Abstract
Kawasaki disease (KD) is an acute febrile illness of early childhood caused by vasculitis. Whether or not peripheral blood T cells are activated in acute KD remains uncertain, as some reports have presented evidence of peripheral blood T cell activation, whereas others suggest that the level of peripheral blood T cell activation is low during acute KD. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4, CD152) is a surface molecule of activated T cells. We therefore investigated intracellular CTLA-4 expression in the peripheral blood T cells of patients with acute KD as a marker of T cell activation. We collected blood samples from 20 patients with KD and six with Epstein–Barr virus infectious mononucleosis (EBV-IM) who were admitted to our hospital, as well as 13 healthy children. We determined the intracellular expression of CTLA-4 in T cells by flow cytometry. We demonstrated that the intracellular expression of CTLA-4 is up-regulated in peripheral blood CD3+ T cells, CD4+ T cells and CD8+ T cells at the early part of the acute stage in KD. However, the mean percentages of intracellular T cells expressing CTLA-4 in EBV-IM patients were about fourfold higher than those in T cells from patients with acute KD. Our results suggested that the level of activation of peripheral blood T cells is very low during acute KD.
Keywords: CD4+ T cells, CD8+ T cells, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), Epstein–Barr virus infectious mononucleosis (EBV-IM), Kawasaki disease (KD), peripheral blood T cell activation
Introduction
Kawasaki disease (KD) is an acute febrile illness that occurs in young children [1]. Activated T cells expressing HLA-DR antigen infiltrate skin lesions of patients with KD [2] and coronary vascular lesions have also been found at autopsy [3]. However, whether or not peripheral blood T cells are activated in patients with acute KD remains uncertain, as some investigators have evidence of significant activation [4,5], whereas others suggest low levels [6–9]. Conflicting data have been reported regarding T cell activation in peripheral blood during acute KD.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4, CD152) is a surface molecule of activated T cells with sequence homology to CD28 [10]. While CD28 is an important co-stimulator of T cell activation, the essential inhibitory function of CTLA-4 is to maintain homeostasis of the immune system [11]. After activation in vitro, CTLA-4 is expressed transiently on both CD4+ and CD8+ T cells [12]. Surface CTLA-4 is rapidly internalized intracellularly through endocytosis, which may explain its low levels of expression on the cell surface [13]. The intracellular expression of CTLA-4 in T cells is a good marker of transient T cell activation in vivo, because CTLA-4 accumulates in the cytoplasm. To clarify the activation of peripheral blood T cells, we compared CTLA-4 expression in these cells during acute KD with that during acute Epstein–Barr virus (EBV) infectious mononucleosis (IM), in which peripheral blood CD4+ and CD8+ T cells are activated significantly [8,14].
Materials And Methods
Subjects
We studied 20 patients with KD and 6 with EBV-IM, who were admitted to our hospital between August 1999 and April 2001. Thirteen healthy children were also studied as normal controls. All of them were Japanese. Informed consent for participation was obtained from the subjects’ parents.
Kawasaki disease
The patients who met the diagnostic criteria for KD [1] comprised 12 boys and eight girls (0·3–7 years of age; mean, 2·6 years). All received intravenous gammaglobulin (i.v.GG; Polyglobin-N, Bayer Co., Osaka, Japan), 1 g/kg/day, for 2 days and oral aspirin (30 mg/kg/day). The onset of illness was defined as the day on which fever appeared. Blood samples were obtained prior to treatment on illness day 2–6 (mean, 4·4), after i.v.GG treatment on illness day 7–13 (mean, 9·4) during the acute stage, and on illness day 19–150 (mean, 51·1) during the convalescent stage. None of the patients had coronary artery lesions (CAL) and none had a second course of i.v.GG.
EBV-IM
This study included four boys and two girls (1·2–11 years of age; mean, 5 years). The diagnosis was based on clinical presentation of a sore throat, fever and bilateral cervical lymphadnopathy accompanied by atypical lymphocytes in the peripheral blood. All patients were positive for IgM and IgG antibodies to EBV capsid antigen, and negative for antibodies to EBV nuclear antigen during the acute stage. The day of onset of fever was considered the first day of illness. Blood samples were taken at the acute stage (mean ± s.d., 9·0 ± 4·4 days) and during the convalescent stage (46·6 ± 23·0 days). No patients required steroid therapy.
Healthy controls
We tested samples from 13 healthy controls consisting of seven boys and six girls (0·8–6 years of age; mean, 3·3 years) in parallel with the samples from the patients.
Measurement of intracellular CTLA-4
We detected intracellular CTLA-4 expression in CD3+ T, CD4+ T and CD8+ T cells by flow cytometry. Whole blood (100 µl) was stained with peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 monoclonal antibodies (moAb) (Becton-Dickinson, Mountain View, CA, USA) and fluorescein isothiocyanate (FITC)-conjugated anti-CD4 moAb (Becton Dickinson) or anti-CD8 moAb (Becton Dickinson). Erythrocytes were incubated with 2 ml of lysing solution (Becton Dickinson) for 10 min. After washing with phosphate-buffered saline containing 0·5% bovine serum albumin and 0·1% NaN3 (washing buffer) leucocytes were suspended in FACS Permeabilizing Solution (Becton Dickinson) for 10 min. The cells were stained with phycoerythrin (PE)-conjugated anti-CD152 (CTLA-4) moAb (Pharmingen) for 30 min, rinsed with washing buffer and resuspended in 1% paraformaldehyde. Stained cells were analysed using a FACScan flow cytometer (Becton Dickinson) equipped with a 15-mW argon ion laser, and a filter set for FITC (530 nm), PE (585 nm) and PerCP (677 nm). Intracellular CTLA-4 was examined in CD3+ T, CD4+ T and CD8+ T cells that were identified and selected by gating. The intracellular expression of CTLA-4 was analysed by PE staining, and the number of cells that were positive for CTLA-4 was expressed as a percentage of CD3+ T cells, CD4+ T or CD8+ T cells. FASTIMMUNE IgG1 PE served as an isotype control (Becton Dickinson).
Statistical analysis
Data were analysed statistically using the Mann–Whitney U-test, the paired Wilcoxon signed-rank test for comparison of means and Spearman's correlation coefficient by rank test.
Results
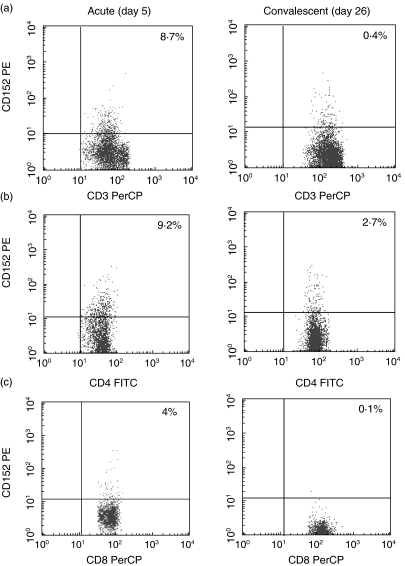
Figure 1 shows flow cytometric results typical of peripheral blood lymphocytes from a 1·9-year-old boy with KD during the acute (day 5) and convalescent (day 26) stages. The level of CTLA-4 expression in this KD patient increased in CD3+ T, CD4+ T and CD8+ T cells during the acute stage. The percentages of intracellular CTLA-4 positive CD3+, CD4+ and CD8+ T cells in all KD patients were significantly higher than those during the convalescent stage (P < 0·01, respectively), and were significantly higher than the levels in healthy children (P < 0·01, respectively) (Table 1). The percentages of CTLA-4 positive T cells decreased to within normal range after I.V.GG treatment.
Fig. 1.
Typical dot plot of intracellular expression in CD3+ T (a), CD4+ T (b) and CD8+ T cells (c) from a 1·9-year-old boy with KD. Percentages are of cells expressing CTLA-4 among CD3+ T, CD4+ T and CD8+ T cells. Upper right quadrant, CTLA-4 positive cells. CTLA-4 expression was increased in all CD3+ T, CD4+ T and CD8+ T cells during the acute stage (day 5), compared with the convalescent stage (day 26).
Table 1.
Intracellular CTLA-4 expression in T cells obtained from patients with KD and EBV-IM and control subjects
| n | CD152+ CD3+ T cells | CD152+ CD4+ T cells | CD152+ CD8+ T cells | |
|---|---|---|---|---|
| Kawasaki disease (KD) | ||||
| Acute stage | ||||
| Before i.v.GG treatment | 20 | 7·4 ± 3·7* | 4·8 ± 3·6* | 6·4 ± 7·2* |
| After i.v.GG treatment | 6 | 4·6 ± 4·0 | 2·9 ± 2·3 | 1·9 ± 2·8 |
| Convalescent stage | 20 | 1·9 ± 1·4 | 1·3 ± 0·9 | 0·7 ± 0·6 |
| Epstein–Barr virus infectious mononucleosis (EBV-IM) | ||||
| Acute stage | 6 | 32·6 ± 18·4** | 22·5 ± 14·0** | 29·9 ± 26·4** |
| Convalescent stage | 6 | 3·2 ± 1·7 | 2·2 ± 1·0 | 1·6 ± 1·9 |
| Controls | 13 | 2·4 ± 1·0 | 1·0 ± 0·6 | 1·2 ± 0·9 |
Values are expressed as means ± s.d.
Significant at P < 0·01 versus convalescent stage and control subjects.
Significant at P < 0·01 versus convalescent stage of IM, acute stage of KD and control subjects. CTLA-4: cytotoxic T lymphocyte-associated antigen 4, CD152. i.v.GG: intravenous gammaglobulin.
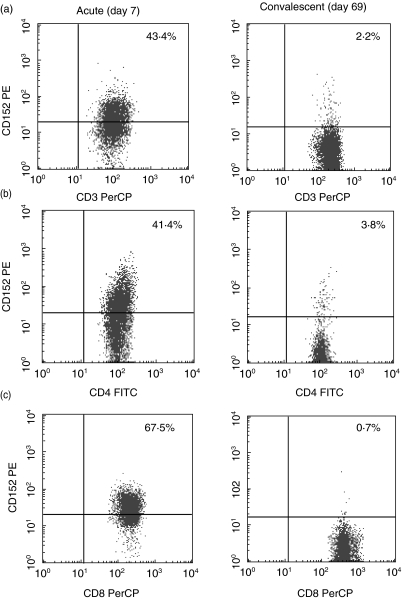
Figure 2 shows obviously increased intracellular CTLA-4 expression in T cells from a 1·7-year-old boy with acute EBV-IM. The percentages of intracellular CTLA-4 positive CD3+, CD4+ and CD8+ T cells were significantly higher during the acute stage of EBV-IM than during the convalescent stage (P < 0·01, respectively) and in controls (P < 0·01, respectively) (Table 1). In addition, the mean percentages of intracellular CTLA-4 positive CD3+, CD4+ and CD8+ T cells were increased significantly in patients with acute EBV-IM than with acute KD (P < 0·01, respectively). The mean percentages of intracellular CTLA-4 positive CD3+, CD4+ and CD8+ T cells in EBV-IM were about fourfold those in acute KD (Table 1).
Fig. 2.
Typical dot plots of intracellular expression in CD3+ T (a), CD4+ T (b) and CD8+ T cells (c) from a 1·7-year-old boy with EBV-IM. Percentages of cells expressing CTLA-4 cells among CD3+ T, CD4+ T and CD8+ T cells. Upper right quadrant, CTLA-4 positive cells. CTLA-4 expression was increased in all CD3+ T, CD4+ T and CD8+ T cells during the acute stage (day 7), compared with the convalescent stage (day 69).
Discussion
In this study, flow cytometry demonstrated that intracellular CTLA-4 expression is up-regulated in peripheral blood CD3+ T, CD4+ T and CD8+ T cells at the acute stage of KD. After i.v.GG treatment, levels of intracellular CTLA-4 expression decreased rapidly to within the normal range. Thus, peripheral blood T cell activation appeared to be maximal at the time of first sampling, when the patients were diagnosed with KD. CTLA-4 is responsible for homeostasis of the immune system through regulatory T cells. The expression of CTLA-4 on T cells depends on cell activation induced by CD28–B7 interaction. Our results suggest that peripheral blood T cells are activated early in the acute stage of KD. In addition, we found that the percentages of intracellular CTLA-4 positive CD4+ and CD8+ T cells in EBV-IM patients are increased during the acute stage, and that the mean percentage of T cells expressing CTLA-4 in EBV-IM was approximately fourfold that in acute KD patients. We reported that peripheral blood CD4+ and CD8+ T cell counts are decreased [6] and that neither CD4+ T nor CD8+ T cells express HLA-DR antigen during acute KD, whereas the numbers of CD4+ T and CD8+ T cells expressing HLA-DR were increased during acute EBV-IM [8]. Our published and present findings indicate that CD4+ and CD8+ T cells in the peripheral blood are less activated in KD than in EBV-IM at the acute stage, suggesting a relatively low level of activation of peripheral blood T cell during acute KD.
We have demonstrated recently a decrease in the number of T cells producing interferon-gamma (Th1-type), but not in that of T cells producing interleukin-4 (Th2-type), in the peripheral blood of patients with acute KD, suggesting an imbalance of peripheral blood T cell function during this stage [15]. We speculate that the increased number of CD4+ T cells expressing CTLA-4 in this study consist of the Th-2 type, as the number of Th1-type T cells was decreased during acute KD.
To the best of our knowledge, we are the first to analyse intracellular CTLA-4 expression in T cells from KD patients. The expression of CTLA-4 on CD4+ T cells is increased in patients with malaria [16], human immunodeficiency virus infection [17] and systemic lupus erythematosus [18]. Levels of CTLA-4 expression are remarkably increased in CD4+ T, but not in CD8+ T cells from patients with these diseases. The slightly increased expression of CTLA-4 in peripheral blood CD8+ T cells in our study may reflect an activated state in KD. Few investigators have examined CD8+ T cells in KD. Macrophages and CD8+ T cells infiltrate coronary artery aneurysms in acute KD [19], and clonal expansion of peripheral blood CD8+ T cells occurs during the acute stage of KD [20]. Further studies are required to clarify the importance of CD8+ T cells in KD.
In conclusion, the findings of the present study support the notion that peripheral blood T cells are activated at low levels during acute KD.
References
- 1.Kawasaki T, Kosaki G, Osawa S, Shigematsu I, Yanagawa S. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6. [PubMed] [Google Scholar]
- 2.Sugawara T, Hattori S, Hirose S, Furukawa S, Yabuta K, Shirai T. Immunopathology of the skin lesions of Kawasaki disease. In: Schulman ST, editor. Kawasaki disease. New York: Alan R. Liss; 1987. pp. 185–92. [PubMed] [Google Scholar]
- 3.Terai M, Kohno Y, Namba M, et al. Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol. 1990;21:231–4. doi: 10.1016/0046-8177(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 4.Leung DYM, Chu ET, Wood N, Grady S, Meade R, Geha RS. Immunoregulatory T cell abnormalities in mucocutaneous lymph node syndrome. J Immunol. 1983;130:2002–4. [PubMed] [Google Scholar]
- 5.Barron K, DeCunto C, Montalvo J, Orson F, Lewis D. Abnormalities of immunoregulation in Kawasaki syndrome. J Rheumatol. 1988;15:1243–9. [PubMed] [Google Scholar]
- 6.Furukawa S, Matsubara T, Yabuta K. Mononuclear cell subsets and coronary artery lesions in Kawasaki disease. Arch Dis Child. 1992;67:706–8. doi: 10.1136/adc.67.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terai M, Kohno Y, Niwa K, Toba T, Sakurai N, Nakajima H. Imbalance among T-cell subsets in patients with coronary arterial aneurysms in Kawasaki disease. Am J Cardiol. 1987;60:555–9. doi: 10.1016/0002-9149(87)90304-3. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa S, Matsubara T, Tsuji K, Motohashi T, Okumura K, Yabuta K. Serum soluble CD4 and CD8 levels in Kawasaki disease. Clin Exp Immunol. 1991;86:134–9. doi: 10.1111/j.1365-2249.1991.tb05785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa S, Matsubara T, Tsuji K, Okumura K, Yabuta K. Transient depletion of T cells with bright CD11a/CD18 expression from peripheral circulation during acute Kawasaki disease. Scand J Immunol. 1993;37:377–80. doi: 10.1111/j.1365-3083.1993.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 10.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 11.Brunner MC, Chambers CA, Chan FK-M, Hanke J, Winoto A, Allison JP. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–20. [PubMed] [Google Scholar]
- 12.Alegre M-L, Noel PJ, Eisfelder BJ, et al. Regulation of surface and intracellular expression of CTLA-4 on mouse T cells. J Immunol. 1996;157:4762–70. [PubMed] [Google Scholar]
- 13.Wang X-B, Zheng C-Y, Giscombe R, Lefvert AK. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand J Immunol. 2001;54:453–8. doi: 10.1046/j.1365-3083.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa S, Matsubara T, Obara T, Imai K, Okumura K, Yabuta K. Soluble CD2 levels in serum during acute Kawasaki disease and infectious mononucleosis. J Infect Dis. 1993;167:778–9. doi: 10.1093/infdis/167.3.778. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara T, Katayama K, Matsuoka T, Fujiwara M, Koga M, Furukawa S. Decreased interferon-gamma-producing T cells in patients with acute Kawasaki disease. Clin Exp Immunol. 1999;116:554–7. doi: 10.1046/j.1365-2249.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlotmann T, Waase I, Julch C, et al. CD4 alpha beta T lymphocytes express high levels of the T lymphocyte antigen CTLA-4 (CD152) in acute malaria. J Infect Dis. 2001;182:367–70. doi: 10.1086/315690. [DOI] [PubMed] [Google Scholar]
- 17.Steiner K, Waase I, Rau T, Dietrich M, Fleischer B, Broker BM. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin Exp Immunol. 1999;115:451–7. doi: 10.1046/j.1365-2249.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu MF, Liu HS, Wang CR, Lei HY. Expression of CTLA-4 molecule in peripheral blood T lymphocytes from patients with systemic lupus erythematosus. J Clin Immunol. 1998;18:392–8. doi: 10.1023/a:1023226621966. [DOI] [PubMed] [Google Scholar]
- 19.Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–3. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 20.Choi IH, Chwae YJ, Shim WS, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–6. [PubMed] [Google Scholar]