Abstract
Complement C6 homozygous deficiency (C6D) has been rarely observed in Caucasians but was reported at higher prevalence among African-Americans. We report on the molecular basis of C6D in seven unrelated black individuals of North or Central Africa descent who live in France. These patients have presented Neisseria meningitidis infection (four cases), focal and segmental glomerulosclerosis with hyalinosis (one case), systemic lupus erythematosus (one case) or Still's disease (one case). All patients exhibited undetectable antigenic C6 by using a sensitive ELISA assay. An additional four cases of complete C6 deficiency with no associated disease have been characterized after family studies. Exons 6, 7 and 12 have been described recently as the location of molecular defects on the C6 gene in randomly chosen black Americans. Genomic DNA from the seven patients were subjected to direct polymerase chain reaction amplification of these three exons. Nucleotide sequencing analysis of the amplified DNA fragments revealed a homozygous single-base deletion (1936delG) in exon 12 in three cases and four compound heterozygous deletions for a single base in exon 7 (1195delC) or in exon 6 (878delA) associated with the same deletion in exon 12 (1936delG). Our observations further establish the restricted pattern of genetic defects associated with homozygous C6 complement deficiency in individuals of African descent.
Keywords: complement deficiency, C6, gene mutation, Neisseria meningitidis
Introduction
Complement C6 is one of five plasma proteins that are incorporated into the potentially lytic terminal complement complex (C5b-9 m) on lipid membranes upon activation of the complement system. The assembly of the terminal complex begins with the proteolytic cleavage of C5 by the C5-convertase and proceeds through the formation of a complex between C5b and C6 and the subsequent and sequential binding of C7, C8 and C9. Components five to nine of complement form the terminal complement pathway resulting in transmembrane channel causing cell death [1,2]. Other sublytic functions on nucleated cells have been described [3]. Recent data suggest also a role of C5b-9 in cell cycle and apoptosis [4,5].
Several families with individuals deficient in proteins of the terminal complement pathway have been reported, originating from different ethnic backgrounds and different geographical regions [6]. Inherited defects affecting individual terminal components are uncommon in the general population, but the prevalence depends largely upon the ethnic origin of the tested population. Thus, the prevalence of C9 deficiency has been reported to be 0·095% in Japan [7], and that of C6 deficiency to be of 0·04% in African Americans [8,9]. C6 deficiency, like most other complement protein deficiencies, is inherited as an autosomal recessive trait.
The gene encoding for C6 spans about 80 kb of DNA and contains 18 exons [10]. Four gene deletions associated with homozygous complete C6 deficiency have been described so far in approximately 25 deficient individuals reported (review in [8]). Deletion 879 delG located in exon 6 represent 70% of the cases that have been found in the western Cape, South Africa [11]. Deletions 1195 delC located in exon 7, 1936delG located in exon 12 and 878 delA located in exon 6 have been found in different geographical areas as well as appearing to be an ancient defect in African populations [11–13]. Combined subtotal deficiencies of C6 and C7 has also been reported from two families [14]. In these cases, the C6 deficiency resulted from a defect at the 5′ splice donor site of intron 15 generating a protein of lower molecular weight with low expressed concentration. This molecular defect has also been found without C7 deficiency [15].
Here we report seven new index cases of complete C6 deficiency with the characterization of the underlying molecular genetic defect. The patients, who were unrelated, presented with one or several of the previously described deletions in the C6 gene, further confirming that C6 deficiency is associated with a restricted pattern of genetic defects.
Patients and Methods
Patients
The patients with C6 deficiency included in this report were seen between 1990 and 2001. Patients’ samples were selected from those found with undetectable CH50 activity upon investigation of the complement system, in the Department of Immunology of the Hôpital Européen Georges Pompidou, Paris. Following informed consent, blood was taken from patients and family members to extract DNA. EDTA plasma samples were stored at − 70°C.
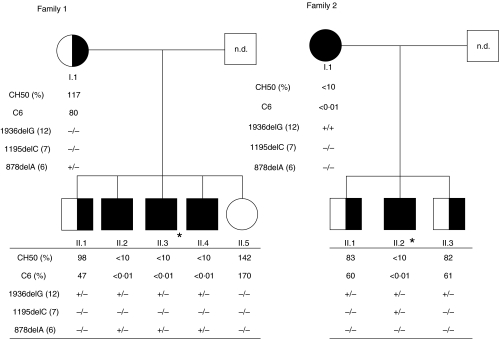
The clinical history of the seven index patients is summarized in Table 1. All patients were of African origin. Four of the seven patients had suffered from systemic infections with Neisseria meningitidis. Episodes of meningococcaemia were associated with a prodromal period of 2 or more days. The infecting serotype was not identified in two of the cases; it was attributable to group Y in one case and to group B in the other. One patient presented with two successive episodes of meningococcaemia at 5-year intervals. The age of the patients at the time of the first infection ranged between 12 and 28 years. All patients were treated with appropriate antibiotic chemotherapy and recovered uneventfully, except for one patient with residual chronic renal failure attributed to the infection. Investigations carried out among family members of patients 1 and 2 allowed for the diagnosis of complete C6 deficiency in four additional and asymptomatic individuals (Fig. 1). Of note, we found the mother of the index patient in family 2 to be homozygous C6-deficient. The three patients who did not present with a systemic infection were diagnosed upon investigation of the complement system in the context of a focal and segmental glomerulosclerosis with hyalinosis, coeliac disease asociated with Still's disease and SLE.
Table 1.
Molecular defects of the C6 gene associated with complete C6 deficiency in index cases in seven unrelated families
| Clinical presentation (age, years) | Origin | CH50 (%) | C6 Ag (%) | Nucleotide A878 in Exon 6* | Nucleotide C1195 in Exon 7* | Nucleotide G1936 in Exon 12* |
|---|---|---|---|---|---|---|
| 1 Recurrent Neisseria meningitidis infection (19) | Central African Republic | <10 | <0·1 | 878delA/878A | 1195C/1195C | 1936delG/1936G |
| 2 Neisseria meningitidis Y infection (18) | Ghana | <10 | <0·1 | 878 A/878A | 1195delC/1195C | 1936delG/1936G |
| 3 Neisseria meningitidis infection (28) | Africa | <10 | <0·1 | 878 A/878A | 1195C/1195C | 1936delG/1936delG |
| 4 Neisseria meningitidis B septicaemia (12) | Africa | <10 | <0·1 | 878 A/878A | 1195delC/1195C | 1936delG/1936G |
| 5 Focal and segmental glomerulosclerosis with hyalinosis | Gabon | <10 | <0·1 | 878 A/878A | 1195C/1195C | 1936delG/1936delG |
| 6 Still's disease and coeliac disease | North Africa | <10 | <0·1 | 878 A/878 | 1195C/1195C | 1936delG/1936delG |
| 7 Systemic lupus erythematosus | Cameroon | <10 | <0·1 | 878 A/878A | 1195delC/1195C | 1936delG/1936G |
cDNA nucleotide numbers refer to Hobart et al. [10].
Fig. 1.
Pedigrees and immunological/genetic data of subjects in families 1 and 2. n.d.: Not done; *index cases. Parentheses indicate the exon involved in the genetic abnormality.
Complement assays
CH50 and C2 haemolytic activity were determined according to standard procedures [16]. Results were expressed as a percentage of the CH50 and C2 activities of a reference plasma pool obtained from 100 healthy donors. Plasma concentrations of C4 and C3 antigens were measured by nephelometry (Dade Behring, Paris La Defense, France). Normal values established with pooled plasma from 100 healthy donors ranged between 220 ± 120 mg/l and 1200 ± 250 mg/l (mean ± 2 s.d.) for C4 and C3, respectively.
The concentration of C6 antigen in serum was measured by means of a double-ligand ELISA, as described previously [17]. Briefly, Nunc MaxiSorp ELISA plates (Nunc, Roskilde, Denmark) were coated with goat antihuman C6 IgG (Calbiochem, Meudon, France). After washing and blocking free reactive sites with PBS containing 1% BSA, the serum to be tested was added at a dilution of 1 : 2000 for 1 h. After washing, the plates were then incubated with biotinylated goat antihuman C6 IgG prior to the addition of streptavidin-biotinylated horseradish peroxidase and further incubation for 30 min at 37°C. Enzymatic activity was revealed using the orthophenyldiamine substrate. The same method was used to measure the C5, C7, C8 and C9 antigen concentrations with the appropriate polyclonal antibodies (Calbiochem, Meudon, France) [15]. The C6 haemolytic activity was measured as described [16].
Genomic C6 DNA sequencing
DNA was extracted from whole blood using the proteinase K/phenol method [18]. Uncloned genomic DNA was amplified by means of a polymerase chain reaction (PCR) using oligonucleotides flanking the exons 6, 7 and 12, as described by Hobart et al. [11]. Direct DNA sequencing of the purified PCR products (QiaQuick PCR Purification Kit, Qiagen SA, Courtaboeuf, France) was then carried out by the Dye terminator cycle sequencing method (Applied Biosystems, Courtaboeuf, France). Sequence analysis was performed using the Sequence Navigator® sofware.
Results
C6 deficiency
In all 11 cases diagnosed as complete C6 deficiencies, the patients’ complement profile was characterized by the lack of detectable CH50 activity, the absence of detectable C6 haemolytic activity, lack of detectable C6 antigen at a 1 : 40 dilution of plasma, with normal antigenic levels of C4, C3, C5, C7, C8 and C9 and normal levels of haemolytic C2 activity (Fig. 1 and Table 1). The addition of normal plasma to the patients’ plasma restored its ability to sustain total haemolytic activity (data not shown). Functional and immunochemical levels of complement components were consistent when investigated for a second time within 3 months (data not shown).
Measurement of C6 antigen in siblings of index cases 1 and 2 suggested three cases of heterozygous C6 deficiency characterized by normal CH50 activity and approximately 50% of the C6 antigenic levels (Fig. 1).
Molecular genetics
The three exons of gene encoding for C6 in chromosome 5 where molecular defects associated with complete C6 deficiency had been described previously were analysed first. The entire exons 6, 7 and 12 of all index cases were amplified and sequenced using forward and reverse primers. In the 14 chromosomes analysed, 10 carried the G deletion in position 1936 (1936 delG) in exon 12, three carried with the C deletion in position 1195 (delC 1195) in exon 7 and one carried the A deletion in position 878 (878 delA) in exon 6 (Table 2). Three patients were homozygous 1936delG, three patients were compound heterozygous for the nul allele 1936delG associated with a 1195 delC in two cases and with the 878 delA in another case (Table 1). No other abnormality was detected in the three exons 6, 7 and 12. Exon-specific sequence analyses were used to investigate the distribution of the C6 gene deletions among members of two patients’ families (Fig. 1). Two members of family 1, individuals II.2 and II.4, were diagnosed with homozygous C6 deficiency in addition to the index case II.3. The latter siblings presented with the same genetic defects as the index case. The sequencing data showed further that the mother and the brother (II.1) of the propositus exhibited the 878 delA deletion (mother) or the 1936 delG (II.1), respectively. No genetic abnormality was found in the sister of the index case (II.5), who presented with normal C6 antigenic levels. In family 2, two brothers (individuals II.1 and II.3) were heterozygous for the 1936 delG deletion (1936 delG). The homozygous-deficient mother in this family carried the homozygous deletion 1936delG in exon 12.
Table 2.
Three C6 deficiency mutations in seven unrelated individuals of North or Central Africa descent who live in France
| Common name | Location of the defect | Number of affected chromosomes | Notes | No. of affected chromosomes described previously in C6-deficient individuals |
|---|---|---|---|---|
| C6Q0/1936delG | Exon 12 | 10 | Frame shift and premature termination codon 9nucleotides downstream | 5 ([9], [11], [12], and [13]) |
| C6Q0/1195delC | Exon 7 | 3 | Frame shift generating 63 novel amino acids andtermination codon in exon 9 | 8 ([11] and [13]) |
| C6Q0/878delA | Exon 6 | 1 | Frame shift generating 45 novel amino acids andtermination codon in exon 7 | 3 ([9]) |
Discussion
Here we report on seven unrelated individuals of African descent living in Europe, with homozygous complete complement C6 deficiency. We have investigated the presence of the deletions previously described in the C6 null alleles. The 1936delG defect in exon 12 and the 1195delC defect in exon 7, resulting in premature termination of the C6 polypeptide, were described first in unrelated African-American families [12,13] and were found exclusively in individuals of African descent [9]. The deletion 878delA located in exon 6 leading to a shift in the reading frame, generating a novel 45 amino acid sequence, followed by a stop codon, was found to be equally distributed among blacks and whites [9]. The prevalence of homozygous C6D deficiency associated with one or several of these three defects may be estimated to be 1 in 1600 African Americans compared with approximately 1 in 40 000 white individuals in the South-eastern part of the United States [8]. The 879delG in exon 6 is restricted to South African or Netherlands patients [11].
In the present study, the gene defects in seven individuals with complete C6 deficiency included a homozygous single-base deletion (1936delG) in exon 12 in three cases, and four compound heterozygous deletions of a single base in exon 7 (1195delC) or in exon 6 (878delA) associated with the same deletion in exon 12 (1936delG). Ten chromosomes among the 14 tested carried a deletion of nucleotide G1936 in exon 12, which causes a shift in the reading frame and generates a stop codon 9 nucleotides downstream. In all cases the C6 antigenic level by using a sensitive ELISA was undetectable (< 0·1% of normal values), suggesting that the truncated RNA and/or the short C6 protein were unstable.
The patients presented with meningococcal infections (four cases) or in the context of the investigation of an autoimmune disease (three cases). The deficiency in any of the proteins of the terminal complex will block the lytic complement pathway. The terminal complement complex plays an important role in neutralizing Neisseria. Deficiencies in components of the terminal pathway are thus associated with the risk of occurrence of neisserial infections. Most of the cases of C6 deficiency described so far have been identified upon screening following the occurrence or recurrence of meningococcal disease [11–13]. Recurrent episodes of meningococcal meningitidis occur typically after the age of 10 years. Infections are associated frequently with septicaemia and are often caused by unusual serotypes of the meningococci. Three of our patients presented with autoimmune diseases and no documented episode of meningococcaemia. Whether individual patients with C6 deficiency have been reported as suffering from systemic [19] or discoid lupus erythematosus [20], Sjögren's syndrome [20] and arthritis with antinuclear antibodies [21], no association of C6 deficiency with SLE has been found, as discussed previously [22]. Screening of large populations has also revealed the existence of asymptomatic C6-deficient individuals [6,12]. In this regard, we identified three cases of complete C6 deficiency in asymptomatic relatives of index cases in two families that could be comprehensively investigated.
Our observations establish further the restricted array of genetic defects associated with homozygous C6 complement deficiency in individuals of African descent.
Acknowledgments
The authors thank the clinicians who referred the patients: C. Casenave MD (Service de Pédiatrie, Centre Hospitalier Jacques Coeur, Bourges), O. Chosidow MD (Service de Médecine Interne 2, Hôpital Pitié-Salpêtrière, Paris), S. Herson MD (Service de Médecine Interne 2, Hôpital Pitié-Salpêtrière, Paris), N. Cailleux MD (Departement de Medecine Interne, CHU de Rouen, France), C. Jacquot MD (Service de Nephrologie, Hôpital Européen Georges Pompidou, Paris), E. Vandemeulebroucke MD (Fédération de Biologie médicale, Centre Hospitalier de Gonesse, Gonesse, France) and A. Fischer MD (Departement de Pédiatrie, unité d’Immunologie et d’Hématologie, Hôpital Necker Enfants Malades, Paris). They also thank N. Poulain and D. Senger for technical support.
References
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264:1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- 5.Nauta AJ, Daha MR, Tijsma O, et al. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–92. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984;63:243–73. [PubMed] [Google Scholar]
- 7.Fukumori Y, Yoshimura K, Ohnoki S, et al. A high incidence of C9 deficiency among healthy blood donors in Osaka. Japan Int Immunol. 1989;1:85–9. doi: 10.1093/intimm/1.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Orren A. Molecular mechanisms of complement component C6 deficiency; a hypervariable exon 6 region responsible for three of six reported defects. Clin Exp Immunol. 2000;119:255–8. doi: 10.1046/j.1365-2249.2000.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Atkinson TP, Hovanky KT, et al. High prevalence of complement component C6 deficiency among African-Americans in the south-eastern USA. Clin Exp Immunol. 2000;119:305–10. doi: 10.1046/j.1365-2249.2000.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobart MJ, Fernie B, DiScipio RG. Structure of the human C6 gene. Biochemistry. 1993;32:6198–205. doi: 10.1021/bi00075a012. [DOI] [PubMed] [Google Scholar]
- 11.Hobart MJ, Fernie BA, Fijen KA, et al. The molecular basis of C6 deficiency in the western Cape, South Africa. Hum Genet. 1998;103:506–12. doi: 10.1007/s004390050858. [DOI] [PubMed] [Google Scholar]
- 12.Nishizaka H, Horiuchi T, Zhu ZB, et al. Molecular bases for inherited human complement component C6 deficiency in two unrelated individuals. J Immunol. 1996;156:2309–15. [PubMed] [Google Scholar]
- 13.Zhu ZB, Totemchokchyakarn K, Atkinson TP, et al. Molecular defects leading to human complement component C6 deficiency in an African-American family. Clin Exp Immunol. 1998;111:91–6. doi: 10.1046/j.1365-2249.1998.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernie BA, Wurzner R, Orren A, et al. Molecular bases of combined subtotal deficiencies of C6 and C7: their effects in combination with other C6 and C7 deficiencies. J Immunol. 1996;157:3648–57. [PubMed] [Google Scholar]
- 15.Wurzner R, Hobart MJ, Fernie BA, et al. Molecular basis of subtotal complement C6 deficiency. A carboxy-terminally truncated but functionally active C6. J Clin Invest. 1995;95:1877–83. doi: 10.1172/JCI117868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazatchkine M, Hauptmann G, Nydegger U. Techniques du complement. Paris: INSERM; 1985. [Google Scholar]
- 17.Perissutti S, Tedesco F. Effect of cytokines on the secretion of the fifth and eighth complement components by HepG2 cells. Int J Clin Laboratory Res. 1994;24:45–8. doi: 10.1007/BF02592409. [DOI] [PubMed] [Google Scholar]
- 18.Jeanpierre M. A rapid method for the purification of DNA from blood. Nucl Acids Res. 1987;15:9611. doi: 10.1093/nar/15.22.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tedesco F, Silvani CM, Agelli M, et al. A lupus-like syndrome in a patient with deficiency of the sixth component of complement. Arthritis Rheum. 1981;24:1438–40. doi: 10.1002/art.1780241119. [DOI] [PubMed] [Google Scholar]
- 20.Trapp RG, Mooney E, Coleman TH, et al. Hereditary complement (C6) deficiency associated with systemic lupus erythematosus, Sjogren's syndrome and hyperthyroidism. J Rheumatol. 1987;14:1030–3. [PubMed] [Google Scholar]
- 21.Reinitz E, Lawrence M, Diamond B, et al. Arthritis and antinuclear antibodies (ANA) with inherited deficiency of the sixth component of complement (C6) Ann Rheum Dis. 1986;45:431–4. doi: 10.1136/ard.45.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurzner R, Orren A, Lachmann PJ. Inherited deficiencies of the terminal components of human complement. Immunodefic Rev. 1992;3:123–47. [PubMed] [Google Scholar]