Abstract
Cytotoxic T lymphocytes (CTL) are key players to suppress viral load (VL) but CTL responses become compromised with progression of HIV-infection/AIDS. Some progressors develop MHC-unrestricted CTL with anti-CD4+ cytocidal activity. Immune activation status of these CTL and its significance in disease progression are unknown. To determine the relationship between VL and T cell activation, a cross-sectional study was carried out using blood samples from 13 HIV-1-infected/AIDS patients at various stages of progression and seven age-matched seronegative controls. We examined expression of HLA-DR and CD38 activation markers on purified CTL. MHC-unrestricted killing by these CTL was also evaluated against uninfected, allogeneic CD4+ T cells as well as several human cell lines. The expression of activation markers correlated inversely (rs = − 0·91, P < 0·0001) with VL of the subjects. CTL effectors of these patients killed targets expressing or lacking CD4+, independently of MHC class I recognition. Interestingly, the patients with higher VL showed an increased number of γδTCR-bearing CTL in blood and their MHC-unrestricted killing activity was blocked significantly (P < 0·01) by γδTCR-specific monoclonal antibody. CD3+ T counts of these patients were also consistently subnormal. Inverse correlation between VL and CD8+ T cell activation markers seems to be an indicator of CTL-associated immunopathogenesis in HIV patients with elevated γδCTL in the peripheral blood.
Keywords: AIDS-pathogenesis, CTL, immune activation markers, MHC-unrestricted HIV load
Introduction
HIV infections present a unique opportunity to study the induction and responsiveness of innate and adaptive immune responses to chronic, persistent viral infections. HIV primary infection is marked by a short and acute febrile phase, followed by a prolonged period of asymptomatic infection. Following seroconversion, strong HIV-specific cytotoxic T lymphocyte (CTL) responses are induced [1] as part of the host cellular defence arsenal. During asymptomatic HIV-infection, virus-specific CTL are the key players in suppressing the virus load [2]. Nevertheless, persistent CD4+ T cell depletion continues along with disease progression, and the advanced-stage individuals also develop CD8+ T lymphopenia. Of note, HIV-mediated cytopathic effects do not explain, alone, the development of CD4+ T lymphocytopenia in low viraemic patients with asymptomatic infection [3]. It has been reported [4,5] that AIDS patients bear CTL subsets capable of lysing CD4+ T cells in an MHC-unrestricted manner; however, whether changes in virus load (VL) relate with this phenomenon has not been examined. Interestingly, elevations in peripheral blood γδT cell numbers in AIDS patients have also been reported [6], but no studies have defined the immunological significance of this finding. Moreover, it is known that T cell homeostasis responds to progressive CD4+ T cell losses by regenerating T cells without the selective distinction of CD4+ or CD8+ subpopulations in order to maintain the total (i.e. CD3+) T cell pool [7], but the status of T cell homeostasis, especially in HIV patients developing MHC-unrestricted CTL activity, is not known. Here we report a negative correlation of HLA-DR and CD38 CTL activation markers with the peripheral HIV-1 RNA burden in these subjects. We also describe the perturbations in peripheral blood CTL immunophenotypes and in T cell homeostasis as correlates of the development of aberrant CTL activity and, eventually, of disease progression.
Materials And Methods
Subjects
Thirteen HIV-1 infected/AIDS patients visiting the AIDS clinic at the University of Montreal's allied medical centre were registered for this cross-sectional study. These individuals were shown to be HIV seropositive by enzyme-linked immunosorbent assay (ELISA) and were confirmed by Western blot. The subjects belonged to various categories regarding the Center for Disease Control (CDC)'s 1993 revised AIDS classification criteria [8]. The virus load in these individuals varied from log10 2·7 to log10 5·34 of HIV-1 RNA copies/ml. These subjects were divided arbitrarily into two groups based on virus load at the time of blood sampling, i.e. low VL group A (log10 2·70–log10 3·58 HIV-1 RNA copies/ml) and high VL group B (log10 3·97–log10 5·34 HIV-1 RNA copies/ml). The associated infections and malignancies in AIDS patients included: Pneumocystis carinii pneumonia, candidiasis, polyadenopathy and Kaposi's sarcoma. All these patients were on various regimen of highly active antiretroviral therapy (HAART). The controls comprised seven HIV-1 seronegative, age-matched healthy individuals. All blood samples were collected following informed written consent of the participants as well as approval of the institutional Ethics Committee.
Monoclonal antibodies
Flow cytometry on purified CTL was carried out using peridinin chlorophyll-A protein (PerCP)-conjugated anti-CD8 (Leu-2a), phycoerythrin (PE)-conjugated anti-HLA-DR (clone G46-6/L243; mouse IgG2a, κ) and fluorescein isothiocyanate (FITC)-conjugated anti-CD38 (Leu-17) (clone H1T2; mouse IgG1, κ) monoclonal antibodies. The CD4+ T subset was stained using PE-conjugated anti-CD4 (Leu-3a) in combination with FITC-conjugated anti-CD3 (Leu-4). FITC-conjugated anti-CD16 and PE-conjugated anti-CD56 monoclonal antibodies were used to measure CD16+ and CD56+ cells in the purified CTL populations, respectively. The αβTCR- (clone: T10B9·1 A-31, isotype: mouse IgM κ) and γδTCR-specific (clone: B1 isotype: mouse IgG1 κ) monoclonal antibodies were used in the TCR-blocking experiments following the manufacturer's instructions. Briefly, the cells were washed twice with sterile PBS and once with RPMI medium containing 2% fetal bovine serum (FBS) before incubation with control and monoclonal antibodies (1 µg/0·2 × 106 cells) on wet ice for 45 min. The cells were washed twice and used as effectors in cytolytic assays. All reagents were purchased from BD-Pharmingen, CA, USA.
Blood samples and flow cytometry
Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood on Ficoll-Hypaque (Pharmacia Chemicals, Montreal, Canada) density gradient (400 g for 25 min, Beckman GPR Centrifuge, USA). Interface cells were collected, washed thrice with RPMI-1640 medium supplemented with 2% FBS and counted. Cells were resuspended (1 × 106 cells per ml) in RPMI-1640 medium containing 2 mm l-glutamine, 10 mm HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% FBS. Cell viability was determined by trypan blue dye exclusion test. Lymphocyte populations were determined by flow cytometry of peripheral whole-blood samples using a standard method and a FACScan flow cytometer with lysys software (Becton Dickinson, San Jose, CA, USA). Briefly, a 150 µl sample was mixed with 20 µl of the specified monoclonals for 20 min at 4°C, followed by FACS lysing solution (2 ml) treatment for 10 min at 26°C. After washing (with PBS containing 1% sodium azide) and fixation (using PBS with 1% paraformaldehyde), samples were analysed by flow cytometry within 24 h of fixation, counting at least 1·5 × 104 lymphocytes per sample.
Lymphocyte activation for cytotoxic assays
To prepare CTL effectors, PBMC from HIV-positive patients and seronegative controls were first incubated with ConA (20 µg/ml, ICN Biomedicals, Montreal, Canada) at 37°C, 5% CO2 and 80% relative humidity for 3 days, and then with IL-2 (100 µg/ml, Proleukin, Cetus Inc., CA, USA) for 4 more days. To prepare CD4+ T cell targets, PBMC procured from HIV-seronegative healthy donors were incubated with phytohemagglutinin (10 µg/ml, ICN Biomedicals, Montreal, Canada) for 3 days, followed by 1-day incubation with IL-2.
Cell purification by immunomagnetic column
CTL effectors and CD4+ T cell targets from fresh as well as mitogen-stimulated PBMC from AIDS and control individuals were purified by negative selection (StemCell Technologies, Vancouver, Canada) following the manufacturer's instructions. Briefly, PBMC were washed thrice with RPMI-1640 medium containing 2% FBS. The pellet was resuspended in 1 ml of the same medium and labelled using the respective antibody cocktails for purification of CTL (‘CD8+ T cell-enrichment cocktail’ containing monoclonal antibodies to CD4, CD16, CD19, CD36 and CD56 cell surface antigens) and of CD4+ T lymphocytes (‘CD4+ T cell-enrichment cocktail’ containing monoclonal antibodies to CD8, CD16, CD19, CD36 and CD56 antigens), each at a concentration of 1 µg/2·5 × 106 cells. Following incubation on wet ice for 30 min, magnetic colloid (microbeads precoated with antimouse IgG1, κ) was added (0·75 µg/2·5 × 106 cells) and reincubated on ice for 30 min. The cells were remixed every 10 min during incubation. Finally, the cells were passed through a magnetic column to obtain the purified cell types. The cell purity for selection markers was determined by FACScan flow cytometry and the preparations contained 89 ± 5·8% CD8+ and 91 ± 4·5% CD4+ T cells. Moreover, CD8+ CD16+ and CD8+ CD56+ T cells in the purified CTL population, as calculated from five independent determinations, were 3·7 ± 0·2% and 2 ± 0·4%, respectively.
Cytotoxicity assay
The assay was carried out essentially as described [9]. Briefly, the effectors were prepared by washing column-purified CTL thrice with RPMI-1640 containing 2% FBS and suspending in RPMI with 10% FBS at 4 × 106 cells/ml concentration. The targets were washed thrice in RPMI with 2% FBS, pelleted and chromium (51Cr) labelled (100 µCi/106 cells for 90 min; New England Nuclear, Boston, MA, USA). The labelled cells were washed four times and re-suspended in RPMI with 10% FBS at 2 × 105 cells/ml concentration. Target cells were added (50 µl/well) in triplicate wells of 96-well, V-bottom microtitre plates (Nunclon, Denmark) for each effector to target (E : T) ratio and for spontaneous and maximum 51Cr-release controls. Accordingly, effectors were added in triplicate wells for each E : T ratios. No effectors were added to the control wells. The volume was made up to 200 µl per well by adding culture medium to the minimum control, and 0·1 N HCl lysis solution to the maximum control wells. The plates were incubated at 37°C (5% CO2, and humidity) for 8 h. Supernatants (100 µl per well) were drawn and used for automated counting (LKB-Wallace CliniGamma 1272 Counter, Wallace, Finland) to yield percent specific lysis derived as:
 |
The various targets used in cytotoxicity assays were: (i) primary CD4+ T lymphocytes, (ii) T lymphoblastoid Jurkat, (iii) EBV-transformed B-lymphoblastoid (B-LCL) and (iv) promonocytic U937 cell lines.
Plasma viral load
The plasma viral load (log10 HIV-1 RNA copies/ml) was quantified by the Amplicor HIV monitor test (Roche Diagnostic System Inc., NJ, USA). Briefly, a 142 base pair region in the gag gene of HIV-1 was amplified by reverse transcription and polymerase chain reaction in a single reaction with rTth DNA polymerase. A synthetic RNA molecule with a known number of copies was used as standard and ELISA was used to detect biotinylated HIV-1 and standard amplicons. RNA copy number was determined by the known input copy number of standard RNA and from the optical densities of standard and sample wells.
Statistical analysis
Differences between groups were analysed for significance using the non-parametric Wilcoxon two-sample test. Shapiro–Wilk's test was used to check normality of distributions. The correlation between VL and the parameters as indicated was determined using Spearman's rank correlation test. P-values of 0·05 or less were considered significant. The prism program (GraphPad Software, Inc., San Diego, CA, USA) was used for the graphic presentations.
Results
Activation markers and T cell subsets in subjects with low and high virus load
We have shown recently that CTL from HIV-1-infected patients lyse CD4+ T cells and their cytocidal potential correlates with the patients’ viral load [10]. We therefore sought to extend this work by examining the relation between virus load and the expression of activation markers on CTL effectors from such patients in parallel to their cytocidal function, the related phenotypic features and CD4+ and CD8+ T cell counts.
As shown in Fig. 1, plasma viral load (log10 of HIV-1 RNA copies per ml) correlated inversely with the expression of CTL activation marker(s) in these subjects (P < 0·01). Representative staining profiles of HLA-DR and CD38 immune activation markers are shown in Fig. 2. Regarding the various T cell subsets, CD4+ T cell counts in HIV-infected individuals (both the low and high VL) were diminished significantly compared to those of the controls. Even though CD4+ T counts varied less (P < 0·076) between the two HIV groups (Fig. 3a), CD8+ T cell number in low VL patients (Fig. 3b) was significantly (P < 0·01) elevated.
Fig. 1.
Correlation between expression of CTL activation markers and virus load in HIV-infected/AIDS patients. Peripheral blood samples (fresh) from 13 HIV-1-infected individuals were analysed by flow cytometry for the expression of HLA-DR and CD38 antigens on CD8+ T cell population. The Spearman's rank correlation (rs) between plasma viral load (log10 HIV-1 RNA copies/ml) and expression of HLA-DR, CD38 and HLA-DRCD38 is shown in (a, b and c), respectively. The inverse correlations: (a) rs = 0·73 (b) rs = 0·88 and (c) rs = 0·91 were statistically significant (P < 0·01).
Fig. 2.
Staining profiles of immune activation markers. Expression of HLA-DR and CD38 activation antigens on CD8+ T cell population was determined by flow cytometry using specific fluorescent dye-conjugated monoclonal antibodies. Samples were gated on CD8-bright T lymphocyte cluster; quadrant markers were adjusted using a negative isotype control, and at least 1·5 × 103 lymphocytes were counted. (a, b) Stainings for negative isotype control and sample from a low virus load HIV patient, respectively; whereas (c) and (d) represent stainings for isotype control and sample from a high virus load HIV patient, respectively.
Fig. 3.
T lymphocyte populations in HIV-infected/AIDS patients and seronegative individuals. Peripheral blood CD4+/CD8+ T lymphocyte populations in 13 HIV-infected/AIDS patients and seven HIV-seronegative healthy donors were measured by flow cytometry and using specific monoclonal antibodies. (a, b) CD4+ and CD8+ T subsets, respectively. The data (median and range) shown are from three replicates from the subjects: six low-VL patients, seven high-VL patients and seven HIV-seronegative controls (as defined previously in legend to Fig. 2). Absolute values were determined from the leucocytic counts. Asterisks (*) and (**) represent P-values < 0·05 and < 0·01, respectively.  , VL = log10 (2·70–3·58) copies; ▪, VL = log10 (3·97–5·34) copies; □, age-matched, HIV-seronegatives.
, VL = log10 (2·70–3·58) copies; ▪, VL = log10 (3·97–5·34) copies; □, age-matched, HIV-seronegatives.
Peripheral blood CTL phenotypes in HIV-1 patients and seronegative individuals
We also sought to determine TCR-immunophenotypes in HIV-1-positive and seronegative individuals. The data show that the αβ- and γδ-CTL in the peripheral blood of low VL patients are comparable (Fig. 4), whereas in the case of high VL patients, both CTL phenotypes showed significant changes in number (Pαβ< 0·05, Pγδ< 0·01).
Fig. 4.
CTL phenotypes in HIV-infected/AIDS patients and seronegative controls. Immunophenotyping of purified CTL from the peripheral blood samples was performed by flow cytometry following standard protocol and using relevant monoclonal antibodies. The data shown (median and range values) are from triplicate determinations. (a) Six low-VL patients (log10 2·70–log10 3·58 copies of HIV-1 RNA/ml); (b) seven high-VL patients (log10 3·97–log10 5·34 copies of HIV-1 RNA/ml); and (c) seven HIV seronegatives. The asterisks (*) and (**) show the significant (P < 0·05 and P < 0·01, respectively) differences from the controls.  , αβ CTL; ▪, γδ CTL.
, αβ CTL; ▪, γδ CTL.
T cell homeostasis is perturbed in high virus load HIV-patients
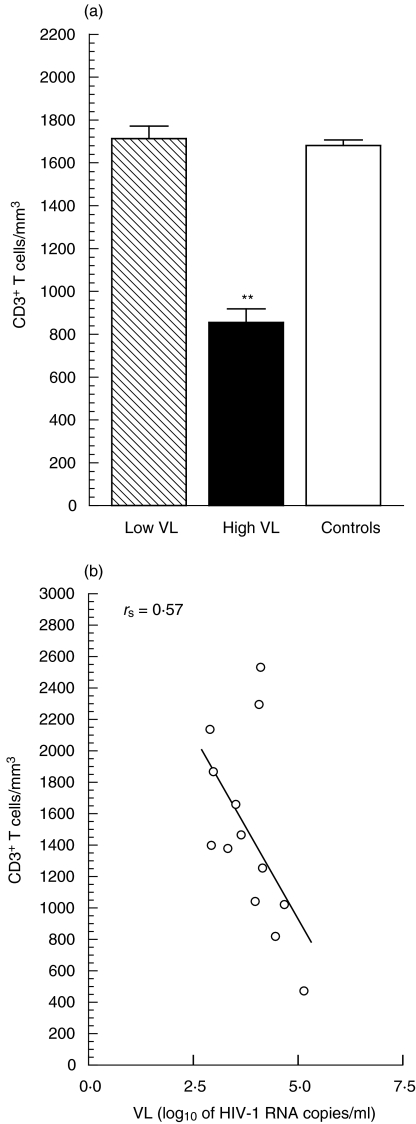
Individuals with a high VL had significantly lower CD3+ T cell numbers (P < 0·01) compared with the patients having a low VL, in whom the total (CD3+) T cells were similar to those of HIV-seronegative controls (Fig. 5a). The correlation between CD3+ T counts and the VL is shown in Fig. 5b. Nevertheless, a similar inverse correlation also existed between VL and CD4+/CD8+ T numbers in these individuals (data not shown).
Fig. 5.
Correlation between CD3+ T cell counts and virus load. Peripheral blood CD3+ T lymphocyte counts were determined by flow cytometry. (a) Data (median and range) from triplicate values regarding six low-VL, seven high-VL and seven seronegative control individuals (as explained above). Highly significant difference (P < 0·01) from the control value is shown by (**). (b) Inverse correlation (rs = 0·57,P < 0·025) between CD3+ T counts and virus load. (a)  , VL = log10 (2·70–3·58) copies; ▪, VL = log10 (3·97–5·34) copies; □, age-matched, HIV-seronegatives.
, VL = log10 (2·70–3·58) copies; ▪, VL = log10 (3·97–5·34) copies; □, age-matched, HIV-seronegatives.
CTL-mediated lysis of CD4+ T lymphocytes as well as various human cell lines, and its blocking by anti-γδ TCR-specific monoclonal antibody
Expanded CTL were employed as effectors against purified CD4+ T targets from age-matched seronegative controls at two E : T ratios (20 : 1 and 40 : 1). The results show that CTL from HIV-1-infected/AIDS patients (summarized in Table 1) lysed CD4+ T cells, whereas CTL from the controls did not. To determine whether these CTL had a broader range of cytocidal activity than that reported earlier, target cells representing different phenotypes and origins were included, i.e. Jurkat, B-LCL and U937. As shown in Fig. 6 all cell line targets were lysed, as did CD4+ T cells but, overall, U937 showed the maximum lysis. The differences between median values for all targets were statistically significant (P < 0·05). The lysis exhibited by these targets was E : T ratio-dependent (the data shown in Fig. 6 represent lysis at E : T ratio of 20 : 1). All targets used were found susceptible to lysis, whether bearing or lacking CD4+ surface molecule. Target sensitivity to CTL effectors in the descending order was: U937, B-LCL, Jurkat and CD4+ T cells. Moreover, using CTL effectors pretreated with antihuman γδ pan-TCR monoclonal antibody in the cytotoxic assays suppressed target cytolysis significantly (Table 2; percentage of lysis shown for CD4+ T lymphocyte targets, P < 0·01). However, pretreatment of effectors with antihuman αβTCR monoclonal antibody did not result in significant blocking of their cytolytic activity.
Table 1.
Characteristics of the study subjects
| Patient ID | Age | Sex | CD4+ counts | Virus load | CD4/CD8 ratio | CDC stage | Antiretroviral therapy | Duration of ART |
|---|---|---|---|---|---|---|---|---|
| Patient 01 | 41 | M | 254 | 4·18 | 0·40 | C3* | Protocol 2 | 6 years |
| Patient 02 | 47 | M | 297 | 3·43 | 0·22 | C2 | Protocol 1 | 4 years |
| Patient 03 | 38 | M | 176 | 3·97 | 0·13 | C3 | Protocol 2 | 6·5 years |
| Patient 04 | 51 | M | 364 | 2·74 | 0·36 | C2† | Protocol 2 | 10 years |
| Patient 05 | 43 | M | 190 | 2·70 | 0·09 | C3† | Protocol 1 | 5 years |
| Patient 06 | 52 | M | 432 | 4·07 | 1·09 | C2† | Protocol 2 | 7 years |
| Patient 07 | 30 | F | 489 | 3·20 | 0·47 | C2 | Protocol 1 | 5·25 years |
| Patient 08 | 48 | M | 299 | 4·54 | 1·13 | C2* | Protocol 2 | 7·5 years |
| Patient 09 | 32 | F | 38 | 5·34 | 0·04 | C3‡ | Protocol 2 | 8 years |
| Patient 10 | 55 | M | 746 | 2·80 | 0·61 | C1 | Protocol 1 | 4 years |
| Patient 11 | 37 | M | 171 | 4·79 | 0·22 | C3§ | Protocol 3 | 3 years |
| Patient 12 | 42 | M | 72 | 3·58 | 0·04 | C3*† | Protocol 2 | 5 years |
| Patient 13 | 65 | M | 578 | 4·11 | 0·75 | C1 | Protocol 2 | 7 years |
Different opportunistic infections/malignancies in our AIDS patients have been shown by various symbols as superscripts.
These are as follows:
Pneumocystis carinii pneumonia
candidiasis
polyadenopathy
Kaposi's sarcoma
The antiretroviral therapy (ART protocols) comprised the following drug combinations: protocol no. 1: ddI, ddC and AZT; protocol no. 2: ddI, 3TC and ritonavir; protocol no. 3: d4T, indinavir and 3TC.
Fig. 6.
MHC-unrestricted CTL-mediated lysis of various cell targets, shown at effector : target ratio of 20 : 1. Immunomagnetically purified, in vitro activated CTL (i.e. with ConA + IL-2) from 13 HIV-1-infected/AIDS patients were used as effectors against the various 51Cr-labelled targets as shown. The specific lysis was detected from gamma counts of the supernatants collected (100 µl) in 8-h microculture plate assay. Each bar represents median and range values of percentage of cytotoxicity as calculated from triplicate values. The respective controls (a, b, c, d) represent lysis of the same target by similarly prepared effectors from seven HIV seronegative, age-matched healthy donors. Differences from the respective control values were highly significant (P < 0·01). All values were normalized with respect to the background lysis. The experiments were also performed at E : T ratio of 40 : 1 that gave similar but relatively higher values (not shown). Cell preparation, purification and treatment procedures were as described in Materials and methods. , Six low-VL patients; ▪, seven high-VL peatients; □, seven HIV-seronegatives.
, Six low-VL patients; ▪, seven high-VL peatients; □, seven HIV-seronegatives.
Table 2.
Percentage of cytotoxicity* shown at two effector : target ratios, following treatment of effectors with TCR-specific monoclonal antibodies
| Effector blocking → Efector: target ratio → | CTL untreated | Treated with αβMoAb | Treated with γδMoAb | Treated with mock antibody | ||||
|---|---|---|---|---|---|---|---|---|
| 20 : 1 | 40 : 1 | 20 : 1 | 40 : 1 | 20 : 1 | 40 : 1 | 20 : 1 | 40 : 1 | |
| Patient 01 (high-VL individual) | 61·7 ± 5·9 | 70·0 ± 4·6 | 59·8 ± 5·4 | 64·2 ± 3·2 | 13·4 ± 2·1 | 14·2 ± 4·9 | 61·4 ± 5·6 | 70·5 ± 4·9 |
| Patient 09 (high-VL individual) | 69·1 ± 4·7 | 90·2 ± 3·8 | 50·9 ± 8·0 | 86·2 ± 7·6 | 5·1 ± 3·2 | 10·3 ± 4·8 | 57·2 ± 5·2 | 87·8 ± 5·3 |
| Patient 11 (high-VL individual) | 50·9 ± 6·2 | 65·7 ± 7·4 | 47·1 ± 5·7 | 60·4 ± 4·3 | 6·9 ± 3·1 | 10·2 ± 2·8 | 52·9 ± 4·4 | 67·0 ± 7·2 |
| Patient 07 (low-VL individual) | 43·0 ± 2·6 | 58·5 ± 5·1 | 45·6 ± 4·3 | 50·1 ± 6·5 | 7·6 ± 1·9 | 12·3 ± 4·2 | 48·8 ± 5·0 | 66·1 ± 6·4 |
| Patient 10 (low-VL individual) | 29·2 ± 3·5 | 42·1 ± 2·0 | 25·7 ± 3·8 | 40·9 ± 6·1 | 2·7 ± 2·0 | 0·8 ± 0·6 | 32·1 ± 3·9 | 44·9 ± 4·7 |
Represented as mean ± s.e.m. from triplicate values. Column mean differences (Mann–Whitney test), compared with mock antibody-treated control, were significant (P = 0·01) for γδ MoAb treatments and were non-significant (P > 0·05) for αβ MoAb treatments at both effector : target ratios. The targets used were allogeneic primary CD4+ T lymphocytes. The patients’ numbers listed here correspond to the respective numbers given in Table 1, and were chosen as representative of high and low virus load individuals for these analyses.
Discussion
Despite the fact that robust CTL activity is induced during host immune response to HIV infection, infected individuals eventually develop AIDS. CTL responses are known to become compromised with the progression of HIV infection [11]. We have shown recently in another study [10] that CTL effectors from HIV-1-infected/AIDS patients lyse primary CD4+ T lymphocytes, as well as CEM.NKR and K562 targets. These findings point to the possibility that this type of CTL killer could play a pathogenic role by lysing various types of immunoregulatory cells in the body and thus contribute to immunosuppression and hasten progression of HIV infection. The present data extend this work by showing for the first time that CTL from individuals with a high VL and with broad-scale killing activity display a reduced expression of HLA-DR/CD38 activation markers compared with the counterpart low-VL group. The expression of HLA-DR on cytotoxic T lymphocytes is considered to have important functional implications for the adaptive immune response of the host to infection. Following seroconversion, dramatic elevations in HLA-DR expression have been reported in HIV infection [12,13]. Human CD38 antigen is a type II transmembrane glycoprotein, classified as a bifunctional ectoenzyme called ADP-ribosyl-cyclase or cyclic ADP-ribose hydrolase [14]. With regard to its functional importance, it has been related to properties of homing and adhesion [15,16]. We found that, unlike high-VL subjects, low-VL individuals had a greater number of cells expressing activation markers and the total (CD3+) T cell pool in these patients was comparable to that of the controls. This could be a result of gradual loss of activated CD8+ T subsets in HIV patients over time. Kestens et al. [12] reported a significant correlation between DR+ CD38+ expression on CD8+ T cells and CD4+ T counts in HIV infection, and Liu et al. [17] found that percentages of activated CD8+ T cells expressing CD38 antigen were predictors of the declining rates of CD+ T cells in AIDS patients. Our findings, i.e. that VL correlates inversely with the expression of CTL activation markers in HIV patients with MHC-unrestricted CTL-mediated killing, are supported by other studies [12,16,18] which reported that changes in the expression of activation markers relate to the clinical stages of HIV infection. On the other hand, studies on paediatric HIV-1 infections report a higher expression of CD38+ on CD8+ T cells in subjects with severe disease [19] or with low percentages of CD4+ counts and high VL [20]. Tilling et al. [21] also reported that CD38+ CD8+ lymphocytes declined rapidly in parallel with VL after the initiation of HAART, whereas Ruiz et al. [22] have described increased levels of expression of CD38 antigen on CD8+ T cells in response to viral rebound in chronic HIV-1 patients on a structured treatment interruption (STI) regimen. Rapid progressors with higher viraemia have also been reported [23] to have a higher mean percentage of DR+ CD38+ CD8+ T cells than the nonrapid progressor group. However, Ho et al. [24] found no relation between CTL activity and the level of DR+ CD38+ T cells in their study subjects. Similarly, non-significant elevations in median levels of immune activation markers (HLA-DR and CD38 on CD8+ T cells) were reported [25] in a cohort of female sex workers in the STD group. Overall, it may be inferred that the levels of expression of these immune activation markers are likely to differ in one group of HIV patients from another in regard to particular clinical settings, and that various co-factors, e.g. VL and quasispecies, CD4+ T decline rate, antiretroviral therapy, concurrent infections and malignancies, mode of disease progression and ongoing immune pathologies such as untoward CTL-mediated killing, etc. may contribute to these changes.
It is also noteworthy that our subjects with higher VL had a deficient CD3+ T cell pool representing diminished T cell homeostasis. This could, however, be explained as an aftermath of the continued T cell depletion attributable to both the cytolytic effects of HIV and MHC-unrestricted CTL-mediated killing of infected and/or bystander cells. It has been reported that, despite the persistent loss of CD4+ T lymphocytes from blood, CD3+ T counts are maintained steadily over the course of asymptomatic HIV infection [26]; however, as reported by others [27,28], T cell homeostasis eventually fails to maintain the total T cell counts in individuals with advanced AIDS.
More importantly, we also confirm here that effector blocking by the antihuman γδTCR monoclonal antibody before the addition of targets diminishes cytolysis remarkably. Further, the patients with high VL had increased γδ T cell numbers in peripheral blood. Taken together, it may be inferred that γδCTL from these HIV patients are important players in effecting MHC-unrestricted killing of the various targets. Our findings are supported by the studies [6,29,30] describing that different γδ subsets are expanded in the peripheral blood of HIV-infected people. Our data reveal that there are comparable numbers of blood γδ T cells both in low VL patients and HIV-seronegatives but, interestingly, they do not match with respect to levels of MHC-unrestricted cytolysis by the CTL. This may result partly from the effect of other factor(s) related to the HIV-1 infection that could be involved, because the use of anti-γδ pan-TCR monoclonal antibody did not completely abrogate target cytolysis. Of note here, not only did the high VL patients (i.e. the AIDS group) show an increased level of CTL-mediated killing of all targets compared with the low VL counterpart group (see the data presented); the higher VL individuals also had CTL expressing a higher cytocidal potential (data not shown). As our study patients were all on HAART, we also cannot rule out possible drug-related effects on the immune effector cells; inclusion of therapy as a covariate in the data had no significant effect on the ‘virus : effector function’ correlation detected. Also, we do not yet know what the killing mechanism of this phenomenon is, as observed in HIV patients. Therefore, further studies are needed to establish: (i) what factors dictate the selective expansion of blood γδCTL during HIV infection, and (ii) what cognate molecule(s) is (are) implicated in target cytolysis.
In conclusion, we have demonstrated an inverse correlation between VL and the CTL activation markers in HIV-infected/AIDS patients. Our data suggest further that increased number of γδCTL in the peripheral blood of HIV patients with a high virus load may be implicated with the depletion of various cell types in these individuals, and hence represents an important aspect of HIV-associated immunopathogenesis.
Acknowledgments
We thank the Canadian Institutes for Health Research for financial support and Isabelle Champagne for secretarial assistance. This work was presented in part at Workshop no. 27: AIDS, the 20th Annual Meeting of the American Society for Virology (Abstract no. W27-7, pp: 111), University of Wisconsin-Madison, Madison, Wisconsin, 21–25 July, 2001.
References
- 1.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–7. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 2.Barrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolthers KC, Schuitemaker H, Miedema F. Rapid CD4+ T cell turnover in HIV-infection: a paradigm revisited. Immunol Today. 1998;19:44–8. doi: 10.1016/s0167-5699(97)01188-2. [DOI] [PubMed] [Google Scholar]
- 4.Grant MD, Smaill FM, Rosenthal KL. Lysis of CD4+ lymphocytes by non-HLA-restricted cytotoxic T lymphocytes from HIV-infected individuals. Clin Exp Immunol. 1993;93:356–62. doi: 10.1111/j.1365-2249.1993.tb08185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienzle D, Smaill FM, Rosenthal KL. Cytotoxic T-lymphocytes from HIV-infected individuals recognize an activation-dependent, non-polymorphic molecule on uninfected CD4+ lymphocytes. AIDS. 1996;10:247–54. doi: 10.1097/00002030-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Boullier S, Cochet M, Poccia F, Gougeon ML. CDR3-independent γδ V δ1+ T cell expansion in the peripheral blood of HIV-infected persons. J Immunol. 1995;154:1418–31. [PubMed] [Google Scholar]
- 7.Cole S. Blind T cell homeostasis and HIV infection. Immunol Toady. 1997;18:505–7. doi: 10.1016/s0167-5699(97)82524-8. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV, infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41:1–19. [PubMed] [Google Scholar]
- 9.Ahmad A, Menezes J. Defective killing activity against gp12-/41-expressing human erythroleukemic K562 cell line by monocytes and natural killer cells from HIV-infected individuals. AIDS. 1996;10:143–9. doi: 10.1097/00002030-199602000-00003. 2003. [DOI] [PubMed] [Google Scholar]
- 10.Sindhu STAK, Ahmad R, Morisset R, Ahmad A, Menezes J. Peripheral blood cytotoxic γδT lymphocytes from patients with HIV-1 infection and AIDS lyse CD4+ T cells and their cytocidal potential correlates with the viral load. J Virol. 2003;77:1848–55. doi: 10.1128/JVI.77.3.1848-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamberg JC, Bowmer MI, Trahey JC, Campbell CM, Pardoe I, Grant MD. Functional and genetic integrity of the CD8 T cell repertoire in advanced HIV infection. AIDS. 1999;13:2043–53. doi: 10.1097/00002030-199910220-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kestens L, Vanham G, Gigase P, et al. Expression of activation antigens, HLA-DR and CD38, on lymphocytes during HIV-1 infection. AIDS. 1992;6:793–7. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bofill M, Mocroft A, Lipman M, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 1996;10:827–34. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Levacher M, Hulstaert F, Tallet S, Ullery S, Pocidalo JJ, Bach A. The significance of activation markers on CD8 lymphocytes in human acquired immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–82. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho HN, Hultin LE, Mitsuyasu RT, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–9. [PubMed] [Google Scholar]
- 16.Hengel RL, Jones BM, Kennedy MS, Hubbard MR, McDougal JS. Markers of lymphocyte homing distinguish CD4 T cell subsets that turn over in response to HIV-1 infection in humans. J Immunol. 1999;163:3539–48. [PubMed] [Google Scholar]
- 17.Liu Z, Hultin LE, Cumberland WG, et al. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry. 1996;26:1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Yagi MJ, Joesten ME, Wallace J, Roboz JB, Bekesi JG. Human immunodeficiency virus type 1 (HIV-1) genomic sequences and distinct changes in CD8+ lymphocytes precede detectable levels of HIV-1 antibodies in high risk homosexuals. J Infect Dis. 1991;164:183–8. doi: 10.1093/infdis/164.1.183. [DOI] [PubMed] [Google Scholar]
- 19.Sherman GG, Scott LE, Galpin JS, et al. CD38 expression on CD8+ T cells as a prognostic marker in vertically HIV-infected pediatric patients. Pediatr Res. 2002;57:740–5. doi: 10.1203/00006450-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Resino GS, Bellon CJM, Navarro CJ, Gurbindo GD, Leon LJA, Munoz-Fernandez MA. T cell subsets variation during clinical and immunological progression in vertically HIV-infected children. Medicina (B Aires) 2001;61:557–65. [PubMed] [Google Scholar]
- 21.Tilling R, Kinloch S, Goh LE, et al. Parallel decline of CD8+/CD38+ T cells and viraemia in response to quadruple highly active antiretroviral therapy in primary HIV infection. AIDS. 2002;16:589–96. doi: 10.1097/00002030-200203080-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz L, Martinez-Picado J, Romeu J, et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS. 2000;14:397–403. doi: 10.1097/00002030-200003100-00013. [DOI] [PubMed] [Google Scholar]
- 23.Paul ME, Shearer WT, Kozinetz CA, Lewis DE. Comparison of CD8+ T cell subsets in HIV-infected rapid progressor children versus non-rapid progressor children. J Allergy Clin Immunol. 2001;108:258–64. doi: 10.1067/mai.2001.117179. [DOI] [PubMed] [Google Scholar]
- 24.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 25.Nkengasong JN, Kestens L, Ghys PD, et al. Human immunodeficiency virus type 1 (HIV-1) plasma virus load and markers of immune activation among HIV-infected female sex workers with sexually transmitted diseases in Abidjan, Côte d’Ivoire. J Infect Dis. 2001;183:1405–8. doi: 10.1086/319855. [DOI] [PubMed] [Google Scholar]
- 26.Margolick JB, Munroz A, Donnenberg AD, et al. Failure of T cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med. 1995;1:674–80. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 27.Galari N, Margolick JB, Astemborski J, Vlahov D. Existence and failure of T cell homeostasis prior to AIDS onset in HIV-infected injection drug users. Clin Immunol Immunopathol. 1996;79:134–41. doi: 10.1006/clin.1996.0060. [DOI] [PubMed] [Google Scholar]
- 28.Gange SJ, Munoz A, Chmiel JS, et al. Identification of inflections in T cell counts among HIV-1-infected individuals and relationship with progression to clinical AIDS. Proc Natl Acad Sci USA. 1998;95:10848–53. doi: 10.1073/pnas.95.18.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinz T, Wesch D, Friese K, Reckziegel A, Arden B, Kabelitz D. T cell receptor gamma delta repertoire in HIV-1-infected individuals. Eur J Immunol. 1994;24:3044–9. doi: 10.1002/eji.1830241219. [DOI] [PubMed] [Google Scholar]
- 30.Hinz T, Marx S, Nerl C, Kabelitz D. Clonal expansion of gamma delta T cells expressing two distinct T cell receptors. Br J Haematol. 1996;94:62–4. doi: 10.1046/j.1365-2141.1996.d01-1779.x. [DOI] [PubMed] [Google Scholar]