Abstract
Human red cells (RBC) coated with IgG anti-D are cleared from the circulation to the spleen by macrophages which express IgG receptors (Fcγ R). Polymorphisms of Fcγ RIIa and Fcγ RIIIa affect IgG binding in vitro, and may alter the efficiency of clearance of immune complexes in vivo. In a RBC clearance study, 22 Rh D-negative subjects were given 100–400 µg human monoclonal or polyclonal IgG anti-D i.m. followed 48 h later by 51Cr-labelled D+ RBC. The half lives of the infused D+ RBC were determined, together with the coating levels of anti-D on the D+ RBC. Fcγ RIIA and FcγRIIIA genotyping was performed. Large ranges of phagocytosis and extracellular lysis of RBC in vitro, and of half lives of RBC in vivo, were observed. Clearance of RBC coated with monoclonal IgG3 anti-D (BRAD-3) was more rapid in five subjects homozygous for Fcγ RIIIa-F/F158 than in three subjects expressing the Fcγ RIIIa-V158 allele (P = 0·024). This effect was not observed, however, for those individuals given polyclonal anti-D. There was also no significant difference in the efficiency of RBC destruction in vitro or of RBC clearance in vivo between the subjects analysed for individual genotypes or alleles or combinations of alleles. In conclusion, the presence of the Fcγ RIIIa-V158 allele compromised the efficiency of removal of RBC coated with IgG3 anti-D.
Keywords: IgG Fc receptor (Fcγ R), Fcγ R polymorphisms, anti-D, red blood cells (RBC), clearance of RBC
Introduction
Human red blood cells (RBC) coated with IgG anti-D (EA-IgG) are removed from the circulation by the spleen [1] following their interaction with mononuclear phagocytic cells. Macrophages express three classes of IgG receptors, Fcγ RI, Fcγ RIIa and Fcγ RIIIa [2]. Any or all of these FcγR could, in theory, participate in the interaction with the opsonized RBC. Infusion of the anti-Fcγ RIIIa antibody, 3G8, to a patient with severe refractory immune thrombocytopenic purpura caused impaired clearance of IgG-sensitized RBC, a doubling of the half life, as well as a dramatic though transient increase in platelet counts [3]. These workers also found clearance of IgG-coated RBC in chimpanzees (using chimpanzee alloantibodies) to be greatly reduced by prior administration of 3G8 [4]. Human spleen cryostat sections bound EA-IgG solely by Fcγ RIIIa, again being inhibited by 3G8 [5]. Although FcγRIIa may also be blocked by 3G8, by the Fc after the Fab has bound to Fcγ RIIIa, it is likely that macrophage Fcγ RIIIa is the main, or primary, receptor for IgG anti-D opsonized RBC in the spleen, resulting in their removal from the circulation [6].
Fcγ RI (CD64) is a nonpolymorphic high affinity receptor which is normally occupied by cytophilic IgG, preventing interaction with immune complexes unless it is displaced, when efficient binding, phagocytosis and extracellular lysis of opsonized cells occurs.
Fcγ RIIa (CD32) represents a low affinity receptor, binding immune complexes or cell-bound IgG. FcγRIIa contains a polymorphism and the allotype Fcγ RIIa-H131 was found to bind human IgG2, unlike Fcγ RIIa-R131 [7]. Fcγ RIIa has a higher affinity for IgG3 than IgG1 [8]. Cells expressing Fcγ RIIa-H131 were shown to mediate greater EA-rosette formation with RBC coated with high levels (100 000 IgG/RBC) of human monoclonal IgG1 or IgG3 anti-D than Fcγ RIIa-R131 transfectants. Moreover, cells expressing any of the Fcγ RIIa allotypes bound more EA-IgG3 than EA-IgG1 [9]. Similar data was obtained with neutrophils, although blocking experiments indicated Fcγ RIIIb contributed to binding of EA-IgG3 [10]. Phagocytosis of target RBC was mediated solely by Fcγ RIIa-H131 transfectants towards EA-IgG3, and only to a modest degree even at high levels of sensitization [11]. At physiologic levels of opsonization, EA-IgG3 but not EA-IgG1 (at 20 000 IgG/RBC) bound to K562 cells (expressing Fcγ RIIa) [12], whereas EA-IgG1 (at 13 000 IgG/RBC) were lysed by monocytes (Fcγ RI+, Fcγ RIIa+) solely through Fcγ RI [9]. Thus it is probable that only EA-IgG3 at high (nonphysiological) sensitization levels of anti-D may interact with Fcγ RIIa.
Fcγ RIIIa (CD16) is also polymorphic at residue 158 in the membrane-proximal domain [13]. This was later found to influence binding of IgG. Fcγ RIIIa effectively binds complexed IgG and has some affinity for monomeric IgG. NK cells (which express Fcγ RIIIa) from FcγRIIIa-V/V158 individuals had more cytophilic IgG than those from Fcγ RIIIa-F/F158 subjects, and bound more IgG1, IgG3 and IgG4 [14]. Similarly, monocytes from Fcγ RIIIa-V/V158 homozygotes bound more IgG1 than monocytes from Fcγ RIIIa-F/F158 individuals [15]. It is possible that the presence of cytophilic IgG could obstruct interaction of the receptor with immune complexes or opsonized cells. Alternatively, the Fcγ RIIIa-V158 allotype with higher affinity for IgG may more efficiently clear immune complexes. Fcγ RIIIa expressed on NK cells and monocytes are differently glycosylated, with only the former having high mannose-type oligosaccharides, which appears to confer a lower affinity for IgG on monocyte (macrophage) Fcγ RIIIa. Whereas NK cell Fcγ RIIIa was blocked by 2 mg/ml IgG, monocyte FcγRIIIa was only partially (30%) blocked at this IgG concentration, which is below normal serum levels [16]. This may enable it to efficiently interact with immune complexes. Thus clearance of EA-IgG (anti-D) in vivo, which is most likely to occur predominantly through monocyte/macrophage Fcγ RIIIa, may be influenced by the polymorphism of this receptor.
The ability of human monoclonal and polyclonal anti-D to mediate clearance of D-positive (D+) RBC transfused into D-negative subjects has been compared [17], as part of a study of the ability of passive monoclonal anti-D to prevent immunization to D+ RBC. Anti-D was injected i.m. two days before infusion of 51Cr-labelled D+ RBC; in this situation the rate of removal of the cells is slower than when RBC are opsonized in vitro before infusion [18]. The number of molecules of anti-D on the RBC was determined by flow cytometry [19], and the ability of the subjects’ monocytes and NK cells to phagocytose and lyse EA-IgG was assessed in in vitro phagocytosis assays and antibody dependent cell-mediated cytotoxicity (ADCC) assays, respectively. A wide range of clearance rates and functional activities were observed in the subjects. Therefore the role of the polymorphisms of Fcγ RIIa and Fcγ RIIIa on the clearance of EA-IgG was analysed.
Materials And Methods
Red cell clearance study
Twenty-two D-negative male volunteers were injected i.m. with 100–400 µg of anti-D (human monoclonal anti-D produced by EBV-transformed B cell lines, BRAD-3 (IgG3) and BRAD-5 (IgG1), or polyclonal IgG anti-D immunoglobulin). Two days later, 3 ml 51Cr-labelled D+ RBC were injected i.v. and samples taken at 3 min and 1, 3, 5, 24, 48 and 72 h for gamma counting and estimation of RBC half life (t50%) and clearance rate (0·693/t50%) [17]. The number of molecules of anti-D on the D+ RBC in samples taken 3 h after injection was determined by flow cytometry for 14 of these subjects [19]. The study was approved by the local Research and Ethics committees and the volunteers gave written informed consent.
Monocyte EA-rosette/phagocytosis assay
Peripheral blood mononuclear cells were isolated from blood taken from the volunteers just prior to the injection of RBC and 24, 48, 72 h and 8 months later, incubated for 1 h at 37°C in wells of a 24-well plate and nonadherent cells removed by washing. D+ RBC were sensitized with serial dilutions of anti-D, washed and adjusted to 1% and 50 µl EA-IgG added to the wells. Unsensitized D+ and D-negative RBC were also used. To standardize the assay, the target D+ red cells were from a single donor (group O R1R2) and the anti-D serum (single donation) was stored frozen in aliquots. After incubation for 2 h at 37°C, cells were fixed in 1% glutaraldehyde, rinsed and stained with 0·4% Trypan Blue. The percentage of monocytes with adherent or phagocytosed RBC was determined microscopically.
NK Cell ADCC
Peripheral blood mononuclear cells were isolated from the same samples of blood that were taken for the monocyte assay, and depleted of adherent cells by incubation for 1 h at 37°C in plastic flasks. The nonadherent cells (6 × 105/well in medium with 10% human AB serum) were incubated in triplicate in U-well microplates with 51Cr-labelled papainized D+ RBC (4 × 104/well) for 16 h at 37°C in the presence or absence of anti-D [20]. The RBC and anti-D were those used for the monocyte assay above. The anti-D was used neat and at four 10-fold dilutions. Maximum lysis was achieved by addition of 1% Triton X-100. Radioactivity was determined in aliquots of supernatants, and the percentage haemolysis calculated:
FcγRIIA and FcγRIIIA genotyping
This was performed by allele-specific polymerase chain reaction genotyping methods [14,21] using DNA prepared from EBV-transformed B cell lines from the subjects. The specificities of the genotyping assays were confirmed by direct sequencing.
Results
Data on the subjects studied, the dose and type of anti-D injected, FcγRIIA and FcγRIIIA genotypes, in vitro monocyte and NK cell function at the time of RBC infusion, RBC half lives in vivo, coating levels of IgG anti-D on the D+ RBC ex vivo, and the RBC clearance rate with respect to bound IgG are given in Table 1.
Table 1.
Experimental and clinical data
| Subject number | Anti-D, dose (µg) | FcγRIIA-131 | Fcγ RIIIA-158 | % monocyte phagocytosis | % NK haemolysis | RBC half life, t50% (hours) | Molecules IgG/RBC | Clearance rate/molecules bound (×10−5) |
|---|---|---|---|---|---|---|---|---|
| 1 | BRAD-3 (100) | RH | FF | 4 | 92 | 41·0 | nd | nd |
| 2 | BRAD-3 (200) | RR | FF | 16 | 82 | 14·6 | nd | nd |
| 4 | BRAD-3 (300) | RH | FF | 28 | 104 | 9·9 | 3 600 | 1·94 |
| 6* | BRAD-3 (300) | RR | FF | 7 | 100 | 28·1 | 1 500 | 1·67 |
| 8* | BRAD-3 (300) | RR | FF | 5 | 81 | 8·7 | 8 000 | 1·00 |
| 9 | BRAD-3 (300) | RH | FF | 39 | 93 | 2·5 | 10 000 | 2·77 |
| 10 | BRAD-3 (300) | HH | FF | 41 | 100 | 10·9 | 3 800 | 1·68 |
| 11* | BRAD-3 (300) | RR | FF | 27 | nd | 4·4 | 4 300 | 3·67 |
| 17 | BRAD-5 (300) | RR | FF | 36 | 95 | 8·7 | 2 700 | 2·96 |
| 21* | Polyclonal (100) | RH | FF | 35 | 80 | 6·9 | nd | nd |
| 23 | Polyclonal (100) | RH | FF | 14 | 76 | 6·6 | nd | nd |
| 24 | Polyclonal (100) | RR | FF | 21 | 62 | 1·2 | nd | nd |
| 25 | Polyclonal (100) | RR | FF | nd | nd | 3·8 | 4 000 | 4·55 |
| 26 | Polyclonal (100) | RR | FF | 64 | 77 | 2·8 | 5 600 | 4·43 |
| 27 | Polyclonal (100) | RH | FF | 21 | 88 | 9·7 | 3 000 | 2·37 |
| 28 | BRAD-3 + 5 (400) | HH | FF | 20 | 107 | 2·7 | 9 300 | 2·76 |
| 29 | BRAD-3 + 5 (400) | RH | FF | 13 | 82 | 32·1 | 5 000 | 0·43 |
| 3 | BRAD-3 (200) | RH | VV | 41 | 87 | 26·7 | nd | nd |
| 20 | Polyclonal (100) | RR | VV | 34 | 72 | 2·3 | nd | nd |
| 5 | BRAD-3 (300) | RH | VF | 36 | 97 | 24·2 | 3 400 | 0·85 |
| 7 | BRAD-3 (300) | HH | VF | 16 | 67 | 12·9 | 3 000 | 1·80 |
| 22 | Polyclonal (100) | RH | VF | 48 | 92 | 6·7 | nd | nd |
HLA B8 DR3 (DR17). nd, not determined. Subject numbers taken from [17]. The figures for percentage phagocytosis and haemolysis are the results obtained using anti-D at 1/2 and 1/10 dilutions in the assays, respectively.
FcγRIIa and FcγRIIIa genotype and allele frequencies
More subjects were homozygous for the FcγRIIA-R/R131 genotype (41%) than the H/H131 genotype (14%) and the allele frequencies were 0·63 (R131) and 0·37 (H131). For the Fcγ RIIIA polymorphism, there were 9% and 77% of subjects homozygous for the V/V158 and F/F158 genotypes, respectively, and the allele frequencies were 0·16 (V158) and 0·84 (F158).
No effect of FcγRIIa and FcγRIIIa genotypes on monocyte and NK cell function
The results for monocyte and NK cell function and RBC half lives are summarized in Table 2. In general, monocyte and NK cell function of the subjects remained relatively constant both during the clearance study and several months later. Data from the subject groups were analysed by the unpaired t-test. There were no significant differences in mean values of in vitro EA-IgG phagocytosis by monocytes or lysis by NK cells or in vivo RBC clearance between any of the groups of subjects, either homozygous or heterozygous for the Fcγ RIIa or Fcγ RIIIa polymorphisms. Although the mean percentage phagocytosis by monocytes appeared to vary with respect to the Fcγ RIIIa-V/F158 genotype, this was not significant and was consistent with the involvement of only Fcγ RI in this assay. Although EA-IgG were lysed by NK cells through interactions with Fcγ RIIIa, there was little variation in the percentage haemolysis between subjects. The numbers of subjects with Fcγ RIIa-H/H131, Fcγ RIIIa-V/V158 and Fcγ RIIIa-V/F158 unfortunately were too small for meaningful statistical analysis. Due to the unequal distribution of genotypes, multiple comparisons were not performed. In addition, there was no difference in in vitro functional activity or the rate of red cell clearance in vivo between the subjects grouped according to their Fcγ RIIa allotype, or combinations of Fcγ RIIa and Fcγ RIIIa haplotypes.
Table 2.
Analysis of experimental and clinical data with respect to FcγRIIA and FcγRIIIA genotypes and alleles of the subjects
| FcγRIIA genotype or allele | FcγRIIIA genotype or allele | Combinations of FcγRIIA and FcγRIIIA alleles | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R/R 131 | R/H 131 | H/H 131 | R 131 | H 131 | V/V 158 | V/F 158 | F/F 158 | V 158 | F 158 | R 131 V 158 | R 131 F 158 | H 131 V 158 | H 131 F 158 | |
| Number of subjects | 9 | 10 | 3 | 19 | 13 | 2 | 3 | 17 | 5 | 20 | 4 | 17 | 4 | 12 |
| (% of total) | (41) | (45) | (14) | (86) | (59) | (9) | (14) | (77) | (23) | (91) | (18) | (77) | (18) | (55) |
| % phagocytosis by monocytes | ||||||||||||||
| Mean | 26·3 | 27·9 | 25·7 | 27·2 | 27·4 | 37·5 | 33·3 | 24·4 | 35·0 | 25·8 | 39·8 | 25·9 | 35·3 | 26·3 |
| SEM | 6·7 | 4·5 | 7·8 | 3·8 | 3·8 | 3·5 | 9·3 | 4·0 | 5·3 | 3·6 | 3·1 | 6·9 | 6·9 | 3·9 |
| % haemolysis by NK cells | ||||||||||||||
| Mean | 81·3 | 89·1 | 91·3 | 85·9 | 89·6 | 79·5 | 85·3 | 87·9 | 83·0 | 87·5 | 87·0 | 86·7 | 85·8 | 89·8 |
| SEM | 4·9 | 2·6 | 12·3 | 2·6 | 3·2 | 7·5 | 9·3 | 3·2 | 5·8 | 3·0 | 5·4 | 2·8 | 6·6 | 3·4 |
| Half life, t50% | ||||||||||||||
| Mean | 8·3 | 16·6 | 8·8 | 12·7 | 14·8 | 14·5 | 14·6 | 10·2 | 14·6 | 10·9 | 15·0 | 12·5 | 17·6 | 13·8 |
| SEM | 2·9 | 4·2 | 3·1 | 2·7 | 3·4 | 12·2 | 5·2 | 2·6 | 4·8 | 2·3 | 6·1 | 2·8 | 4·7 | 3·5 |
Association between expression of the FcγRIIIa-V158 allele and reduced in vivo RBC clearance of EA-IgG3
Fcγ RIIIa has been proposed the primary receptor utilized in clearance or haemolysis of EA-IgG, and polymorphisms may influence either of these functions. When comparing all subjects for Fcγ RIIIa genotype or allele using the unpaired t-test, there were no significant differences in RBC half lives (Table 2). In contrast, it was observed that in subjects given BRAD-3 (monoclonal IgG3 anti-D) the RBC half lives were significantly different when categorizing the subjects into two groups, those who were homozygous for Fcγ RIIIa-F/F158 (five individuals) and those who expressed the Fcγ RIIIa-V158 allele (three individuals). Clearance of RBC was observed to be more rapid in the Fcγ RIIIa-F/F158 homozygotes (Fig. 1). Three subjects (numbers 1, 6 and 8) given BRAD-3 were excluded from this analysis; their monocytes exhibited very low phagocytic ability (4%, 7% and 5% of their monocytes phagocytosed EA-IgG in vitro) compared to the eight included subjects (mean 30·5%). Subjects 6 and 8 with low phagocytosis expressed the HLA haplotype B8 DR3 (DR17) (Table 1) [17], and it is known that some individuals with this haplotype exhibit reduced monocyte phagocytosis [22] and slower clearance [23] of anti-D-coated RBC. Although subject 11 was also HLA B8 DR3 (DR17), phagocytosis by his monocytes was within the range of the other included subjects. In addition, subject 1 was given a low dose of anti-D and had a markedly low plasma concentration of anti-D (1·8 ng/ml) [17] compared to a mean of 10·65 ng/ml (range 4·9–16·4 ng/ml) [17] for the eight included subjects, and this would have severely restricted the amount of anti-D bound to the infused D+ RBC. The mean RBC half life was observed to be 8·5 ± 2·2 h for the five included Fcγ RIIIa-F/F158 subjects, and 21·3 ± 4·2 h for the three possessing the Fcγ RIIIa-V158 allele (P = 0·024). This difference was not attributable to variation in coating levels of BRAD-3 on the RBC in vivo, as the mean clearance rate/molecules of anti-D bound was not different between the two groups (2·5 ± 0·5, and 1·3 ± 0·5, respectively, P = 0·181). The in vitro functional activities of the subjects were also similar.
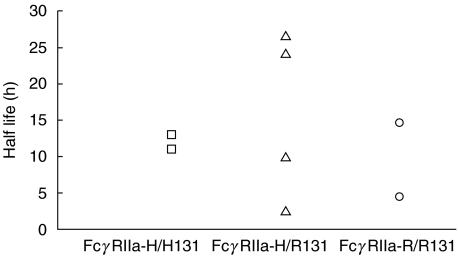
Fig. 1.
Half lives of RBC after injection of BRAD-3 in eight subjects, stratified according to FcγRIIIA genotypes. Subject 3 (▪, -V/V158), subjects 5 and 7 (▴, -V/F158) and subjects 2, 4, 9, 10 and 11 (•, -F/F158).
Polymorphisms of FcγRIIa do not affect RBC clearance rates
When the subjects shown in Fig. 1 were grouped according to their FcγRIIA genotypes, no difference was apparent in the half lives of RBC in vivo (Fig. 2).
Fig. 2.
Half lives of RBC after injection of BRAD-3 in eight subjects, stratified according to FcγRIIA genotypes. Subjects 7 and 10 (□, -H/H131), subjects 3, 4, 5 and 9 (▵, -H/R131) and subjects 2 and 11 (○, -R/R131).
Comparison of RBC destruction in vitro and in vivo
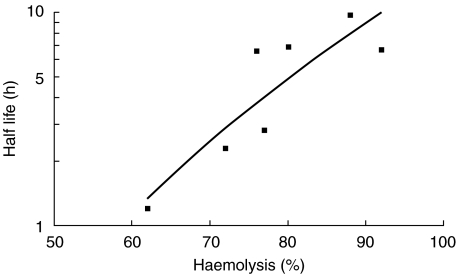
Large differences in RBC half life were obtained between subjects with the same FcγRIIIA genotype, but there was less variation with haemolysis in vitro. It was observed, however, that when comparing one group of subjects, those given polyclonal anti-D (subjects 20–27, Table 1), there was a correlation between the half life of EA-IgG in vivo and the NK cell-mediated haemolysis of EA-IgG in vitro elicited by polyclonal anti-D (Fig. 3).
Fig. 3.
Relation between haemolysis of EA-IgG by NK cells in vitro and clearance of EA-IgG in vivo by seven subjects (20, 21, 22, 23, 24, 26, 27) injected with polyclonal anti-D. Slope R2 = 0·7631.
Discussion
The genotypes of both FcγRIIA and FcγRIIIA in this small sample of Caucasians from the south-west of England differed slightly in frequency from most published studies. There was an increased representation of the FcγRIIa-R/R131 and FcγRIIIa-F/F158 genotypes, although when larger numbers of subjects from this region were genotyped, results were similar to previous reports. The FcγRIIA genotypes (33% R/R131 and 16% H/H131) and allele frequencies (0·59 R131, 0·41 H131) of 78 subjects were slightly outside the range of genotypes (16–32% R/R131 and 18–29% H/H131) and allele frequencies (0·42–0·59 R131 and 0·45–0·58 H131) found in 10 other studies [24–33]. The FcγRIIIA genotypes (9% V/V158 and 59% F/F158) and allele frequencies (0·25 V158, 0·75 F158) of 32 subjects were similar to published data (7–13% V/V158, 26–66% F/F158, 0·33–0·44 V158, 0·56–0·62 F158) [14, 15, 33, 34].
The situation whereby allogeneic D+ RBC are cleared from the circulation after binding passive anti-D in vivo is more complex than binding of IgG to isolated leucocytes under experimental conditions. Many factors may affect the overall clearance observed, including the amount of IgG on the RBC [35,36], the expression of FcγRIIa and FcγRIIIa [37] and regulation of adhesion molecules by cytokines [38]. Furthermore, the nature of the anti-D may influence interaction with FcγR as, for example, monoclonal IgG1 anti-D's differ in their capacity to promote clearance [39]. The type of clearance study may determine the outcome; antibodies that promote clearance when bound to RBC in vitro before injection may not do so when injected i.m., which was the procedure used here. For instance, BRAD-3 cleared D+ RBC more rapidly than polyclonal anti-D when precoated autologous RBC were infused into D+ subjects [36] but more slowly when injected i.m. into D-negative subjects two days before D+ RBC [17]. In these reports, removal of RBC from the circulation started within minutes or hours, respectively. The clearance study analysed here (RBC and anti-D injected separately) is not strictly comparable to most previous ones when RBC precoated with polyclonal anti-D were infused [23, 33, 37]. However, it more closely represents the clinical situation whereby passive anti-D is given to D-negative women to prevent immunization to fetal D+ RBC [39].
The lack of association of FcγRIIA genotypes or alleles with clearance of RBC coated with anti-D found in our study may be because there is little interaction of these cells with Fcγ RIIa+ effector cells [11]. At physiological levels of opsonization (10 000–20 000 IgG/cell) only about 5% of monocyte phagocytosis of EA-IgG3 was shown to be a result of activation of Fcγ RIIa, while this receptor was not utilized for interaction with EA-IgG1 anti-D [40]. In contrast to our data obtained with normal subjects (Fig. 2), a small association between polymorphisms of Fcγ RIIa and clearance of cells precoated with anti-D has been reported in patients with SLE [33].
The use of monoclonal IgG3 anti-D (BRAD-3) in some subjects may have presented a more sensitive test of the roles of FcγR polymorphisms than that of polyclonal anti-D, which comprises both IgG1 and IgG3, but predominantly IgG1. This is because IgG1 and IgG3 anti-D display a dichotomy of functional activity in vitro. In rosette assays, BRAD-3 was found more efficient than IgG1 or polyclonal anti-D at mediating binding of RBC to to Fcγ RI on monocytes [12], to Fcγ RIIa on K562 cells or transfectants [9] and to Fcγ RIIIa on splenic macrophages [41] or NK cells [42]. However, BRAD-3 mediated little haemolysis by Fcγ RIIIa+ NK cells in ADCC assays, in contrast to polyclonal anti-D or some IgG1 monoclonal anti-D [17, 41, 43]. The initial step in splenic clearance of RBC may be adherence of EA-IgG to Fcγ RIIIa on macrophages, because the high affinity Fcγ RI would be blocked in vivo by cytophilic IgG [6]. After binding of EA-IgG to macrophages cytophilic IgG could be displaced from Fcγ RI allowing signalling through this receptor. If binding of EA-IgG to Fcγ RIIIa is compromised, and because EA-IgG3 signal poorly through Fcγ RIIIa, the net result may be slow clearance of EA-IgG3 from the circulation. This may be the explanation why polymorphisms of Fcγ RIIIa were seen to influence clearance of RBC by BRAD-3 but not by polyclonal anti-D.
The observed association of reduced clearance of RBC mediated by IgG3 anti-D with expression of the Fcγ RIIIa-V158 allele may well have clinical relevance. It has been documented that donors whose NK cells readily bind IgG express this allele, whereas donors with a low binding phenotype are F/F homozygotes [14,15]. Furthermore, patients with systemic lupus erythematosus (SLE) and haematologic cytopenias were more likely to have the Fcγ RIIIa-F/F158 genotype, i.e. expression of the V158 allele appeared to confer protection from autoantibody mediated cell clearance and destruction [33]. In contrast, fewer patients with idiopathic thrombocytopenic purpura (ITP) had the Fcγ RIIIa-F/F158 genotype [34]. Even if Fcγ RIIIa is not the sole FcγR recruited for EA–IgG interactions, any reduction in the ability of Fcγ RIIIa to bind EA-IgG may affect the total response achieved. The evidence so far suggests that NK cells, monocytes and macrophages expressing Fcγ RIIIa-V158 may bind more monomeric cytophilic IgG, blocking the receptor, and partially preventing interactions with IgG-coated cells. In contrast, macrophages expressing Fcγ RIIIa-F/F158 may have unoccupied receptors and may therefore be able to bind immune complexes more readily than those expressing Fcγ RIIIa-V/V158, leading to an increased ability to bind and clear these immune complexes and opsonized particles. This hypothesis is supported by results of the clearance study reported here showing that subjects expressing the Fcγ RIIIa-V158 allele removed RBC coated with IgG3 anti-D from the circulation more slowly than individuals homozygous for Fcγ RIIIa-F/F158.
These findings are, however, unlikely to affect the design of monoclonal anti-D for Rh D prophylaxis to prevent Rh D immunization of Rh D-negative women and subsequent haemolytic disease of the newborn. A 3 : 1 blend of BRAD-5:BRAD-3 has an IgG anti-D subclass profile of 75% IgG1 and 25% IgG3, approximating that of polyclonal anti-D [43] where IgG3 is only a minor component. This mixture of monoclonal antibodies has shown efficacy at preventing Rh D immunization in a large multicentre study [44,45] where the FcγRIIIa-V/F158 genotypes of the recipients were unknown.
Acknowledgments
We are grateful to Nomdo Westerdaal (Department of Immunology, University Medical Centre Utrecht) for genotyping some samples.
References
- 1.Hughes-Jones NC, Mollison PL, Veall N. Removal of incompatible red cells by the spleen. Br J Haematol. 1957;3:125–33. doi: 10.1111/j.1365-2141.1957.tb05779.x. [DOI] [PubMed] [Google Scholar]
- 2.van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity; molecular aspects and clinical implications. Immunol Today. 1993;14:215–21. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson SB, Bussel JB, Kimberly RP, Valinsky JE, Nachman RL, Unkeless JC. Treatment of refractory immune thrombocytopenic purpura with an anti-Fcγ-receptor antibody. New Engl J Med. 1986;314:1236–9. doi: 10.1056/NEJM198605083141907. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson SB, Kimberly RP, Valinsky JE, Witmer MD, Bussel JB, Nachman RL, Unkeless JC. Blockade of clearance of immune complexes by an anti-Fcγ receptor monoclonal antibody. J Exp Med. 1986;164:474–89. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport RD, Kunkel SL. IgG receptor roles in red cell binding to monocytes and macrophages. Transfusion. 1994;34(Suppl.):79S. [Google Scholar]
- 6.Engelfriet CP. The immune destruction of red cells. Transfus Med. 1992;2:1–6. doi: 10.1111/j.1365-3148.1992.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Warmerdam PAM, van de Winkel JGJ, Vlug A, Westerdaal NAC, Capel PJA. A single amino acid in the second Ig-like domain of the human Fcγ receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–43. [PubMed] [Google Scholar]
- 8.Parren PWHI, Warmerdam PAM, Boeije LCM, et al. On the interaction of IgG subclasses with the low affinity Fcγ RIIa (CD32) on human monocytes, neutrophils and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992;90:1537–46. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumpel BM, van de Winkel JGJ, Westerdaal NAC, Hadley AG, Dugoujon JM, Blancher A. Antigen topography is critical for interaction of IgG2 anti-red cell antibodies with Fcγ receptors. Br J Haematol. 1996;94:175–83. doi: 10.1046/j.1365-2141.1996.d01-1764.x. [DOI] [PubMed] [Google Scholar]
- 10.Bredius RGM, Fijen CAP, de Haas M Kuiper EJ, Weening RS, van de Winkel JGJ, Out TA. Role of neutrophil Fcγ RIIa (CD32) and Fcγ RIIIB (CD16) polymorphic forms in phagocytosis of human IgG1-and IgG3-opsonized bacteria and erythrocytes. Immunology. 1994;83:624–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Wiener E, Dellow RA, Mawas F, Rodeck CH. Role of Fcγ RIIa (CD32) in IgG anti-RhD-mediated red cell phagocytosis in vitro. Transfus Med. 1996;6:235–41. doi: 10.1111/j.1365-3148.1996.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumpel BM, Hadley AG. Functional interactions of red cells sensitised by IgG1 and IgG3 human monoclonal anti-D with enzyme-modified human monocytes and FcR-bearing cell lines. Mol Immunol. 1990;27:247–56. doi: 10.1016/0161-5890(90)90137-o. [DOI] [PubMed] [Google Scholar]
- 13.Ravetch JV, Perussia B. Alternative membrane forms of Fcγ RIII (CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AEGKr, de Haas M. Fcγ RIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fcγ RIIIa, independently of the Fcγ RIIIa-48L/R/H phenotype. Blood. 1997;90:1109–14. [PubMed] [Google Scholar]
- 15.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of Fcγ RIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edberg JC, Kimberley RP. Cell type-specific glycoforms of Fcγ RIIIa (CD16). Differential ligand binding. J Immunol. 1997;159:3849–57. [PubMed] [Google Scholar]
- 17.Kumpel BM, Goodrick MJ, Pamphilon DH, et al. Human Rh D monoclonal antibodies (BRAD-3 and BRAD-5) cause accelerated clearance of Rh D+ red blood cells and suppression of Rh D immunization in Rh D– volunteers. Blood. 1995;86:1701–9. [PubMed] [Google Scholar]
- 18.Chapman GE. A pharmacokinetic/pharmacodynamic model for the action of anti-D immunoglobulin in effecting circulatory clearance of Rh D+ red cells. Transfus Med. 1996;6:227–33. doi: 10.1111/j.1365-3148.1996.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumpel BM, Judson PA. Quantification of IgG anti-D bound to D-positive red cells infused into D-negative subjects after intramuscular injection of monoclonal anti-D. Transfus Med. 1995;5:105–12. doi: 10.1111/j.1365-3148.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumpel BM, Leader KA, Merry AH, et al. Heterogeneity in the ability of IgG1 monoclonal anti-D to promote lymphocyte-mediated red cell lysis. Eur J Immunol. 1989;19:2283–8. doi: 10.1002/eji.1830191216. [DOI] [PubMed] [Google Scholar]
- 21.Flesch BK, Bauer F, Neppert J. Rapid typing of the human Fcγ receptor IIA polymorphism by polymerase chain reaction amplification with allele-specific primers. Transfusion. 1998;38:174–6. doi: 10.1046/j.1537-2995.1998.38298193100.x. [DOI] [PubMed] [Google Scholar]
- 22.Salmon JE, Kimberly RP, Gibofsky A, Fotino M. Altered phagocytosis by monocytes from HLA-DR2 and DR3-positive healthy adults is Fcγ receptor specific. J Immunol. 1986;136:3625–30. [PubMed] [Google Scholar]
- 23.Lawley TJ, Hall RP, Fauci AS, Katz SI, Hamburger MI, Frank MM. Defective Fc-receptor functions associated with the HLA-B8/DRw3 haplotype; studies in patients with Dermatitis Herpetiformis and normal subjects. N Engl J Med. 1981;304:185–92. doi: 10.1056/NEJM198101223040401. [DOI] [PubMed] [Google Scholar]
- 24.Sanders LAM, van de Winkel JGJ, Rijkers GT, Voorhorst-Ogink MM, de Haas M, Capel PJA, Zegers BJM. Fcγ receptor IIa (CD32) heterogeneity in patients with recurrent bacterial respiratory tract infections. J Infect Dis. 1994;170:854–61. doi: 10.1093/infdis/170.4.854. [DOI] [PubMed] [Google Scholar]
- 25.Osborne JM, Chacko GW, Brandt JT, Anderson CL. Ethnic variation in frequency of an allelic polymorphism of human Fcγ RIIA determined with allele specific oligonucleotide probes. J Immunol Meths. 1994;173:207–17. doi: 10.1016/0022-1759(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 26.Duits AJ, Bootsma H, Derksen RHWM, et al. Skewed distribution of IgG Fc receptor IIA (CD32) polymorphism is associated with renal disease in systemic lupus erythematosus patients. Arthritis Rheum. 1996;97:1348–54. doi: 10.1002/art.1780381217. [DOI] [PubMed] [Google Scholar]
- 27.Botto M, Theodoridis E, Thompson EM, Beynon HLC, Briggs D, Isenberg DA, Walport MJ, Davies KA. Fcγ RIIa polymorphism in systemic lupus erythematosus (SLE): no association with disease. Clin Exp Immunol. 1996;104:264–8. doi: 10.1046/j.1365-2249.1996.33740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmon JE, Millard S, Schachter LA, et al. Fcγ RIIA alleles are heritable risk factors for lupus nephritis in African Americans. J Clin Invest. 1996;97:1348–54. doi: 10.1172/JCI118552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arepally G, McKenzie SE, Jiang X-M, Poncz M, Cines DB. Fcγ RIIA H/R131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis. Blood. 1997;89:370–5. [PubMed] [Google Scholar]
- 30.Smyth LJ, Snowden N, Carthy D, Papasteriades C, Hajeer A, Ollier WE. Fcγ RIIa polymorphism in systemic lupus erythematosus. Ann Rheum Dis. 1997;56:744–6. doi: 10.1136/ard.56.12.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joutsi L, Javela K, Partanen J, Kekomaki R. Genetic polymorphism H131R of Fcγ receptor type IIA (Fcγ RIIA) in a healthy Finnish population and in patients with or without platelet-associated IgG. Eur J Haematol. 1998;61:183–9. doi: 10.1111/j.1600-0609.1998.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 32.Manger K, Repp R, Spriewald BM, et al. Fcγ RIIa polymorphism in Caucasian patients with systemic lupus erythematosus: association with clinical symptoms. Arthritis Rheum. 1998;41:1181–9. doi: 10.1002/1529-0131(199807)41:7<1181::AID-ART6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 33.Dijstelbloem HM, Bijl M, Fijnheer R, et al. Fcγ receptor polymorphisms in systemic lupus erythematosus. Arthritis Rheum. 2000;43:2793–800. doi: 10.1002/1529-0131(200012)43:12<2793::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto T-T, Inoue M, Shimomura T, Fujimura K. Involvement of Fcγ receptor polymorphism in the therapeutic response of idiopathic thrombocytopenic purpura. Br J Haematol. 2001;115:125–30. doi: 10.1046/j.1365-2141.2001.03109.x. [DOI] [PubMed] [Google Scholar]
- 35.Mollison PL, Crome P, Hughes-Jones NC, Rochna E. Rate of removal from the circulation of red cells sensitized with different amounts of antibody. Br J Haematol. 1965;11:461–70. doi: 10.1111/j.1365-2141.1965.tb06609.x. [DOI] [PubMed] [Google Scholar]
- 36.Thomson A, Contreras M, Gorick B, et al. Clearance of Rh D-positive red cells with monoclonal anti-D. Lancet. 1990;336:1147–50. doi: 10.1016/0140-6736(90)92767-c. [DOI] [PubMed] [Google Scholar]
- 37.Seres T, Csipo I, Kiss E, Szegedi G, Kavai M. Correlation of Fcγ receptor expression of monocytes with clearance function by macrophages in systemic lupus erythematosus. Scand J Immunol. 1998;48:307–11. doi: 10.1046/j.1365-3083.1998.00383.x. [DOI] [PubMed] [Google Scholar]
- 38.Palermo MS, Alves Rosa F, Fernandez Alonso G, Isturiz MA. Fcγ receptor-dependent clearance is enhanced following lipopolysaccharide in vivo treatment. Immunology. 1997;92:536–43. doi: 10.1046/j.1365-2567.1997.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumpel BM, Elson CJ. Mechanism of anti-d-mediated immune suppression – a paradox awaiting resolution? Trends Immunol. 2001;22:26–31. doi: 10.1016/s1471-4906(00)01801-9. [DOI] [PubMed] [Google Scholar]
- 40.Kumpel BM, Beliard R, Brossard Y, et al. Section 1C. Assessment of the functional activity and IgG Fc receptor utilisation of 64 IgG Rh monoclonal antibodies. Coordinator's report. Transfus Clin Biol. 2002;9:45–53. doi: 10.1016/s1246-7820(01)00215-4. [DOI] [PubMed] [Google Scholar]
- 41.Kumpel BM, Davenport RD. Comparison of two Fcγ RIII-mediated assays of anti-D functional activity, using spleen and K cells. Transfus Med. 1996;6(Suppl. 2):20. [Google Scholar]
- 42.Hadley AG, Zupanska B, Kumpel BM, Leader KA. The functional activity of Fcγ RII and Fcγ RIII on subsets of human lymphocytes. Immunology. 1992;76:446–51. [PMC free article] [PubMed] [Google Scholar]
- 43.Kumpel BM. In vitro functional activity of IgG1 and IgG3 polyclonal and monoclonal anti-D. Vox Sang. 1997;72:45–51. doi: 10.1046/j.1423-0410.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 44.Smith NA, Ala FA, Lee D, et al. A multi-centre trial of monoclonal anti-D in the prevention of Rh-immunisation of RhD- male volunteers by RhD+ red cells. Transfus Med. 2001;10(Suppl. 1):8. [Google Scholar]
- 45.Kumpel BM. Monoclonal anti-D development programme. Transpl Immunol. 2002;10:199–204. doi: 10.1016/s0966-3274(02)00066-7. [DOI] [PubMed] [Google Scholar]