Abstract
The availability of tetrameric complexes of HLA class I molecules folded with immunodominant peptides makes it possible to utilize flow cytometry for rapid and highly specific visualization of virus specific CD8+ T cells. An alternate technique is to incubate whole blood with specific antigens and to subsequently detect and characterize responding T cells (e.g. by performing intracellular staining of interferon-gamma). By using an HLA-A2 tetramer construct folded with the same immunodominant CMV-peptide as that used for peptide pulsing, we monitored both the presence and functional capacity of CMV-specific CD8+ T cells. In addition T cell activation was assayed by determination of CD38 and CD69 expression. Twelve organ transplant patients and 31 healthy blood donors with latent CMV infection were investigated using CMV pp65 tetramer staining and intracellular staining of interferon-gamma after CMV pp65 peptide pulsing or CMV lysate pulsing. CMV-specific T cells were detected in similar absolute numbers as well as frequencies of T cells in the two groups investigated. However, the CMV-specific CD8+ T cells in immunosuppressed individuals showed a decreased functional response to the CMV-peptide, as evidenced by reduced interferon-gamma production when compared to healthy blood donors (19%; 42%, P < 0·005). In addition, CD38 expression was markedly higher in immunosuppressed patients compared to healthy blood donors (24%; 6%, P < 0·005). In a case report we demonstrate that reactivation of CMV can occur in an immunosuppressed patient with high number of CMV-specific T cells, but without functional capacity. Hence, these findings reflect impaired activation of cytotoxic T cells controlling latent CMV infection in immunosuppressed patients.
Keywords: CD8-positive T lymphocytes, CMV, cytomegalovirus, HLA, flow cytometry, MHC
Introduction
The introduction of new techniques to visualize antigen-specific T lymphocytes offers unique possibilities to characterize the cellular immune response to defined antigens of viruses and tumour cells. Cytotoxic (CD8+) and helper (CD4+) T lymphocytes are of crucial importance in the acute phase of viral diseases and for keeping lifelong viral infections, such as cytomegalovirus (CMV), in a latent phase [1–4]. However, in order to understand the mechanisms of reactivated CMV infection in immunosuppressed patients, the precise role of these antigen specific T lymphocytes remains to be fully explored.
Previous studies using MHC tetramers for quantification of virus specific cells have reported similar frequencies of these cells in immunosuppressed and in non-immunosuppressed individuals with latent infection [5–7]. This suggests that functional characterization of the tetramer positive T cells is also required in order to assess antiviral capacity at the individual level.
An alternative to the MHC tetramer technique is incubation of whole blood with antigen, either as short selected peptides representing immunodominant epitopes presented by MHC class I, or as crude unprocessed viral lysates for proteolytic processing and antigen presentation by MHC class II on antigen presenting cells present in blood. Subsequently, T lymphocytes of either the CD8+ or the CD4+ subset will produce cytokines (e.g. interferon-gamma (IFN-γ)) when stimulated with specific antigen. By using Brefeldin to stop granule secretion and membrane permeabilization agents, cells producing IFN-γ in response to antigen challenge can be detected readily using flow cytometry [8,9]. Studies have shown a strong positive correlation between the frequencies of peptide activated cells expressing IFN-γ and the specific lysis of peptide pulsed targets in a 51Cr release assay [10].
This technique has, for example, been used to monitor the level of CMV-specific T lymphocytes in HIV and kidney transplanted patients [3,11,12].
However, the secretion of IFN-γ in response to antigen is dependent both on the presence and functional capacity of antigen specific cells. When using this technique alone an unknown number of antigen-specific cells will not be visualized, particularly if the patient's IFN-γ secretion capacity is reduced due to the use of immunosuppressive drugs or an acquired/inherited immunodeficiency.
By using HLA-A2 tetramers folded with the same peptide as that used for peptide pulsing, it is possible to monitor both the frequency and the functional capacity of antigen-specific T cells by determining the ratio of tetramer positive cells responding to peptide pulsing.
In this study we used three different techniques to visualize CMV-specific T cells on immunosuppressed (organ transplant patients) as well as non-immunosuppressed individuals with latent CMV infection. By combining the techniques of HLA-A2/CMVpp65 tetramer staining, pulsing with CMVpp65 peptide or a lysate of CMV-infected fibroblasts, we investigated whether CMV seropositive immunosuppressed patients, who have an increased risk of symptomatic recurrent CMV infection, differ in the functional capacity of their CMV-specific T cells compared to healthy controls.
Participants, Materials and Methods
Healthy blood donors and immunosuppressed patients
This study was approved by the Ethics Committee at the University Hospital, Uppsala, Sweden. All participants gave their informed consent for the use of blood samples. CMV status was determined by CMV IgG/IgM capture ELISA (Messer Griesheim GMBH, Schwalbach ATs, Germany). HLA class I haplotypes were established for each individual using serological techniques. For individuals that were both HLA-A2- and CMV-positive, sequence-based typing of the HLA-A locus was performed as described previously [13].
Twelve HLA*A0201 and CMV-seropositive immunosuppressed patients were included in the study (11 patients with kidney transplants and one with a liver transplant). The average time-point since transplantation was 31 months (3–216 months). Immunosuppression consisted of tacrolimus alone (n = 1), tacrolimus and steroids (n = 2), cyclosporine (CsA) and steroids (n = 4), tacrolimus, mycophenolate mofetil (MMF) and steroids (n = 4), or tacrolimus, azathioprin and steroids (n = 1). All drugs were given at maintenance dosages. Blood samples were obtained from these patients 12 h after the last dose of calcinurin inhibitors. The concentrations of CsA and tacrolimus were measured at the time of the assay in 10 of 12 cases. The concentration of CsA ranged from 100 to 220 ng/ml (mean 210) and the concentration of tacrolimus ranged from 3·7 to 15·0 ng/ml (mean 9·0). Three patients had received treatment for recurrent and one for primary CMV infection after transplantation. After treatment, all four of these patients tested negative for CMV–DNA in blood using polymerase chain reaction (PCR). At the time of the investigation, none of the transplant patients or healthy blood donors had clinical signs of ongoing CMV infection. In addition, all patients were examined using quantitative CMV–PCR [14]. Forty-one healthy blood donors were also included in the study. Participants were selected based on tissue-type and serological CMV status; 24 individuals were HLA-A2-positive and CMV-seropositive, six were HLA-A2-positive and CMV-seronegative, seven were HLA-A2-negative and CMV-seropositive and four were HLA-A2-negative and CMV-seronegative. All HLA-A2-positive individuals were HLA*A0201.
HLA-A2/pp65 tetramer staining of whole blood
EDTA or sodium-heparinized whole blood from each participant was used for tetramer staining as described previously [5]. The CMVpp65 tetramer is a construction of HLA-A2 folded with the same CMVpp65 peptide (NLVPMVATV) used for peptide pulsing. The staining was performed as follows: 100 µl whole blood was incubated with 1 µl HLA-A2pp65-phycoerythrin (PE) (100 µg/ml, Immunotech, Marseilles, France), CD3-allophycocyanin (APC) (Becton Dickinson, Mountain View, CA, USA) and CD8 peridinin chlorophyll protein (PerCP) (Becton Dickinson) for 30 min at room temperature (RT). Antibodies and MHC tetramers were used at optimal dilutions after titration of samples with known tetramer-positive cells. Control experiments were performed for each individual by omitting the tetramers. After incubation, 1 ml lysing solution (BD-perm, Beckton Dickinson) was added followed by incubation for 10 min at RT. The suspension was centrifuged at 200 g for 5 min and resuspended in 250 µl PBS containing 1% formaldehyde and 1% fetal calf serum. Samples were analysed on a Becton Dickinson FACSCalibur flow cytometer using Cell-Quest software according to standard procedures. Results are presented as the percentage of A2/pp65 tetramer-positive cells within the CD3 positive population.
Cytokine detection after antigen pulsing
The assay used is a modification of a protocol developed by Dr L. E. Nomura and coworkers at BD Biosciences, San Jose, CA, USA [9]. Briefly, 10 µl CMVpp65 peptide (NLVPMVATV; 0·5 mg/ml, kindly provided by Dr Florian Kern (Institut für Medizinische Immunologie der Charité, Berlin, Germany) or 10 µl CMV lysate (0·5 mg/ml, Advanced Biotechnologies, Columbia, MD, USA) was added to 0·5 ml sodium-heparinized whole blood. Controls contained no antigen. All assays were performed in duplicate. One additional resting control was used for flow cytometer setup, as described below. When comparing blood donors with patients, three additional tubes were used for double staining with A2/pp65 tetramer and IFN-γ (Table 1). In these tubes, tetramer (5 µl of 100 µg/ml) was added 30 min before incubation with either pp65 peptide or CMV lysate.
Table 1.
Antibodies used in the antigen-specific cytokine assay
| Tube no. | |||
|---|---|---|---|
| Stain | 1 | 2 | 3 (comparison) |
| APC | CD3 | CD3 | CD3 |
| PerCP | CD8 (CD4 for CMV-lysate) | CD8 (CD4 for CMV-lysate) | CD8 (CD4 for CMV-lysate) |
| PE | CD69 | CD38 | A2/pp65 tetramer |
| FITC | IFN-γ | IFN-γ | IFN-γ |
Whole blood was distributed among six tubes (nine tubes in experiments designed to make a comparison between immunosuppressed and non-immunosuppressed individuals). After incubation with peptide or CMV-lysate, blood was lysated, permeabilized and incubated with antibodies. In tube 3, tetramers were added 30 min before peptide/lysate.
The incubation was performed for 2 h at 37°C, 5% CO2 at a 5° slant after which 10 µl Brefeldin A (0·5 mg/ml in EtOH, Sigma Chemicals, St Louis, MO, USA) was added followed by a 4-h incubation at 37°C. After the final incubation, samples were stored at 4°C overnight; 100 µl of 0·2 m EDTA (Sigma) was added, samples were vortexed, incubated for 15 min at RT and then vortexed again. For optimal lysing, 250 µl aliquots of sample were added to 4·5 ml lysing solution (Beckton Dickinson) and incubated for 10 min at RT. Next, the samples were centrifuged at 200 g for 5 min, decanted and washed once with 1% BSA in PBS, and supplemented with 0·5 ml permeabilizing solution (BD-perm, Beckton Dickinson), followed by 10 min incubation at RT in the dark. After washing, staining was performed for 30 min at RT using antibodies as described in Table 1. Blood intended for instrument setup was distributed into five tubes. Of these, one remained unstained while anti-CD3, conjugated to either fluorescein–isothiocyanate (FITC), PE, PerCP or APC, was added at optimal dilution to the other tubes. Cell suspensions were incubated with antibodies for 30 min at RT, washed once, and then fixed by adding 250 µl PBS containing 1% paraformaldehyde and 0·1% sodium azide. Samples were analysed on a FACSCalibur flow cytometer (Beckton Dickinson). The threshold was set to exclude CD3-negative events. Typically, 30–50 000 events were analysed for each tube. For evaluation purposes, a lymphocyte gate according to forward scatter/side scatter (FSC/SSC) was used. Results are presented as the percentage of IFN-γ or tetramer-positive T cells (CD3+ lymphocytes). Results are not presented as the percentage of CD8 or CD4 positive T lymphocytes as antigen stimulation and subsequent IFN-γ production weakens CD8 and CD4 expression [15], making it difficult to set cut-off values. Individuals with a distinct population of at least 0·1% T lymphocytes positive over the background were considered positive. For statistical analysis, cell populations <0·1% were considered equal to 0·05% because positive populations between 0 and 0·1% could not be excluded.
Determination of the functional response of CMV tetramer positive cells
The direct number of CMV tetramer- and IFN-γ-positive cells/ml whole blood was calculated using flow-count beads (Coulter Corporation, Miami, FL, USA), according to the manufacturer's instructions. Because HLA-tetramer staining inhibits the production of IFN-γ (as discussed below), tetramer staining and peptide pulsing was performed in separate tubes. The ratio of CMV tetramer-positive cells responding with IFN-γ secretion was calculated from the obtained absolute values.
Influence of cyclosporin on secretion of IFN-γ after peptide and lysate stimulation of whole blood
Heparinized whole blood was drawn from four healthy HLA A2-positive CMV-seropositive blood donors, known from previous experiments to have IFN-γ responding cells. Cyclosporine (for i.v. distribution) was added to 2 × 8 × 0·25 ml of blood before peptide or lysate pulsing. The concentration of CsA ranged from 0 to 1000 ng/ml. After pulsing and staining as described above, the percentage of IFN-γ-secreting T lymphocytes in each tube was determined.
Statistical analysis
Results are expressed as mean ± standard error of the mean (s.e.m.). Comparisons between the three different techniques in healthy blood donors were evaluated using the Wilcoxon test. Comparisons between immunosuppressed patients and healthy blood donors were evaluated using the Mann–Whitney test.
Results
All but one patient were negative for viral replication using quantitative CMV–PCR [14]. The remaining patient was just above the cut-off value for the test used (600 copies/ml). This is in concurrence with previous findings that a low level of CMV–DNA can be seen in the blood of transplant patients without clinical signs of ongoing CMV infection [16,17].
HLA-A2/pp65 tetramer staining and antigen pulsing of healthy CMV-seropositive blood donors
Tetramer or IFN-g-positive cells could be detected clearly as distinct positive populations (Fig. 1). The mean fluorescence intensity (MFI) did not differ between immunosuppressed and non-immunosuppressed blood donors (data not shown).
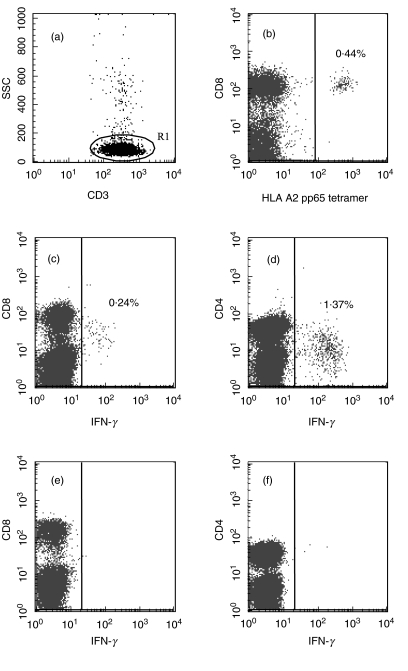
Fig. 1.
Detection of CMV-specific T lymphocytes using flow-cytometry in a healthy HLA A2-positive CMV-seropositive blood donor. T lymphocytes were defined as CD3 high; side-scatter low (a). CMVpp65 tetramer-positive T lymphocytes were all CD8+ (b). IFN-γ-positive T lymphocytes after pulsing with CMVpp65-peptide showing decreased expression of CD8 (c). IFN-γ positive T lymphocytes after pulsing with CMV lysate showing decreased expression of CD4 (d). Unpulsed CD8+ (e) or CD4+ (f) T lymphocytes displaying low background-staining.
Tetramer binding T lymphocytes were seen in 10 of the 31 CMV-seropositive individuals (0·73% ± 0·29/CD3+) (Table 2). These individuals were all HLA-A2-positive. In seven of these 10 patients, IFN-γ production in response to pulsing with CMVpp65 peptide was noted (0·44% ± 0·14/CD3+). In 27 of the 31 CMV-seropositive individuals, IFN-γ production in response to pulsing with CMV lysate was observed (0·89% ± 0·14/CD3+). Of these, 20 were HLA-A2-positive.
Table 2.
Characteristics of non-immunosuppressed participants for comparison of A2/pp65 tetramer staining with the antigen pulsing technique
| Participants (n = 41) | n | HLA-A2/ pp65 tetramer+ | pp65-peptide (responders) | CMV-lysate (responders) |
|---|---|---|---|---|
| HLA A2+ CMV+ | 24 | 10 | 7 | 20 |
| HLA A2+ CMV– | 6 | 0 | 0 | 0 |
| HLA A2– CMV+ | 7 | 0 | 0 | 7 |
| HLA A2– CMV– | 4 | 0 | 0 | 0 |
Healthy participants (n = 41) were selected based on tissue-type and serological CMV status. Whole blood was used for HLA-A2/pp65 tetramer staining, pulsing with NLVPMVATV pp65-peptide and CMV-lysate.
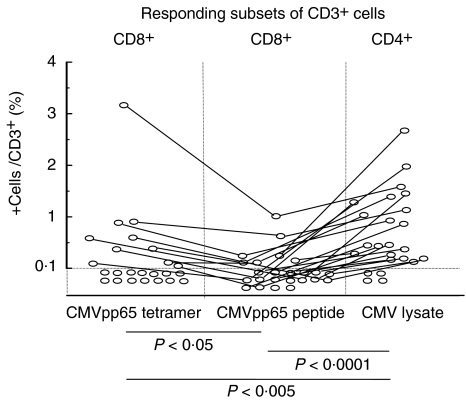
In the HLA-A2-positive and CMV-seropositive participants (n = 24), theoretically all three techniques should work. CMV-responsive cells were detected (>0·1%/CD3+) in 83% of these individuals using CMV lysate pulsing, 42% using A2/pp65 tetramer and 29% using the pp65 peptide pulsing technique. Also, the frequency of specific cells differed between the techniques, with lysate pulsing displaying the highest value, followed by tetramer staining and peptide pulsing (Fig. 2). No CMV-seronegative individual displayed a positive response in any of the three techniques evaluated. In addition, neither tetramer binding nor INF-γ secretion after pp65 peptide pulsing were observed in HLA-A2 negative individuals.
Fig. 2.
Comparison of HLA-A2/pp65 tetramer staining with CMVpp65 peptide- and CMV lysate-pulsing techniques for the detection of CMV-specific T lymphocytes in HLA-A2-positive, CMV-seropositive healthy individuals. The frequency of CMV-specific T lymphocytes was highest using the lysate pulsing technique, followed by tetramer staining and CMVpp65 peptide pulsing. Four individuals did not display CMV-specific T cells using any of the techniques.
Expression of CD8, CD4, CD69 and CD38 among IFN-γ positive T lymphocytes in healthy CMV-seropositive blood donors
IFN-γ-positive cells detected were CD3+ CD8 intermediate after pp65 peptide pulsing and CD3+ CD4 intermediate after CMV lysate pulsing. In both cases, the majority (>90%) of all IFN-γ-positive cells were CD69-positive. For technical reasons, CD38 expression was analysed for only 24 of 27 individuals with IFN-γ positive cells. In all but one individual, the majority of T cells (>75%) were CD38 negative. In the remaining individual, 20% of the T cells were CD38-negative. The expression of CD69 and CD38 did not differ between CD8 and CD4-positive T cells after peptide or lysate pulsing.
Comparison of CMV-seropositive healthy blood donors and immunosuppressed patients using A2/pp65 tetramer staining and CMV antigen pulsing
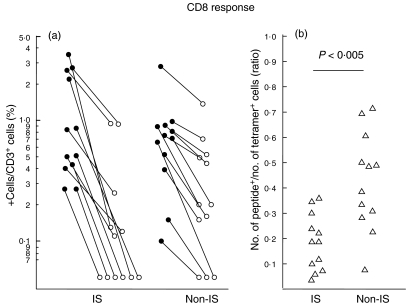
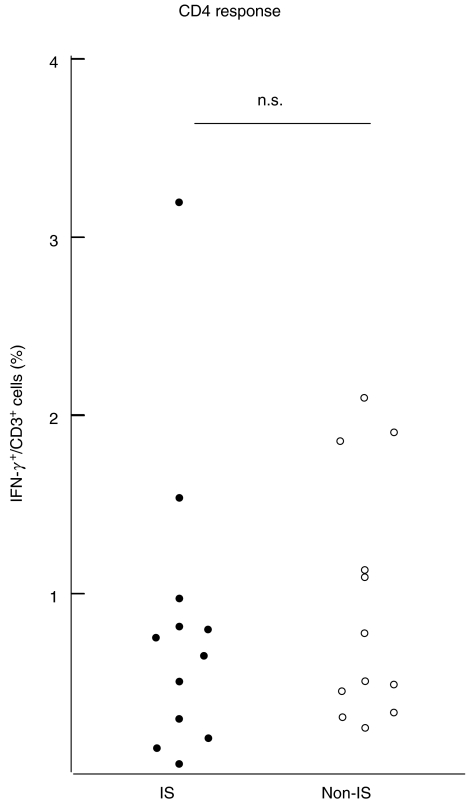
Healthy blood donors (n = 12) and immunosuppressed patients (n = 12) were selected based on the presence of tetramer positive T cells. The percentage of tetramer positive T cells did not differ between blood donors and immunosuppressed patients (2·07% ± 0·36; 1·26% ± 0·33, P = 0·47, Fig. 3a). Also, the absolute number of tetramer positive cells did not differ between the groups (19·89 cells/µl ± 10·88; 30·01 cells/µl ± 9·43, P = 0·51). However, the ratio of tetramer-positive T cells responding to CMVpp65 peptide stimulation in immunosuppressed patients was about half that of healthy blood donors (19%; 42%, P < 0·005, Fig. 3b). The percentage of IFN-γ-positive T lymphocytes after CMV lysate pulsing was higher than after CMV peptide pulsing (P < 0·005), with no clear difference between immunosuppressed patients and healthy blood donors (0·82%; 0·94%, Fig. 4).
Fig. 3.
Visualization of a reduced functional capacity of CD8+ CMV-specific T cells in immunosuppressed patients. In HLA-A2-positive and CMV-seropositive individuals, the fraction of tetramer-positive T cells responding to peptide pulsing by IFN-γ synthesis was lower in immunosuppressed patients compared with non-immunosuppressed blood donors. Results are shown as the percentage of CMV pp65 tetramer binding cells and CMV pp65 peptide responding cells for each individual (a). The ratio of CMV tetramer-positive cells responding with IFN-γ secretion was calculated after determining the absolute number of tetramer- and IFN-γ positive cells (b). IS: immunosuppressed (n = 12); non-IS: non-immunosuppressed (n = 12) •, Tetramer staining; ○, peptide pulsing; ▵, ratio.
Fig. 4.
IFN-γ-secreting CD4+ T lymphocytes after pulsing with CMV lysate. In HLA-A2-positive and CMV-seropositive individuals, no significant difference could be seen between immunosuppressed patients compared with non-immunosuppressed blood donors. IS: immunosuppressed (n = 12); non-IS: non-immunosuppressed (n = 12)
Attempts to stain CMV peptide stimulated cells with A2/pp65 tetramer and IFN-γ in the same test tube were unsuccessful because the frequency of IFN-γ-producing cells was reduced by about 50% when the tetramer was added before the peptide when compared to incubation with peptide only (data not shown). These results suggest that tetramer binding to the TCR induces steric hindrance and partial blocking of responding T lymphocytes, making the combined use of tetramers and peptide in the same test tube less attractive.
Comparison of CD69 and CD38 expression on IFN-γ-positive T lymphocytes between immunosuppressed and healthy blood donors
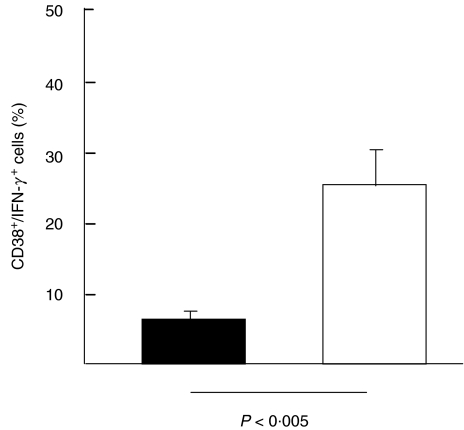
In both groups, the majority (> 90%) of all IFN-γ-positive T lymphocytes expressed CD69. Due to the small numbers of responding T lymphocytes in immunosuppressed individuals after CMVpp65 peptide stimulation, the expression of CD38 was evaluated only on CMV lysate responding T cells. The CD38 expression was markedly higher in immunosuppressed patients when compared to blood donors (24%; 6%, P < 0·005, Fig. 5).
Fig. 5.
Expression of CD38 on CMV lysate-responding T lymphocytes. The CD38 expression was markedly higher in immunosuppressed patients than in healthy blood donors (P < 0·005). ▪, Non-immunosuppressed (n = 12); □, immunosuppressed (n = 12).
Influence of cyclosporin on secretion of IFN-γ after peptide- and lysate stimulation of whole blood
The secretion of IFN-γ after peptide or lysate pulsing in healthy HLA A2-positive CMV-seropositive blood donors was not affected by CsA concentrations up to 400 ng/ml. At higher concentrations, the percentage of secreting cells was progressively reduced, with about 70% reduction at 1000 ng/ml (data not shown).
Case report (Fig. 6)
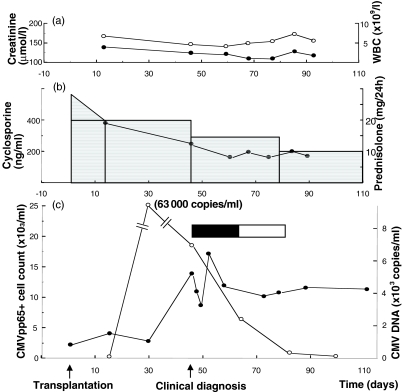
Fig. 6.
Reactivated CMV infection in CMV-seropositive recipient of a kidney transplant from a CMV-seropositive donor. After CMV reactivation, the absolute number of tetramer-positive cells increases and remain high during antiviral treatment. However, functional response in terms of IFN-γ secretion after peptide or lysate pulsing has not been seen in this patient, suggesting that this individual may have an impaired ability to keep the CMV infection in a latent stage. (a) •, Creatinine (µmol/l); ○, WBC (×109). (b) □, Prednisolone (mg/24 h); •, cyclosporine (ng/ml). (c) ○, CMV–DNA (×103 copies/ml; •, CMVpp65+ (×103/ml); ▪, ganciclovir; □, valaciclovir.
A 64-year-old CMV-seropositive female with polycystic kidney disease received a kidney transplant from a CMV-seropositive donor. Immunosuppression consisted of cyclosporine and steroids. Blood samples were obtained at regular intervals and were analysed for the presence and function of CMV-specific T cells. Viral load was determined using quantitative CMV–PCR. The clinical course was uneventful until 6 weeks after transplantation. At this time the patient developed signs of CMV infection (fever, thrombocytopenia and elevated transaminases). CMV–PCR was negative (<100 copies/ml) 2 weeks after transplantation but was found positive (63 000 copies/ml) 2 weeks later (before clinical symptoms). When the patient developed signs of CMV infection CMV–PCR showed 7600 copies/ml and treatment with i.v. ganciclovir (350 mg × 2) was given for 2 weeks, followed by 2 weeks’ treatment with oral valaciclovir (500 mg × 2). During the first weeks of treatment intermittent fever was noted, but this gradually disappeared and laboratory values were normalized.
CMV tetramer staining, CMV peptide- and CMV lysate pulsing was performed repeatedly during this period. The absolute number of CMV tetramer-positive cells, the number of CMV–DNA copies, the level of immunosuppression, the creatinine level and the white blood cell count (WBC) are presented in Fig. 6. IFN-γ-positive cells after either CMV peptide- or CMV lysate pulsing were never seen in this patient and is consequently not presented.
Discussion
In the immediate period after stem cell transplantation, the recovery of CMVpp65-specific CD8+ T cells seems to correspond with a reduced risk of developing CMV disease [18,19]. It should be noted, however, that this group of patients have almost no circulating leucocytes and are therefore not directly comparable to other groups of immunosuppressed patients with normal levels of white blood cells. Past results using the tetramer staining technique have indicated that the frequency of CMV-specific T cells is surprisingly high in immunosuppressed individuals with latent CMV infection [5, 7, 12, 20]. This most probably reflects a compensatory mechanism for impaired T cell function due to the immunosuppressive state. Alternatively, or in addition, the subclinical viral replication as seen in immunosuppressed patients, constantly stimulates further proliferation of virus-specific cells. As a consequence, further characterization of virus-specific cells in immunosuppressed patients is warranted.
In this study we used tetramer staining and peptide pulsing for visualization of CD8+ T cells and lysate pulsing for CD4+ T cells. For the functional analysis of CD8+ T cells we combined the use of tetramer staining and peptide pulsing. Although the frequency and absolute number of CMV-specific T cells did not differ between healthy blood donors and immunosuppressed patients with latent CMV infection, the CD8+ T cells in immunosuppressed individuals showed a decreased functional response to CMV peptide, as evidenced by a reduction in IFN-γ secretion when compared to non-immunosuppressed blood donors.
In a previous study combining tetramer staining and peptide pulsing for the functional analysis of CD8+ T cells, higher frequencies of tetramer binding and IFN-γ-secreting cells were found in renal transplant patients compared to non-immunosuppressed participants [20]. The frequency of tetramer binding T cells responding with IFN-γ production after pp65 peptide stimulation (approx. 20%) was the same between the groups. However, quantification of absolute numbers of functionally active CMV-specific cells were not performed. Hence, a direct comparison between the results in the present study and the results of Gamadia et al. cannot be conducted.
In this study, the induction of IFN-γ in CD4+ T cells pulsed with CMV-lysate was the same in both groups investigated. Technical failures, such as interference between the two detection techniques (discussed previously in the Results section) are unlikely, because tetramer staining and peptide pulsing were performed in separate tubes. However, a direct effect of the different immunosuppressive drugs may have affected the functional assay. All patients were being treated with calcineurin inhibitors and all but one were being treated with steroids. No effect on IFN-γ secretion was noted with increasing concentrations of CsA (up to 400 ng/ml) in samples retrieved from healthy blood donors with known frequencies of CMV-specific T cells. Notably, the levels of calcineurin inhibitors in the patients were all well below the concentration needed to directly suppress IFN-γ production in vitro after retrieval of the blood samples. However, the reduced functional capacity of the CMV-specific CD8+ T cells demonstrated in the present study may be a consequence of a repeated exposure of high concentrations of immunosuppressive drugs encountered the first hours after medication. Of the 12 patients studied, four were being treated with MMF and one with azathioprin but, since these drugs are purely antiproliferative [21,22], it is not likely that they would influence secretion of IFN-γ.
Although pp65 protein is an important target for both CD4 and CD8 T cells in the majority of individuals [23], the MHC tetramers and the peptide pulsing techniques have their limitations in terms of HLA restriction. Hence, inherent in the use of MHC tetramers loaded with a defined epitope of an antigen, or pulsing T cells with this same peptide, is the failure to detect T lymphocytes restricted to alternative CMV epitopes or to other HLA alleles expressed in a particular individual. Also, in the initial phase of primary infections, polyclonal expansion of antigen-specific T lymphocytes occurs [24]. With time, a selective expansion of epitope-specific T lymphocytes with a high affinity to the T cell receptor (TCR) is usually observed [25,26].
In clinical practice, restriction to a defined immunodominant epitope and a specific HLA allele makes the tetramer and peptide pulsing technique less attractive. These limitations may be overcome by using a combination of several different immunodominant peptides [27]. However, the viral lysate pulsing technique allows the visualization of CMV-specific cells irrespective of tissue type and selected viral epitopes. This is a major advantage in clinical practice and at present the CMV lysate technique seems to be the only one of the three used in this study that could serve as a diagnostic tool.
In this study, CD69 and CD38 were included as T cell activation markers. CD69 is known to be an early activation marker on T lymphocytes that precedes cytokine secretion after antigenic stimulation [28]. In all cases investigated, the vast majority (>90%) of IFN-γ secreting cells were CD69-positive. The results suggest an early and specific up-regulation of CD69 on cells specific for CMV. However, as CD69 up-regulation was found also on CMV tetramer-positive, IFN-γ-negative T cells (data not shown), this early activation marker does not seem to correlate with functional maturation into effector T cells.
Higher frequencies of CD38+, tetramer-positive T lymphocytes in immunosuppressed patients compared to healthy participants have been reported previously [5,7]. In this study, CD38 expression on IFN-γ-secreting T lymphocytes was also higher in immunosuppressed individuals when compared to healthy blood donors. Although the functional role of CD38 is unknown, an increased expression of CD38 on virus-specific T cells has been associated with active viral disease [29–31]. The present findings of increased expression of CD38 on CD4+, CMV-specific T cells, together with a reduced frequency of IFN-γ-secreting pp65-specific CD8+ T cells, may reflect impaired activation of functional cytotoxic T cells controlling latent CMV infection in immunosuppressed patients.
The case report describes a CMV-seropositive patient transplanted with a kidney from a CMV-seropositive donor (Fig. 6). After transplantation and stabilization with immunosuppressive treatment, the patient has the same number of CMV tetramer-positive cells as before treatment with immunosuppressive drugs (2 tetra + cells/µl). This finding is in agreement with tetramer studies on immunosuppressed patients where the number of virus specific cells remains at the same level as non-immunosuppressed controls [5–7].
After CMV reactivation, the number of tetramer-positive cells increases notably (14 tetramer+/µl) and these high numbers persist during the antiviral treatment and suppression of viral load. This may reflect the compensatory proliferation of virus-specific cells during viral replication. Notably, a functional response in terms of IFN-γ secretion after peptide or lysate pulsing has not been seen in this patient, suggesting that this individual may have an impaired ability to keep the CMV infection in a latent stage. However, this finding needs to be confirmed on more patients undergoing primary or reactivated CMV infection before reliable conclusions can be drawn.
In conclusion, the MHC tetramer technique offers a rapid and highly specific assay for the presence of antigen-specific cells. However, in immunosuppressed patients it may be insufficient to detect only the number of CMV reactive cells. From the results presented, it seems equally important to monitor the functional capacity of these cells. It is suggested that the proportion of IFN-γ-secreting tetramer-positive T cells better reflects the antiviral capacity at the individual level than only the number of tetramer positive T cells. However, whether this information may prove useful for identifying patients at risk of reactivated CMV infection/disease remains to be evaluated.
Acknowledgments
The authors wish to thank Mrs Ulrika Johansson and Mrs Selina Bari for excellent technical assistance. This study was supported by grants from the Swedish Medical Research Council (16P-13568 and 16X-12219), the Åke Wiberg Foundation, the Nordic Insulin Fund, the Torsten and Ragnar Söderbergs Foundation, the Ernfors Family Fund, Barn Diabetes Fonden, the Göran Gustafsson Foundation, the Swedish Diabetes Association, the Juvenile Diabetes Foundation International and the Knut and Alice Wallenberg foundation.
References
- 1.Reusser P, Cathomas G, Attenhofer R, Tamm M, Thiel G. Cytomegalovirus (CMV)-specific T cell immunity after renal transplantation mediates protection from CMV disease by limiting the systemic virus load. J Infect Dis. 1999;180:247–53. doi: 10.1086/314879. [DOI] [PubMed] [Google Scholar]
- 2.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 3.Komanduri KV, Viswanathan MN, Wieder ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–6. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 4.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–9. [PubMed] [Google Scholar]
- 5.Engstrand M, Tournay C, Peyrat MA, et al. Characterization of CMVpp65-specific CD8+ T lymphocytes using MHC tetramers in kidney transplant patients and healthy participants. Transplantation. 2000;69:2243–50. doi: 10.1097/00007890-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kostense S, Ogg GS, Manting EH, et al. High viral burden in the presence of major HIV-specific CD8 (+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31(3):677–86. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Hassan-Walker AF, Vargas Cuero AL, Mattes FM, et al. CD8+ cytotoxic lymphocyte responses against cytomegalovirus after liver transplantation: correlation with time from transplant to receipt of tacrolimus. J Infect Dis. 2001;183:835–43. doi: 10.1086/319260. [DOI] [PubMed] [Google Scholar]
- 8.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–50. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura LE, Walker JM, Maecker HT. Optimization of whole blood antigen-specific cytokine assays for CD4 (+) T cells. Cytometry. 2000;40:60–8. doi: 10.1002/(sici)1097-0320(20000501)40:1<60::aid-cyto8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Ghanekar SA, Nomura LE, Suni MA, Picker LJ, Maecker HT, Maino VC. Gamma interferon expression in CD8 (+) T cells is a marker for circulating cytotoxic T lymphocytes that recognize an HLA A2-restricted epitope of human cytomegalovirus phosphoprotein pp65. Clin Diagn Laboratory Immunol. 2001;8:628–31. doi: 10.1128/CDLI.8.3.628-631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279:459–70. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 12.Sester M, Sester U, Gartner B, et al. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71(9):1287–94. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- 13.Turner S, Ellexson ME, Hickman HD, et al. Sequence-based typing provides a new look at HLA-C diversity. J Immunol. 1998;161:1406–13. [PubMed] [Google Scholar]
- 14.Yun Z, Lewensohn-Fuchs I, Ljungman P, Vahlne A. Real-time monitoring of cytomegalovirus infections after stem cell transplantation using the TaqMan polymerase chain reaction assays. Transplantation. 2000;69:1733–6. doi: 10.1097/00007890-200004270-00037. [DOI] [PubMed] [Google Scholar]
- 15.Robbins PA, McMichael AJ. Immune recognition of HLA molecules downmodulates CD8 expression on cytotoxic T lymphocytes. J Exp Med. 1991;173:221–30. doi: 10.1084/jem.173.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyoda M, Carlos JB, Galera OA, et al. Correlation of cytomegalovirus DNA levels with response to antiviral therapy in cardiac and renal allograft recipients. Transplantation. 1997;63:957–63. doi: 10.1097/00007890-199704150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bitsch A, Kirchner H, Dennin R, et al. The long persistence of CMV DNA in the blood of renal transplant patients after recovery from CMV infection. Transplantation. 1993;56:108–13. doi: 10.1097/00007890-199307000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Gratama JW, van Esser JW, Lamers CH, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98:1358–64. doi: 10.1182/blood.v98.5.1358. [DOI] [PubMed] [Google Scholar]
- 19.Aubert G, Hassan-Walker AF, Madrigal JA, et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis. 2001;184:955–63. doi: 10.1086/323354. [DOI] [PubMed] [Google Scholar]
- 20.Gamadia LE, Rentenaar RJ, Baars PA, et al. Differentiation of cytomegalovirus-specific CD8 (+) T cells in healthy and immunosuppressed virus carriers. Blood. 2001;98:754–61. doi: 10.1182/blood.v98.3.754. [DOI] [PubMed] [Google Scholar]
- 21.Malamud D, Gonzalez EM, Chiu H, Malt RA. Inhibition of cell proliferation by azathioprine. Cancer Res. 1972;32:1226–9. [PubMed] [Google Scholar]
- 22.Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51:278–98. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185:1709–16. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 24.Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–8. [PubMed] [Google Scholar]
- 25.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–10. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern F, Faulhaber N, Frommel C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–82. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Picker LJ, Singh MK, Zdraveski Z, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 29.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein–Barr virus in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belles-Isles M, Houde I, Lachance JG, Noel R, Kingma I, Roy R. Monitoring of cytomegalovirus infections by the CD8+CD38+ T cell subset in kidney transplant recipients. Transplantation. 1998;65:279–82. doi: 10.1097/00007890-199801270-00026. [DOI] [PubMed] [Google Scholar]
- 31.Benz C, Utermohlen O, Wulf A, et al. Activated virus-specific T cells are early indicators of anti-CMV immune reactions in liver transplant patients. Gastroenterology. 2002;122:1201–15. doi: 10.1053/gast.2002.33021. [DOI] [PubMed] [Google Scholar]