indeed, KGF moderately decreases ICAM-1 and VCAM-1 cell expression and inhibited the neutrophil adherence to activated BEAS-2B cells. By this mechanism, this growth factor may play a role in the control of inflammatory reaction, reducing leucocyte recruitment and activation.
References
- 1.White SR. Epithelium as a target. In: Kay AB, editor. Asthma. 1. Physiology, immunopharmacology, and treatment. Oxford: Blackwell Scientific Publications; 1997. pp. 875–99. [Google Scholar]
- 2.Holgate ST. The inflammation repair cycle in asthma: the pivotal role of the airway epithelium. Clin Exp Allergy. 1998;28:S97–103. doi: 10.1046/j.1365-2222.1998.028s5097.x. [DOI] [PubMed] [Google Scholar]
- 3.Jeffery PK. Differences and similarities between chronic obstructive pulmonary disease and asthma. Clin Exp Allergy. 1999;29:S14–26. [PubMed] [Google Scholar]
- 4.Lamblin C, Gosset P, Tillie-Leblond I, et al. Bronchial neutrophilia in patients with non infectious status asthmaticus. Am Rev Respir Crit Care Med. 1998;157:394–402. doi: 10.1164/ajrccm.157.2.97-02099. [DOI] [PubMed] [Google Scholar]
- 5.Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4:195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 6.Robbins RA, Koyama S, Spurzem JR, et al. Modulation of neutrophil and mononuclear cell adherence to bronchial epithelial cells. Am J Respir Cell Mol Biol. 1992;7:19–29. doi: 10.1165/ajrcmb/7.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Bloemen PGM, Van den Tweel MC Henricks PAJ, et al. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1993;9:586–93. doi: 10.1165/ajrcmb/9.6.586. [DOI] [PubMed] [Google Scholar]
- 8.Tosi MF, Stark JM, Smith CW, Hamedani A, Gruenert DC, Infeld MD. Induction of ICAM-1 expression on human airway epithelials by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am J Respir Cell Mol Biol. 1992;7:214–21. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- 9.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothelein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456–9. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- 10.Canonica GW, Ciprandi C, Pesce GP, Buscaglia S, Paolieri F, Bagnasco M. ICAM-1 on epithelial cells in allergic subjects: a hallmark of allergic inflammation. Int Arch Allergy Immunol. 1995;107:99–102. doi: 10.1159/000236943. [DOI] [PubMed] [Google Scholar]
- 11.Gosset P, Tillie-Leblond I, Janin A, et al. Expression of E-selectin, ICAM-1 and VCAM-1 on bronchial biopsies from allergic and non-allergic asthmatic patients. Int Arch Allergy Immunol. 1995;106:69–77. doi: 10.1159/000236892. [DOI] [PubMed] [Google Scholar]
- 12.Riise GC, Larsson S, Lofdalh CG, Andersson BA. Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur Respir J. 1994;7:1673–7. doi: 10.1183/09031936.94.07091673. [DOI] [PubMed] [Google Scholar]
- 13.Marlin SD, Staunton DE, Springer TA, Stratowa C, Sommergruber W, Merluzzi VJ. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990;344:70–2. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- 14.Meyer DM, Dustin ML, Carron CP. Characterization of Intercellular adhesion molecule-1 ectodomain (sICAM-1) as an inhibitor of lymphocyte function-associated molecule−1 interaction with ICAM-1. J Immunol. 1995;155:3578–84. [PubMed] [Google Scholar]
- 15.Becker JC, Termeer C, Schmidt CE, Bröcker EB. Soluble intercellular adhesion molecule-1 inhibits MHC-restricted specific T cell/tumor interaction. J Immunol. 1993;151:7224–32. [PubMed] [Google Scholar]
- 16.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–6. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–65. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 18.Gillis P, Salva U, Volpert OV, et al. Keratinocyte growth factor induces angiogenesis and protects endothelial barrier function. J Cell Sci. 1999;112:2049–57. doi: 10.1242/jcs.112.12.2049. [DOI] [PubMed] [Google Scholar]
- 19.Michelson PH, Tigue M, Panos RJ, Sporn PH. Keratinocyte growth factor stimulates bronchial epithelial cell proliferation in vitro and in vivo. Am J Physiol. 1999;277:L737–42. doi: 10.1152/ajplung.1999.277.4.L737. [DOI] [PubMed] [Google Scholar]
- 20.Ulich TR, Yi ES, Longmuir K, et al. Keratinocyte growth factor is a growth factor for type II pneumocyte in vivo. J Clin Invest. 1994;93:1298–306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu KI, Pollack N, Panos RJ, Sporn RJ, Kamp DW. Keratinocyte growth factor promotes alveolar epithelial cell DNA repair after H2O2 exposure. Am J Physiol. 1998;275:L780–7. doi: 10.1152/ajplung.1998.275.4.L780. [DOI] [PubMed] [Google Scholar]
- 22.Waters CM, Salva U. Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J Cell Physiol. 1999;181:424–32. doi: 10.1002/(SICI)1097-4652(199912)181:3<424::AID-JCP6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol. 1998;186:90–8. doi: 10.1002/(SICI)1096-9896(199809)186:1<90::AID-PATH137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Takeoka M, Ward WF, Pollack H, Kamp DW, Panos RJ. KGF facilitates repair of radiation-induced DNA damage in alveolar epithelial cells. Am J Physiol. 1997;272:L1174–80. doi: 10.1152/ajplung.1997.272.6.L1174. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Yi ES, Havill AM, et al. Intravenous keratinocyte growth factor protects against experimental pulmonary injury. Am J Physiol. 1998;275:L800–5. doi: 10.1152/ajplung.1998.275.4.L800. [DOI] [PubMed] [Google Scholar]
- 26.Mason CM, Guery BP, Summer WR, Nelson S. Keratinocyte growth factor attenuates lung leak induced by alpha-naphtylthiourea in rats. Crit Care Med. 1996;24:925–31. doi: 10.1097/00003246-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Brauchle M, Agermeyer K, Hübner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9:3199–204. [PubMed] [Google Scholar]
- 28.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am J Respir Crit Care Med. 1998;158:386–94. doi: 10.1164/ajrccm.158.2.9711111. [DOI] [PubMed] [Google Scholar]
- 29.Atsuta J, Sterbinsky SA, Plitt J, Schwiebert LM, Bochner BS, Schleimer RP. Phenotyping and cytokine regulation of the BEAS-2B human bronchial epithelial cell: demonstration of inducible expression of the adhesion molecules VCAM-1 and ICAM-1. Am J Respir Cell Mol Biol. 1997;17:571–82. doi: 10.1165/ajrcmb.17.5.2685. [DOI] [PubMed] [Google Scholar]
- 30.Yao PM, Buhler JM, d’Ortho MP, et al. Expression of matrix metalloproteinases A and B by cultured epithelial cells from human bronchial explants. J Biol Chem. 1996;271:15580–9. doi: 10.1074/jbc.271.26.15580. [DOI] [PubMed] [Google Scholar]
- 31.Abdelaziz MM, Devalia JL, Khair OA, Calderon M, Spasford RJ, Davies RJ. The effect of conditionned medium from cultured human bronchial epithelial cells on eosinophil and neutrophil chemotaxis and adherence in vitro. Am J Respir Cell Mol Biol. 1995;13:728–37. doi: 10.1165/ajrcmb.13.6.7576711. [DOI] [PubMed] [Google Scholar]
- 32.Becker EL, Showell HJ, Henson PM, Hsu LS. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974;112:2074–54. [PubMed] [Google Scholar]
- 33.Nagata N, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–202. [PubMed] [Google Scholar]
- 34.Giancotti FG, Ruoslathi E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 35.Szebenyi G, Fallon JF. Fibroblast growth factors as multifunctional signaling factors. Int Rev Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- 36.Takizawa H, Abe S, Ohtoshi T, et al. Diesel exhaust particles up-regulate expression of intercellular adhesion molecule-1 (ICAM-1) in human bronchial epithelial cells. Clin Exp Immunol. 2000;120:356–62. doi: 10.1046/j.1365-2249.2000.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusterer K, Bojunga J, Enghofer M, et al. Soluble ICAM-1 reduces leucocytes adhesion to vascular endothelium in ischemia–reperfusion injury in mice. Am J Physiol. 1998;275:G377–80. doi: 10.1152/ajpgi.1998.275.2.G377. [DOI] [PubMed] [Google Scholar]
- 38.Schmal H, Czermak BJ, Lentsch AB, et al. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J Immunol. 1998;161:3685–93. 1997 875–99. [PubMed] [Google Scholar]