Abstract
In sarcoidosis, a T helper 1 (Th1) response is an essential event and the up-regulation of interleukin-12 (IL-12) has been detected in affected disease sites. In order to investigate the clinical usefulness of circulating IL-12, we measured the serum concentrations of IL-12 by ELISA and performed immunohistochemistry using specific MoAbs for IL-12 in the lungs and scalene lymph nodes of patients with sarcoidosis. The serum concentration of IL-12 p40 was detectable in all 45 patients with pulmonary sarcoidosis and 18 normal controls, whereas that of IL-12 p70 was undetectable. The serum concentrations of IL-12 p40 in pulmonary sarcoidosis were significantly higher than those of the normal controls, especially in cases with abnormal intrathoracic findings detected by chest roentogenogram. The serum concentrations of interferon-γ (IFN-γ) also increased compared with those of normal controls and there was a significant positive correlation between the serum concentrations of IL-12 p40 and IFN-γ. Furthermore, serum angiotensin-converting enzyme (ACE) and lysozyme, which are known to be useful markers for disease activity in sarcoidosis, correlated well with the serum concentrations of IL-12 p40. The positive 67Ga scan group (for lung field) had significantly elevated serum IL-12 p40 levels compared with those of the negative group. No bioactivity of IL-12 p70 was detected in three sarcoid cases sera by using the IL-12 responsive cell line. Finally, the immunohistochemical approach revealed that IL-12 p40 was expressed in the epithelioid cells and macrophages of sarcoid lungs and lymph nodes. We concluded that the production of IL-12 p40 was far greater in the sera and we have demonstrated this to be a useful clinical marker for disease activity and the Th1 response in pulmonary sarcoidosis.
Keywords: IL-12 p40, Th1 predominance, pulmonary sarcoidosis
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown cause which is characterized by histological findings, including non-caseating epithelioid cell granulomas and infiltrates of CD4+ T cells and macrophages. Recent immunological advances have revealed a predominance of Th1 reaction in its immunopathogenesis. IFN-γ and other Th1 cytokines play important roles in the formation of inflamed sarcoid lesions. Many chemokines that prime Th1 reactions [including monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and interferon inducible protein 10 (IP-10)] are also up-regulated in sarcoid lesions [1,2].
IL-12 is an essential cytokine in the Th1 response, which makes naive T cells [T helper potential (Thp) cells] differentiate into Th1 cells, induces IFN-γ production from Th1 cells, and enhances the cytotoxicity of activated T cells and NK cells [3]. In sarcoidosis, up-regulation of IL-12 was reported and is important in the immunopathogenesis of sarcoidosis [4,5]. IL-12 has three different isotypes, the p40 monomer, the p40 homodimer and bioactive p70 heterodimer composed of the p40 and p35 subunits [6,7]. A recent investigation [8] has demonstrated that a novel p19 protein engages IL-12 p40 to form IL-23 (p19p40 heterodimer). Similar to IL-12 p70, human IL-23 stimulates IFN-γ production and proliferation in PHA blast T cells. The role of p40 in IL-12 is well understood, but the role of IL-23 in human disorders has not been fully studied.
In this study, we measured the concentrations of serum IL-12 p40 and IL-12 p70; however, serum IL-12 p70 levels were below our threshold of sensitivity. To evaluate the clinical significance of serum IL-12 p40, we compared circulating IL-12 p40 with the established disease markers of sarcoidosis and investigated the bioactivity of IL-12 p40 in serum using the IL-12 responsive cell line (NK0 cell).
Materials And Methods
Study population
The diagnosis of sarcoidosis was established in 45 individuals (17 males, mean age; 28 and 28 females, mean age 55 years). All of them were histologically proven cases (non-caseating epithelioid cell granulomas) showing without any evidence of mycobacterial, fungal or parasitic infection. None of the individuals had any history of exposure to organic or inorganic materials known to cause granulomatous lung diseases. No patients had received corticosteroid therapy at the start of the study. Thirty-four patients had abnormal chest X-ray findings; 18 patients demonstrated hilar lymphoadenopathy alone (stage I), 10 patients showed hilar lymphoadenopathy and interstitial infiltrates in the lung (stage II) and six patients showed interstitial infiltrates in the lung (stage III) or pulmonary fibrosis (stage IV). Eleven patients had no abnormal chest X-ray findings; however, they had abnormal lung biopsy findings (non-caseating epithelioid cell granulomas) and uveitis (stage 0). The assessment of disease included clinical features, high resolution computed tomography, lung function tests, a 67Ga scan, bronchoalveolar lavage (BAL) and routine blood studies.
For comparison, 18 age- and sex-matched normal individuals were examined. None had a history of cardiopulmonary or other illness. All results of the physical examination, chest X-ray and lung function tests were normal. Informed consent was obtained from both the patients and normal volunteers.
Blood samples were collected at the time of diagnosis and serum samples were cryopreserved at −80°C until use.
IL-12 and IFN-γ assay
ELISA for determination of serum IL-12 and IFN-γ levels was performed using commercial ELISA kits [IL-12 p70: R&D systems, Minneapolis, USA, sensitivity 0·5 pg/ml, IL-12 (p40 and p70): Endogen, Woburn, USA, sensitivity 5 pg/ml and IFN-γ: high sensitive interferon-gamma human ELISA system, Amersham Life Science, Bucks, UK, sensitivity 0·10 pg/ml] according to the manufacture's recommendation. For the measurement of IL-12 (p40 and p70), we diluted serum samples by 10-fold to fall within measurable concentrations. ELISA for IFN-γ measurement of culture supernatants in NK0 cells was performed using another commercial kit (BioSource International, CA, USA, sensitivity 4 pg/ml).
Enzyme assays
Angiotensin-converting enzyme (ACE) activity was measured using a method based on colourimetry of the quinoneimine dye produced from the substrate hippuryl-l-histidyl-l-leicine [9]. Lysozyme activity was measured using a turbidimetric method described by Ensink and Haeringen [10]. In this study, serum samples were incubated with a suspension of Micrococcus lysodeikticus (Sigma, St Louis, MO, USA). Lysis of the bacteria with lysozyme was measured in a spectrophotometer.
Immunohistochemistry of IL-12
IL-12 monoclonal antibodies (MoAbs: 20C2, a p40 in p70 heterodimer, rat IgG1 and 4D6, a p40 monomer and homodimer, rat IgG1, all donated generously by Dr Gately, Hoffmann-La Roche) were used to detect the expression of IL-12. Open lung and scalene node biopsy specimens from sarcoid cases (open lung biopsy: five cases; scalene lymph node biopsy: two cases of five lung biopsy cases, respectively) were fixed in 10% buffered formalin and were paraffin-embedded. Sections of 5 µm thickness were deparaffinized with xylene and were microwaved three times in 0·01 m sodium citrate buffer (pH 6·0) for 5 min, for antigen retrieval. The sections were incubated subsequently with the appropriate dilutions of MoAbs and washed with phosphate-buffered saline (pH 7·4). Positive cells were detected by sequential reaction with avidin–biotin complexes and DAB substrate (ABC kit; Dako, Glatrup, Denmark). No positive cells were identified when primary antibodies were replaced by isotype-matched control immunoglobulins.
Culture of cell line
The human NK0 cell line was a subclone of the NK92 cell line [11] and kindly donated by Dr Hoshino (Kurume University Medical School, Kurume, Japan) with IL-2. NK0 cells were maintained in supplement with RPMI-1640 medium containing 10% fetal calf serum (FCS) and 200 U/ml IL-2. For assays, 5 × 105 NK0 cells were suspended in 1 ml of RPMI-1640 containing 10% FCS in 24-well plates with different concentrations of IL-12 p70 or 10% v/v serum with 200 U/ml IL-2. After 24 h at 37°C, the culture of supernatants were collected for IFN-γ measurement.
Statistical analysis
Data were expressed as mean ± s.e.m. Student's t-test was used to determine the difference between groups. The one-way factorial anova or the Kruskal–Wallis method were used to test for differences in the levels of serum IL-12 or serum IFN-γ among each group, respectively. Significant differences were identified using a sequential rejection Bonferroni test procedure with Kruskal–Wallis methods. Spearman's rank correlation coefficient analysis was used to evaluate serum IL-12 versus serum IFN-γ levels. Peason's product-moment correlation coefficient analysis was used to evaluate serum IL-12 versus serum ACE or lysozyme levels. The P-value level of significance was P < 0·05.
Results
Serum IL-12 levels of patients with sarcoidosis and normal controls
We measured the serum IL-12 concentrations of patients with sarcoidosis and healthy controls. IL-12 (p40 and p70) was detected in all samples of patients with sarcoidosis and normal controls. Conversely, no detactable concentrations of IL-12 p70 were found in any serum samples. These results demonstrated that serum IL-12 (p40 and p70) detected in patients with sarcoidosis and normal controls were virtually attributable to IL-12 p40 alone. Therefore, we will concentrate on IL-12 p40 instead of IL-12 (p40 and p70).
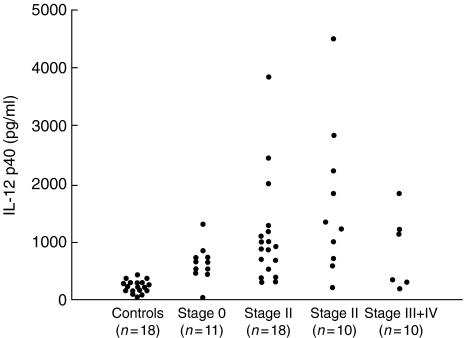
The levels of serum IL-12 p40 in patients with sarcoidosis (1080 ± 136 pg/ml) were significantly higher than those of normal controls (331 ± 34·4 pg/ml, P = 0·0011). When comparing different radiographic chest X-ray stages and normal controls, stages I and II showed significantly elevated serum IL-12 p40 levels [stage 0; 641 ± 95·3 pg/ml (P = 0·279), stage I; 1110 ± 208 pg/ml (P = 0·0024), stage II; 1660 ± 402 pg/ml (P < 0·0001) and stage III + IV; 846 ± 271 pg/ml (P = 0·145)] (Fig. 1).
Fig. 1.
Serum IL-12 p40 levels of patients with sarcoidosis grouped according to radiographic stage and normal controls.
Serum IFN-γ levels of patients with sarcoidosis and normal controls
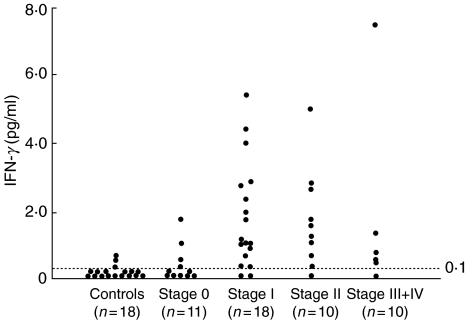
We also measured serum IFN-γ levels of the same patients and normal controls (Fig. 2). In 15 of 18 healthy controls, the levels of serum IFN-γ were very low (<0·10 pg/ml). The levels of the three remaining healthy controls were 050 ± 0·12 pg/ml. In stage 0 sarcoid cases, the levels of seven cases were below the lower detection limit and those of the remaining patients were 0·95 ± 0·32 pg/ml and the levels of stage 0 cases were not elevated significantly compared to controls (P = 0·323). In stage I sarcoid cases, the levels of two patients were below the lower detectable limit and the average of the remaining nine patients was 2·03 ± 0·38 pg/ml. The average serum IFN-γ levels of the nine stage II cases were 1·94 ± 0·47 pg/ml and five stage III + IV cases were 2·15 ± 1·34 pg/ml (except one patient who was below the lower limit). The levels of stage I and stage II cases were increased significantly compared with those of normal controls (P < 0·0001, respectively), and stage 0 cases (P = 0·0021 and P = 0·0047, respectively), and the levels of stage III + IV cases were elevated significantly compared to those of normal controls (P = 0·0011).
Fig. 2.
Serum IFN-γ levels of patients with sarcoidosis grouped according to radiographic stage and normal controls. The dashed line indicates the level of sensitivity in this assay.
Correlation between serum IL-12 p40 and serum IFN-γ of patients with sarcoidosis
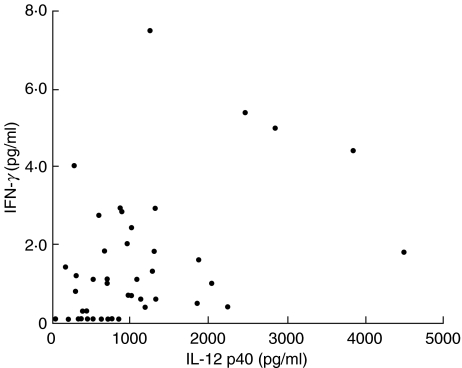
We examined the correlation between levels of serum IL-12 p40 and IFN-γ (Fig. 3). The serum IL-12 p40 levels of patients with sarcoidosis correlated significantly with those of serum IFN-γ levels (rs = 0·431, P = 0·0047).
Fig. 3.
Correlation between serum IL-12 p40 and serum IFN-γ in patients with sarcoidosis. r = 0·431; P = 0·0047; n = 45.
Evaluation of IL-12 p40 as markers of disease activity in sarcoidosis
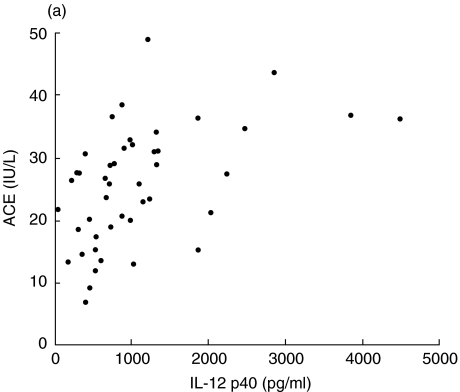
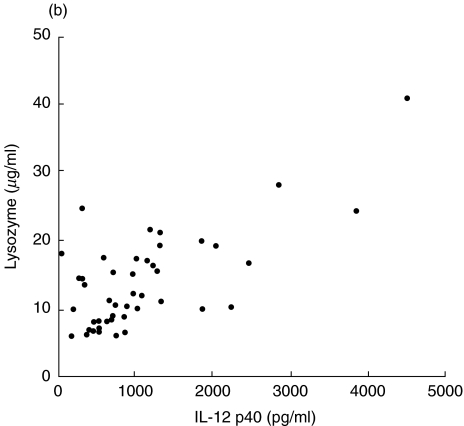
We investigated the clinical usefulness of serum IL-12 p40 as a marker of disease activity in sarcoidosis. Serum ACE and lysozyme activity are well-established sarcoidosis disease markers. The levels of IL-12 p40 correlated significantly with the activity of serum ACE (r = 0·484, P = 0·0008, Fig. 4a) and serum lysozyme (r = 0·693, P < 0·0001, Fig. 4b). Next, we compared the serum IL-12 p40 levels between 67Ga scan positive (positive uptake for lung fields) and negative groups (negative uptake for lung fields). The group having a positive 67Ga scan had significantly elevated serum IL-12 p40 levels (1600 ± 470 pg/ml, n = 10) compared with those of the negative group (935 ± 107 pg/ml, n = 35, P = 0·0408). Serum IL-12 p40 was demonstrated to have potential as a clinical marker for disease activity in sarcoidosis.
Fig. 4.
Evaluation of IL-12 p40 as a marker of disease activity (compared to serum ACE and lysozyme levels) in sarcoidosis. (a) Correlation between serum IL-12 p40 and ACE. (b) Correlation between serum IL-12 p40 and lysozyme. (a) r = 0·484; P = 0·0008; n = 45. (b) r = 0·693; P = 0·0001; n = 45.
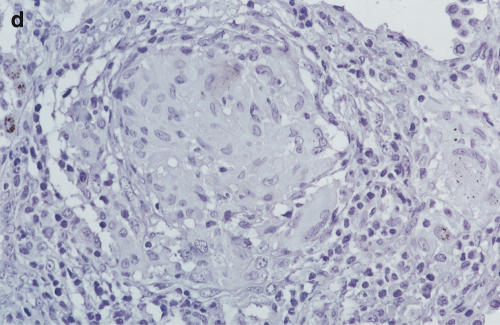
Immunohistochemistry of IL-12 p40 in the sarcoid lung and scalene node
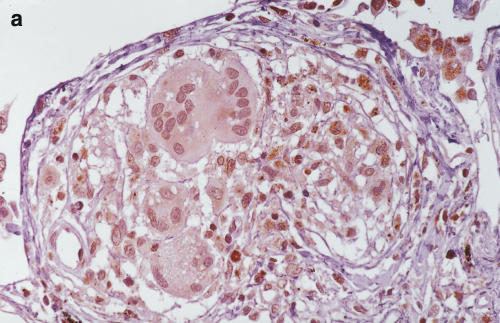
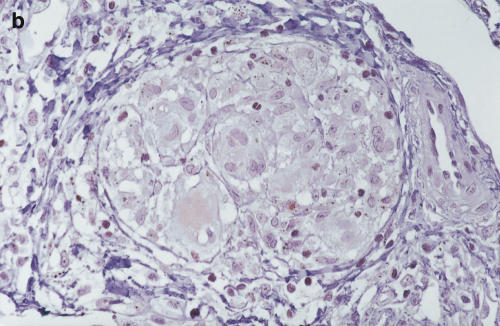
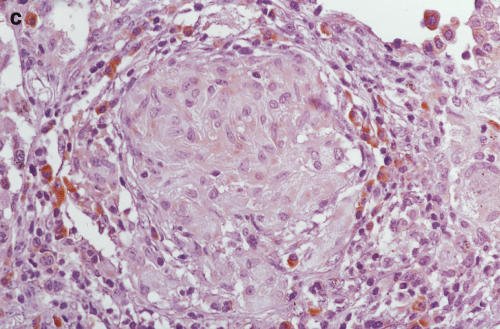
Positive staining of IL-12 p40 (4D6) was found in epithelioid cells, giant cells and macrophages of the sarcoid lung. Lymphocytes and fibroblasts showed no staining. The results of 20C2 (IL-12 p70) were almost the same as those of 4D6 (IL-12 p40), although epithelioid cells showed somewhat weaker staining (Fig. 5c, d). The findings of scalene lymph nodes were the same as those of sarcoid lungs. These results suggest that the increase in IL-12 p40 was, at least partially, derived from these positively stained cells in the saroid lung and lymph nodes.
Fig. 5.
Immunohistochemistry of IL-12 (p70 and p40) in the sarcoid lungs. (a) Anti-human IL-12 p40 MoAb 4D6 (a p40 monomer and homodimer, rat IgG1) and (c) IL-12 p70 MoAb 20C2 (a p40 in p70 heterodimer, rat IgG1) were applied to paraffin-embedded sections using the avidin–biotin complex and developed using the DAB substrate. (b,c) Control immunohistochemical reactions using rat IgG1. A–D: × 340 original magnification.
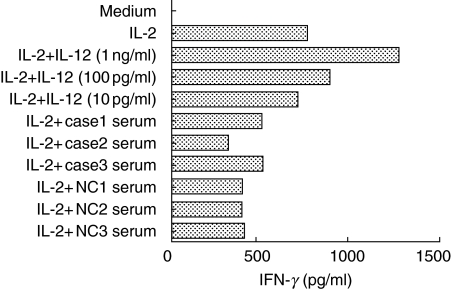
Bioactivity of circulating IL-12 p40 in patients with sarcoidosis using NK0 cells
To examine further the circulating IL-12 p40 of patients with sarcoidosis, we performed Western blot analysis of patient serum. No findings corresponding to IL-12 and its subunits were detected (data not shown). Next, we examined the bioactivity of circulating IL-12 p40 in patients with sarcoidosis. The NK0 cell, which is a subclone of the NK92 cell line, is dependent on IL-2 and IL-12. Co-administration with IL-2 and IL-12 in NK0 cells, IFN-γ production synergistically augments. We used the serum samples of three sarcoid cases. Circulating IL-12 p40 levels of three sarcoid cases as follows: case 1, 1330 pg/ml; case 2, 1850 pg/ml; case 3, 890 pg/ml. Recombinant human (rh) IL-12 p70 was administered at concentrations of 10 pg/ml, 100 pg/ml and 1 ng/ml as positive controls. The results of IFN-γ measurement showed as follows: medium only, < 4 pg/ml; IL-2 (200 U/ml), 755 pg/ml; IL-2 + IL-12 (10 pg/ml), 702 pg/ml; IL-2 + IL-12 (100 pg/ml), 880 pg/ml; IL-2 + IL-12 (1 ng/ml), 1270 pg/ml; IL-2 + case 1 serum, 503 pg/ml; IL-2 + case 2 serum, 314 pg/ml; IL-2 + case 3 serum, 509 pg/ml. The average of three normal controls was 395 ± 3·7 pg/ml (Fig. 6). These results demonstrated that the patient serums with sarcoidosis (and normal controls serum) had no bioactivity of IFN-γ induction.
Fig. 6.
IFN-γ production in NK0 cells stimulated with sarcoid cases (and normal controls) sera. Cases 1–3: three patients with sarcoidosis and NC 1–3: three normal controls.
Discussion
In this study, we have demonstrated that the serum levels of IL-12 p40 and IFN-γ in patients with sarcoidosis were increased when compared with those of normal controls, especially in patients with obvious intrathoracic abnormal chest roentogenogram findings. The 67Ga scan positive sarcoidosis group had significantly elevated serum IL-12 p40 levels compared of those of 67Ga scan negative group. The concentrations of serum IL-12 p40 in sarcoid cases correlated further with those of serum IFN-γ in addition to the activity of serum ACE and lysozyme. Serum levels of IL-12 p40 were demonstrated to be useful markers of disease activity in pulmonary sarcoidosis. The present study revealed immunohistologically that epithelioid cells expressed the IL-12 p40 and p70 heterodimer, although the detection of IL-12 p40 and p35 mRNA was not confirmed. It is suspected that monocytes from the bloodstream differentiate into the epithelioid cells in inflamed sarcoid lesions [12]. Epithelioid cell function in sarcoid lesions is not understood fully. Recent studies [13–15] have revealed that lipopolysaccharide or bacterial stimulation and the interaction between CD40 and the CD40 ligand on antigen-presenting cells (APC, macrophages and dendritic cells) and CD4+ T cells can induce IL-12 p40 production. The interaction between APC and CD4+ T cells via the major histocompatibility complex class II and T cell receptor can also induce IL-12 p35 [16]. We speculate that epithelioid cells of sarcoid granulomas might produce IL-12 and play an important role in the Th1 response in sarcoidosis.
Recent immunological advances have demonstrated that IL-12 was up-regulated in active sarcoid lesions and the cytokine profile in sarcoidosis showed a Th1 predominance in granuloma formation. IL-12 has been reported to be present in three different forms: p40 monomer, p40 homodimer and p70 heterodimer (p35p40). IL-12 p70 is the bioactive form binding and causing signal transduction through the IL-12 receptor (IL-12R), composed of β1 and β2 chains. IL-12 p40 homodimer acts as an antagonist of IL-12 p70 in vitro by blocking the interaction of IL-12 p70 and IL-12Rβ1. Binding studies of the mouse p40 dimer have demonstrated a poor affinity for IL-12Rβ2 [17]. In vivo, treatment with the p40 dimer protected mice from IL-12-dependent shock [18] and p40 transgenic mice showed a reduced Th1 response [19]. Oppmann et al. [8] have documented that a novel 4-α helix cytokine p19, related to IL-12 p35, bound to IL-12 p40 to form a p19p40 heterodimer (IL-23). IL-23 is produced by activated dendritic cells and binds to IL-12Rβ1. IL-23 enhances the proliferation and production of IFN-γ in activated human PHA blast T cells. p40 is important in the formation of IL-12 p70 (p35p40 heterodimer) and IL-23 (p19p40 heterodimer).
Generally, IL-12 p70 is undetectable in the circulation, although it has been measured in Th1 dominant disorders [e.g. rheumatoid arthritis (RA), juvenile chronic arthritis (JCA) and tuberculosis] [20–22]. The production of IL-12 p40 is considered to be far greater than that of IL-12 p70. It has been shown that IL-12 p40 secretion is 10–100-fold greater than IL-12 p70 secretion from cultured macrophages and the Epstein–Barr virus-infected B lymphoblastoid cell line [23]. Further, freezing and thawing of a serum sample may further degrade IL-12 p70 because of its inherent instability [4]. In this study, in spite of the agonistic action of the IL-12 p70 heterodimer, the levels of IL-12 p40 closely followed the pulmonary sarcoidosis disease activity and there was, at least, no bioactivity of IL-12 p70 in the patients’ serum using the IL-12-responsive cell line (NK0 cell). A similar result was reported in RA and JCA for tumour necrosis factor-α (TNF-α). TNF-α is considered to be a major cytokine in the pathogenesis of these diseases and is barely detectable in the serum samples of these patients. In contrast, the levels of TNF soluble receptors (p55 and p75) that neutralize the biological activity of TNF-α are increased in the sera of these diseases and correlated with the clinical markers of disease activity [21,24]. Another possibility concerning the detection of IL-12 p40 in sera may be considered to participate partially in IL-23 production. A recent study by Cooper et al. [25] showed that the IL-12 p35 knockout mice showed high p19 and p40 mRNA expression and protective immunity for mycobacterial infection. However, no methods for measuring p19p40 protein products quantitatively in mouse or human systems have been developed.
We conclude that IL-12 p40 was easily detectable in patient sera and was a useful clinical marker of sarcoidosis disease activity showing a Th1 predominance.
Acknowledgments
We thank Dr Gately for generously donating the antibodies (20C2 and 4D6) and Dr Hoshino for IL-12 responsive cell line (NK0). We would also like to thank Mr Kubo and his colleagues in the Sapporo Hospital of the Hokkaido Railway Company for their help in patients’ serum sampling.
References
- 1.American Thoracic Society. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Quernheim J. Sarcoidosis: immunopathogenetic concepts and their clinical application. Eur Respir J. 1998;12:716–38. doi: 10.1183/09031936.98.12030716. [DOI] [PubMed] [Google Scholar]
- 3.Trinecheri G. Interleukin-12: a cytokine produced by antigen presenting cells with immunoregulatory functions in the generation of T helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 4.Moller DR, Forman JD, Liu MC, et al. Enhanced expression of IL-12 associated with Th1 cytokine profile in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–61. [PubMed] [Google Scholar]
- 5.Shigehara K, Shijubo N, Ohmichi M, et al. IL-12 and IL-18 are increased and stimulate IFN-γ production in sarcoid lungs. J Immunol. 2001;166:642–9. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 6.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but dose not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 7.Heinzel FP, Hujer AM, Ahmed FN, et al. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–8. [PubMed] [Google Scholar]
- 8.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Kasahara Y, Ashihara Y. Colorimetry of ACE activity in serum. Clin Chem. 1981;27:1922–5. [PubMed] [Google Scholar]
- 10.Ensink FTE, Haeringen NJ. Pitfalls in the assay of lysozyme in human tear fluid. Ophthalmic Res. 1977;9:366–73. [Google Scholar]
- 11.Hoshino T, Winkler-Pickett RT, Mason AT, et al. IL-13 production by NK cells: IL-13-producing NK and T cells are present in vivo in the absence of IFN-γ. J Immunol. 1999;162:51–9. [PubMed] [Google Scholar]
- 12.Semenzato G, Agostini C. Immunologic events in the development of interstitial lung disease: the paradigm of sarcoidosis. In: Schwarz MI, King TE Jr, editors. Interstitial Lung Disease. 3. Ontario: BC Dekker Publ.; 1998. pp. 229–50. [Google Scholar]
- 13.Cleveland MG, Gorham JD, Murphy TL, et al. Lipoteichoic acid preparation of Gram-positive bacteria induce interleukin-12 through a CD14-dependent pathways. Infect Immun. 1996;64:1906–12. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cella M, Sheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T, Hakamada R, Yamane H, et al. Induction of IL-12 p40 mRNA expression and IL-12 production of macrophages via CD40–CD40 ligand interaction. J Immunol. 1996;156:3932–8. [PubMed] [Google Scholar]
- 16.Yamane H, Kato T, Nariuuchi H. Effective stimulation for IL-12 p35 mRNA accumulation and bioactive IL-12 production of antigen-presenting cells interacted with Th cells. J Immunol. 1999;162:6433–41. [PubMed] [Google Scholar]
- 17.Wang X, Wilkinson VL, Podlaski FJ, et al. Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits. Eur J Immunol. 1999;29:2007–13. doi: 10.1002/(SICI)1521-4141(199906)29:06<2007::AID-IMMU2007>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Mattner F, Ozmen L, Podlaski FJ, et al. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–7. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto T, Wang C-R, Yoneto T, et al. Reduced T helper 1 responses in IL-12 p40 transgenic mice. J Immunol. 1998;160:588–94. [PubMed] [Google Scholar]
- 20.Bucht A, Larsson P, Weisbort L, et al. Expression of interferon gamma (IFN-γ), IL-10, IL-12 and transforming growth factor-beta (TGF-β) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis (RA) Clin Exp Immunol. 1996;103:357–67. doi: 10.1111/j.1365-2249.1996.tb08288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattorno M, Picco P, Vinnola S, et al. Serum interleukin 12 concentration in juvenile chronic arthritis. Ann Rheum Dis. 1998;57:425–8. doi: 10.1136/ard.57.7.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Gately MK, Wang E, et al. Interleukin 12 at the site of disease in tuberculosis. J Clin Invest. 1994;93:1733–9. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Andrea A, Regaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattorno M, Picco P, Buoncompagni A, et al. Serum p55 and p75 tumor necrosis factor receptors as markers of disease activity in juvenile chronic arthritis. Ann Rheum Dis. 1996;55:243–7. doi: 10.1136/ard.55.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper AM, Kipnis A, Turner J, et al. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]