Abstract
Heroin treatment or abusive drug addiction influences many physiological functions, including the reactions of the immune system. Although suppression of various manifestations of the immune system after heroin (or morphine) administration has been reported, we show here that production of proinflammatory cytokines and nitric oxide (NO) was enhanced and allotransplantation reactions were accelerated significantly in heroin-treated recipients. Mice were treated by a subcutaneous administration of heroin (diacetylmorphine) given in one or repeated daily doses. The ability of spleen cells from treated mice to respond in vitro to alloantigens and to produce IL-2, IL-4, IL-10 and IFN-γ, and the production of IL-1β, IL-12 and NO by peritoneal macrophages, were tested. Within 2 h after heroin administration, proliferative responses to alloantigens and the production of IL-1β, IFN-γ, IL-12 and NO were enhanced significantly. In contrast, the production of anti-inflammatory cytokines IL-4 and IL-10 was at the same time rather decreased. As a consequence, skin allografts in heroin-treated mice were rejected more promptly than in untreated or vehicle-treated recipients. Similarly, the growth of allogeneic tumours induced by high doses of tumour cells was suppressed significantly in heroin-treated mice. The enhancing effects of heroin on the production of proinflammatory cytokines were antagonized by naltrexone, a specific inhibitor of classic opioid receptors. These results show that heroin treatment augments production of proinflammatory cytokines and accelerates allotransplantation reactions. The observations thus illustrate the complexity of the effects of heroin on the immune system and should be taken into account during medical treatment of opiate addicts and in the use of morphine to decrease pain in various clinical situations.
Keywords: cytokine production, heroin, naltrexone, skin allografts, tumour growth
Introduction
Heroin (diacetylmorphine) is the most common opiate drug, with several million abusers worldwide. It influences different physiological functions in the body, including the reactions of the immune system. The complexity of the heroin's effects results from the wide distribution of opioid receptors. These receptors have been demonstrated on various cell types, including cells of both the nervous and immune system. Heroin can thus regulate functions of the immune system either directly by acting on the opioid receptors on lymphocytes and macrophages, or can influence the reactions of the immune system through its effects on the nervous system. Previous studies have shown that heroin (or morphine) administration depresses many parameters of the immune system. However, Pacifici et al. [1,2] described that production of various cytokines and nitric oxide (NO) was enhanced within a few minutes after morphine administration but after 24 h a decrease in proliferation of T cells and in the production of Th1 cytokines was observed. Peng et al. [3] demonstrated augmented production of interleukin (IL)-12 and some proinflammatory cytokines in mice treated with morphine. Enhancement of the contact hypersensitivity reaction and increased IFN-γ mRNA levels in the inflamed tissue by acute morphine administration was reported by Nelson et al. [4]. Elevated levels of plasma IL-6 after morphine treatment were observed in various models [5,6]. Similarly, Roy et al. [7] reported that morphine-treated mice injected with bacterial lipopolysaccharide (LPS) had increased production of IL-6 and TNF-α. The same conclusion was reached by Kapasi et al. [8] who showed that morphine stimulates TNF-α and nitrite production in mouse cells. It thus appears that the functions of macrophages and production of proinflammatory cytokines are augmented after heroin ad-ministration while T cell-mediated functions are rather decreased [9–11].
Recent studies on transplantation immunity suggest that macrophages, delayed-type hypersensitivity and inflammatory reactions, rather than cytotoxic T cells, are the main effector mechanisms of allograft and xenograft rejection [12–14]. To analyse the effects of heroin administration on cytokine production in vitro and on effector transplantation reactions in vivo, we employed experimental models of skin grafting and allogeneic tumour growth in mice. We show that acute or subchronic administration of heroin augmented production of proinflammatory cytokines and simultaneously depressed secretion of IL-4 and IL-10. We also show that allotransplantation reactions were significantly accelerated in heroin-treated recipients. The effects of heroin were antagonized by naltrexone, indicating the involvement of classic opioid receptors in heroin-induced augmentation of transplantation reactions.
Materials and Methods
Animals
Mice of inbred strains BALB/c and C57BL/10Sn (B10) of both sexes at the age of 7–9 weeks were used in the experiments. All animals were from the breeding unit of the Institute of Molecular Genetics, Prague.
Drugs
Heroin (6-diacetylmorphine HCl monohydrate) was obtained from Alltech (State College, PA, USA). Naltrexone HCl was purchased from Sigma Chemical Co. (St Louis, MO, USA). Before each experiment, the drugs were dissolved freshly at the required concentrations in phosphate buffered saline (PBS) and a volume of 0·2 ml was injected subcutaneously (s.c.) on two sides. Control mice were untreated or treated with the same volume of PBS administred s.c. If not stated otherwise, heroin was injected at a dose of 20 mg/kg and naltrexone at a dose of 10 mg/kg.
Skin grafting
Recipient BALB/c mice were grafted with B10 skin according to the technique of Billingham et al. [15]. The recipients were either untreated or were treated with daily doses of heroin (20 mg/kg/day) or PBS from day − 14 to day − 1 (the day of grafting noted as 0) or were treated with heroin (or PBS) daily from day 1 after grafting until the grafts were rejected.
Allogeneic tumour growth
BALB/c mice were injected s.c. in a dorsal part of their body with 107 cells of fibrosarcoma MC11 which was induced originally by methylcholanthrene in a B10 male mouse [16]. The cells were grown in vitro in tissue culture and were injected in 0·2 ml of PBS. The size of the growing tumours was measured every other day. The number of tumour cells was selected on the basis of preliminary experiments showing that 107 is the lowest number of cells that will ensure the progressive tumour growth in allogeneic recipients. As we have demonstrated earlier, MC11 sarcomas induced with 107 cells did not grow in specifically sensitized recipients, but grew rapidly in tolerized or immunosuppressed BALB/c mice [17]. Each individual group of mice was treated with heroin (or PBS) before or after injection of tumour cells at the same schedule and on the same doses as the recipients of skin allografts. One group of mice was injected s.c. with naltrexone (10 mg/kg) 15 min before each treatment with heroin.
Cytokine production and detection
Spleens from control, PBS- or heroin-treated mice were obtained at the indicated time interval after the last heroin dose and single cell suspensions were prepared in RPMI-1640 medium (Sigma) containing 10% heat-inactivated fetal calf serum (Sigma), antibiotics (100 U/ml of penicillin, 100 µg/ml streptomycin), 10 mm HEPES buffer and 5 × 10−5 m 2-mercaptoethanol (hereinafter complete RPMI-1640 medium). Spleen cells (1·25 × 106 cells/ml) from tested BALB/c mice were cultivated in a volume of 1·2 ml of complete RPMI-1640 medium in 48-well plates (Costar, Corning, NY, USA) unstimulated or were stimulated with irradiated (3000 R) spleen cells (1·25 × 106 cells/ml) from B10 mice. The supernatants were harvested after 48-h (IL-2 determination), 72-h (IFN-γ) or 96-h (IL-4, IL-10) incubation periods. To produce and detect IL-1 and IL-12, peritoneal exudate cells (PEC) obtained by washing the peritoneal cavity of tested mice were adjusted to a concentration of 1 × 106 cells/ml and were stimulated with LPS (1·5 µg/ml, Difco Laboratories, Detroit, MI, USA) plus mrIFN-γ (400 U/ml). The supernatants were harvested after a 48-h incubation. The presence of IL-2, IL-4, IL-10 and IFN-γ in the supernatants was determined by ELISA using sets of cytokine-specific capture and detection monoclonal antibodies purchased from PharMingen (San Diego, MA, USA) as we have described previously [18]. For quantification of cytokine levels, standards for IL-2, IL-4, IL-10 and IFN-γ (all purchased from Genzyme, Boston, MA, USA) were included in all ELISA determinations. Production of IL-1 and IL-12 was measured using ELISA DuoSet kits for mouse IL-1β and IL-12p40 obtained from R&D System (Minneapolis, MN, USA).
NO assay
PEC were obtained by washing the peritoneal cavity of individual control or heroin-treated mice. For NO production, PEC (1 × 106 cell/ml) were incubated in 0·4 ml of complete RPMI-1640 medium in 48-well tissue culture plates (Costar) unstimulated or were stimulated with LPS (1·5 µg/ml) plus mrIFN-γ (400 U/ml). The concentrations of NO in the supernatants were measured after 48-h incubation by spectrophotometry using the Griess reaction [19].
Statistics
The statistical significance of the difference between the control and experimental groups was calculated using the Student's t-test.
Results
Enhanced production of proinflammatory cytokines in heroin-treated mice
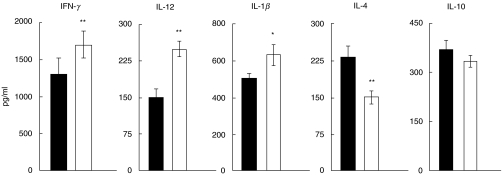
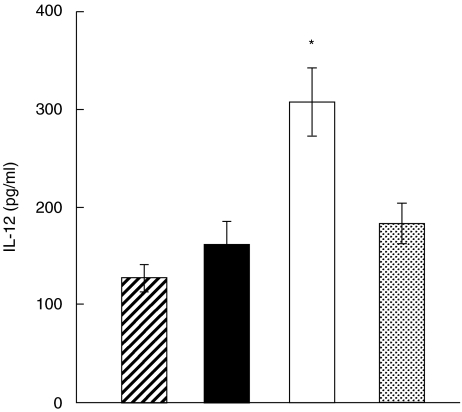
Mice were injected s.c. with heroin (20 mg/kg) or PBS and the ability of their spleen cells or PEC to produce cytokines was determined after 20 min. This time interval was selected on the basis of preliminary experiments which showed the peak of enhanced cytokine production between 15 and 30 min after heroin administration and a decline in the cytokine production until 2 h after the treatment (data not shown). The production of proinflammatory cytokines IFN-γ, IL-12 and IL-1β was enhanced significantly, while production of IL-4 and IL-10 was decreased (Fig. 1). Secretion of IL-2 was comparable in the PBS- and heroin-treated groups (not shown). To test whether the enhancing effect of heroin on IL-12 production was mediated through classic opioid receptors the mice were injected with naltrexone, an opioid receptor antagonist, 15 min before treatment with heroin. Figure 2 shows that treatment with naltrexone abolished the enhancing effect of heroin on IL-12 production.
Fig. 1.
Production of IFN-γ, IL-12, IL-1β, IL-4 and IL-10 by mouse spleen cells 20 min after treatment with heroin. BALB/c mice were treated with heroin (□) or PBS (▪) and their spleen cells were stimulated with irradiated allogeneic cells (production of IFN-γ, IL-4 and IL-10) or their PEC were stimulated with LPS/IFN-γ (production of IL-12 and IL-1β). The production of IL-12 and IL-1β was measured after 48-h stimulation, IFN-γ after 72 h stimulation and IL-4 and IL-10 after 96-h stimulation by ELISA. Each bar represents the mean ± s.e. from three independent experiments. Values with asterisks represent significant (*P < 0·05, **P < 0·01) difference between PBS and heroin-treated group.
Fig. 2.
Effect of treatment with naltrexone on heroin-induced enhancement of IL-12 production. BALB/mice were untreated  or treated with PBS (▪), heroin (□) or with naltrexone 15 min before heroin injection
or treated with PBS (▪), heroin (□) or with naltrexone 15 min before heroin injection  . PEC were harvested 20 min after the heroin administration and were stimulated with LPS/IFN-γ. The production of IL-12 was determined after 48 h by ELISA. Each bar represents the mean ± s.e. from three experiments. Value with asterisk represents statistically significant (P < 0·01) enhancement of IL-12 production in comparison with control untreated mice.
. PEC were harvested 20 min after the heroin administration and were stimulated with LPS/IFN-γ. The production of IL-12 was determined after 48 h by ELISA. Each bar represents the mean ± s.e. from three experiments. Value with asterisk represents statistically significant (P < 0·01) enhancement of IL-12 production in comparison with control untreated mice.
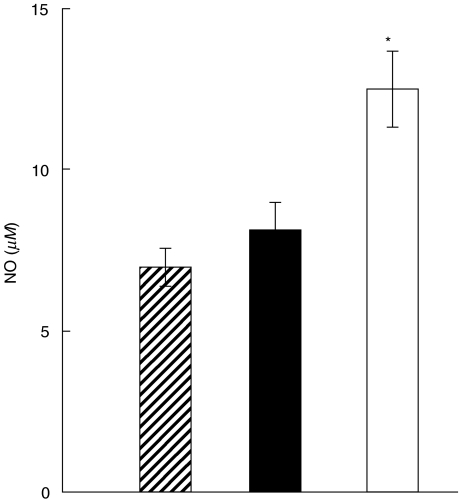
Enhanced NO production after heroin treatment
Production of NO by LPS/IFN-γ stimulated PEC from heroin-treated mice was enhanced 20 min after the treatment (Fig. 3). The enhanced production of NO lasted for 2 h and was no longer detectable after 4, 8 or 24 h (data not shown).
Fig. 3.
Enhanced NO production after heroin administration. BALB/c mice were untreated( ) or treated with PBS (▪) or heroin (□) and their PEC were harvested after 20 min. The cells were stimulated with LPS/IFN-γ and the presence of NO in the supernatants was detected after 48 h using a Griess reaction. Each bar represents the mean ± s.e. from three experiments. Values with asterisk represent NO production that was statistically different (P < 0·01) from untreated controls.
) or treated with PBS (▪) or heroin (□) and their PEC were harvested after 20 min. The cells were stimulated with LPS/IFN-γ and the presence of NO in the supernatants was detected after 48 h using a Griess reaction. Each bar represents the mean ± s.e. from three experiments. Values with asterisk represent NO production that was statistically different (P < 0·01) from untreated controls.
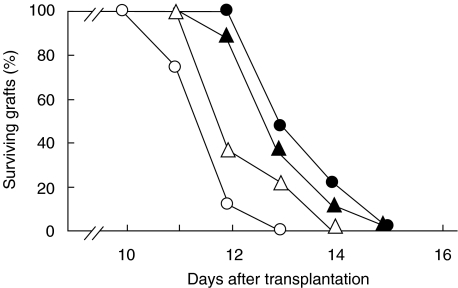
Effect of heroin treatment on skin allograft rejection
A group of mice was treated daily with heroin (or PBS) from days −14 to −1 (day of transplantation noted as 0) and other groups of mice were treated with heroin (or PBS) from day 1 after transplantation until the grafts were rejected. As demonstrated in Fig. 4, heroin-treated mice rejected allografts more promptly than mice treated with PBS. The mean graft survival times for mice treated before transplantation were 10·9 ± 0·4 and 12·8 ± 0·5 days for mice treated with heroin and PBS, respectively, and 11·3 ± 0·6 and 12·4 ± 0·4 days for mice treated with heroin and PBS, respectively, after transplantation. Although these differences were relatively small, they were statistically significant: P < 0·01 for mice treated with heroin before transplantation and P < 0·05 for mice treated after grafting. Also repeated experiments confirmed the accelerated skin allograft rejections in heroin-treated mice.
Fig. 4.
Effects of heroin treatment on rejection of skin allografts. BALB/c mice were grafted with B10 skin and were treated daily with PBS (•) or with 20 mg/kg/day of heroin (○) for 2 weeks before transplantation, or with daily doses of PBS (▴) or heroin (▵) after transplantation until the grafts were rejected. Each group consisted of 7–8 mice.
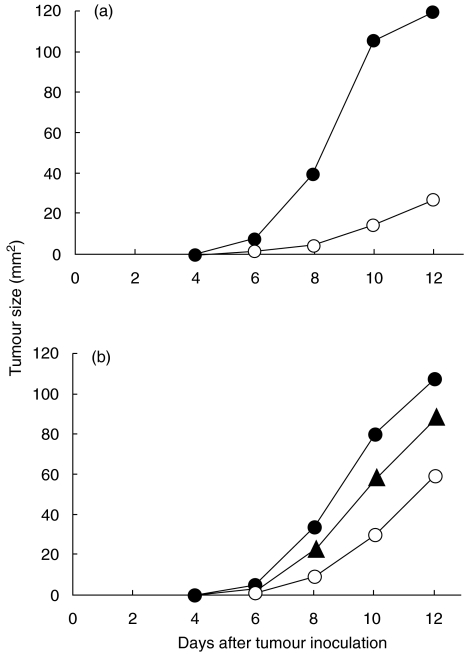
Retarded growth of allogeneic tumours in heroin-treated mice
Mice were treated with heroin (or PBS) for two weeks before or after inoculation of 107 allogeneic tumour cells. As demonstrated in Fig. 5a, treatment with heroin before inoculation of tumour cells significantly (P < 0·001) inhibited tumour growth. Similarly, treatment of mice with heroin after tumour cell inoculation resulted in a suppression of tumour growth (P < 0·01) (Fig. 5b). Figure 5b also shows that treatment with naltrexone 15 min before administration of heroin significantly (P < 0·05) reduced the effect of heroin.
Fig. 5.
Effects of heroin treatment on the growth of allogeneic tumours. BALB/c mice were treated daily with PBS (•) or with 20 mg/kg/day of heroin (○) for 2 weeks before inoculation of 107 cells of MC11 sarcoma (a), or were treated with PBS (•), 20 mg/kg/day of heroin (○) or with naltrexone (10 mg/kg/day) plus heroin (▴) after inoculation with 107 MC11 tumour cells. Each group consisted of 8–10 mice.
Discussion
The increased incidence of various infections and other diseases in heroin addicts has led to the conclusion that heroin administration suppresses the protective functions of the immune system [20]. This was supported by the numerous observations that heroin (or morphine) inhibits proliferation of T lymphocytes in vitro and that mice or rats treated with morphine had decreased responsiveness of lymphocytes and NK cells in vitro [9,10]. It was shown in vivo that treatment with morphine resulted in a decrease in thymus and spleen weight [21] and in reduced resistance to oral Salmonella typhimurium infection [22]. However, using µ opioid receptor KO mice Roy et al. [23] showed that at least some of these inhibitory effects of morphine were not mediated through µ opioid receptors.
Recent studies show that some functions of the immune system, especially those dependent on macrophages or in which inflammatory reactions are involved, are enhanced after morphine administration [3–8]. We confirmed the results of Pacifici et al. [1,2], who described augmented production of cytokines after mitogen stimulation of spleen cells from mice treated with morphine. We extended their results to other cytokines and for antigen-specific system (alloantigens). We showed that while the production of proinflammatory cytokines was enhanced, production of anti-inflammatory cytokines IL-4 and IL-10 was decreased. The alterations in cytokine production were limited to the first 2 h after heroin treatment. However, we have never observed such profound suppression of cytokine production at the later time intervals (24 h), as described by Pacifici et al. [1]. We suggest that the significantly enhanced production of IL-12 (a cytokine responsible for determination of the Th1 type of the immune response) observed after heroin administration might be responsible for pushing the immune response to the Th1 direction and for accellerated allograft rejection. This is also supported by our observation that heroin treatment for a longer time period before transplantation had more profound effect on the allograft rejection than the treatment after grafting. Simultaneously, we showed that production of anti-inflammatory cytokines IL-4 and IL-10, which are representative of Th2 type of the immune response, was decreased. These alterations in cytokine production could be responsible for the modulations of allotransplantation reactions that we described. We also showed that the growth of allogeneic tumours induced by high doses of allogeneic tumour cells was retarded significantly in heroin-treated mice. The finding of augmented proinflammatory reactions and of consequently accelerated allograft rejections after heroin treatment is consistent with the recent observations that delayed-type hypersensitivity and inflammatory reactions represent one of the most important mechanisms of allograft rejection [13,14].
The complexity of the actions of opiates on the functioning of the immune system might be associated with the different levels on which they act. The opiates can affect cells of the immune system directly through opioid receptors which have been demonstrated on lymphocytes and macrophages [24]. In addition, opiates can regulate the functions of the immune system through the nervous system [25,26]. It has been shown that enhanced production of IL-6 depends on the activity of the hypothalamic–pituitary–adrenal (HPA) axis and can be blocked by adrenalectomy [6]. On the contrary, the suppression by morphine of lymphocyte proliferation appeared to be independent of the HPA axis and was resistant to adrenalectomy [6]. In this respect it has been shown that natural anti-inflammatory effects of the HPA axis mediated through corticosteroids are disrupted by IL-1β, the levels of which are increased after heroin treatment [27]. We showed that immunostimulatory effects of heroin were antagonized by naltrexone, a classic opioid receptor antagonist. All the effects of heroin in our model were thus mediated through opioid receptors, but some actions may result from direct effects of heroin on the immune system and some may be mediated indirectly through the effects of heroin on the nervous system.
Finally, our results show clearly that allotransplantation reactions are accelerated in heroin-treated recipients. This is in accordance with the observation that heroin treatment prolongs survival time and retards tumour growth in mice [28]. Augmentation of immune functions and modifications of cytokine responses in morphine-treated patients during postoperative periods have been also reported [29]. We suggest that inflammatory and contact hypersensitivity reactions, which are enhanced after heroin or morphine treatment, are responsible for the observed stronger allotransplantation reaction. In addition to the proinflammatory cytokines, the production of NO was significantly enhanced in heroin-treated mice. It has been demonstrated that the levels of NO produced by macrophages are sufficiently high to kill surrounding cells [30] and NO was shown as an important effector molecule in allograft rejection [12]. In addition to its function as a toxic effector molecule, NO can modulate immune reactions [31]. It has been shown that the inhibition of T cell function described after high-dose morphine was associated with an increase in the inducible NO synthase mRNA expression [32] and NO was suggested to be responsible for the depression of T cell-mediated functions described in various models after morphine treatment [33]. Thus, although the expression of some T cell functions is depressed after heroin application, production of effector molecules, such as IL-1β, TNF-α and NO, is increased. These molecules may be responsible for the accelerated rejections of skin or tumour allografts in heroin-treated recipients.
At the present time it is difficult to compare doses of heroin used in animal studies and in humans. Doses used in mice were more than 10 times higher than those used in humans. In our parallel studies (unpublished results) we compared cytokine production of peripheral blood leucocytes from 12 human heroin addicts and 10 controls. No suppression of cytokine production in heroin addicts was found. Rather, a slight enhancement in the production of some cytokine and a significant increase in IL-6 secretion was recorded. Similarly, Kim et al. [29] observed enhancement of cytokine production in patients treated with morphine to decrease pain after abdominal hysterectomy. Our recent results and the observations of others thus show that the phenomenon of enhanced cytokine production after heroin (or morphine) treatment is common in both humans and in experimental models.
Acknowledgments
This work was supported by grants NF/6824–3 and NJ/6632–3 from the Ministry of Health of the Czech Republic, and projects LN00A026 and MSM 113100003 from the Ministry of Education of the Czech Republic.
References
- 1.Pacifici R, Di Carlo S, Bacosi A, Pichini S, Zuccaro P. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol. 2000;22:603–14. doi: 10.1016/s0192-0561(00)00023-0. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici R, Minetti M, Zuccaro P, Pietraforte D. Morphine affects cytostatic activity of macrophages by the modulation of nitric oxide release. Int J Immunopharmacol. 1995;17:771–7. doi: 10.1016/0192-0561(95)00046-5. [DOI] [PubMed] [Google Scholar]
- 3.Peng X, Mosser DM, Adler MW, Rogers TJ, Meisser JJ, Eisenstein TK. Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol. 2000;68:723–8. [PubMed] [Google Scholar]
- 4.Nelson CJ, How T, Lysle DT. Enhancement of the contact hypersensitivity reaction by acute morphine administration at the elicitation phase. Clin Immunol. 1999;93:176–83. doi: 10.1006/clim.1999.4783. [DOI] [PubMed] [Google Scholar]
- 5.Zubelewicz B, Braczkowski R, Renshaw D, Harbuz MS. Central injection of morphine stimulates plasma corticosterone and interleukin (IL) -6 and IL-6 R mRNAs in the pituitary and adrenals in adjuvant-induced arthritis. J Biol Regul Homeost Agents. 1999;13:103–9. [PubMed] [Google Scholar]
- 6.Houghtling RA, Mellon RD, Tan RJ, Bayer BM. Acute effects of morphine on blood lymphocyte proliferation and plasma IL-6 levels. Ann N Y Acad Sci. 2000;917:771–7. doi: 10.1111/j.1749-6632.2000.tb05442.x. [DOI] [PubMed] [Google Scholar]
- 7.Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulated NF kappa B activation in macrophages. Biochem Biophys Res Commun. 1998;245:392–6. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- 8.Kapasi AA, Gibbons N, Mattana J, Singhal PC. Morphine stimulates mesangial cell TNF-alfa and nitrite production. Inflammation. 2000;24:463–76. doi: 10.1023/a:1007016329300. [DOI] [PubMed] [Google Scholar]
- 9.Bayer BM, Gastonguay MR, Hernandez MC. Distinction between the in vitro and in vivo inhibiotory effects of morphine on lymphocyte proliferation based on agonist sensitivity and naltrexone reversibility. Immunopharmacology. 1992;23:117–24. doi: 10.1016/0162-3109(92)90035-b. [DOI] [PubMed] [Google Scholar]
- 10.Bryant HU, Bernton EW, Holaday JW. Immunosuppressive effects of chronic morphine treatment in mice. Life Sci. 1987;41:1731–8. doi: 10.1016/0024-3205(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 11.Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. 1993;265:1071–8. [PubMed] [Google Scholar]
- 12.Krulová M, Zajícová A, Frič J, Holáň V. Alloantigen-induced, T-cell-dependent production of nitric oxide by macrophages infiltrating skin allografts in mice. Transpl Int. 2002;15:108–16. doi: 10.1007/s00147-002-0378-0. [DOI] [PubMed] [Google Scholar]
- 13.Ito A, Minagava M, Tomiyama K, Ito M, Kawai K. Cytotoxic pathways in the skin allograft rejection by CD4+ T cells. Transplantation. 1999;68:97–100. doi: 10.1097/00007890-199907150-00019. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Einaga-Naito K, Kuriyama M, Kawada Y, Yoshida R. Cellular basis of skin allograft rejecion. Specific lysis of allogeneic skin components by non-T cells. Transplantation. 1998;65:818–25. doi: 10.1097/00007890-199803270-00009. [DOI] [PubMed] [Google Scholar]
- 15.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. The survival times of skin homografts exchanged between members of different inbred strains of mice. Proc Roy Soc Lond. 1954;143:43–55. doi: 10.1098/rspb.1954.0053. [DOI] [PubMed] [Google Scholar]
- 16.Bubeník J, Indrová M, Němečková Š, et al. Solubilized tumour-associated antigens of methylcholanthrene-induced sarcomas. Comparative studies by in vitro sensitization of lymph-node cells, macrophage electrophoretic mobility assay and transplantation tests. Int J Cancer. 1987;21:348–55. doi: 10.1002/ijc.2910210316. [DOI] [PubMed] [Google Scholar]
- 17.Holáň V, Zajícová A, Krulová M, Plšková J, Frič J, Filipec M. Induction of specific transplantation immunity by oral immunization with allogeneic cells. Immunology. 2000;101:404–11. doi: 10.1046/j.1365-2567.2000.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holáň V, Kuffová L, Zajícová A, et al. Urocanic acid enhances IL-10 production in activated CD4+ T cells. J Immunol. 1998;161:3237–41. [PubMed] [Google Scholar]
- 19.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 20.Govitrapong P, Suttitum T, Kotchabhakdi N, Uneklabh T. Alterations of immune functions in heroin addicts and heroin withdrawal subjects. J Pharmacol Exp Ther. 1998;286:883–9. [PubMed] [Google Scholar]
- 21.Bhargava HN, Thomas PT, Thorat S, House RV. Effect of morphine tolerance and abstinence on cellular immune function. Brain Res. 1994;642:1–10. doi: 10.1016/0006-8993(94)90899-0. [DOI] [PubMed] [Google Scholar]
- 22.MacFarlane AS, Peng X, Meissler JJ, Jr, et al. Morphine increases susceptibility to oral Salmonella typhimurium infection. J Infect Dis. 2000;181:1350–8. doi: 10.1086/315403. [DOI] [PubMed] [Google Scholar]
- 23.Roy S, Barke RA, Loh HH. Mu-opioid receptor-knockout mice. role of mu-opioid receptor in morphine mediated immune functions. Mol Brain Res. 1998;61:190–4. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 24.Carr DJ, Bost KL, Blalock JE. The production of antibodies which recognize opiate receptors on murine leukocytes. Life Sci. 1988;42:2615–24. doi: 10.1016/0024-3205(88)90331-1. [DOI] [PubMed] [Google Scholar]
- 25.Houghtling RA, Bayer BM. Rapid elevation of plasma interleukin-6 by morphine is dependent on autonomic stimulation of adrenal gland. J Pharmacol Exp Ther. 2002;300:213–9. doi: 10.1124/jpet.300.1.213. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. The immunosuppressive effects of chronic morphine treatment are partially dependent on corticosterone and mediated by the mu-opioid receptor. J Leukoc Biol. 2002;71:782–90. [PubMed] [Google Scholar]
- 27.House SD, Mao X, Wu G, Espinelli D, Li WX, Chang SL. Chronic morphine potentiates the inflammatory response by disrupting interleukin-1beta modulation of the hypothalamic–pituitary–adrenal axis. J Neuroimmunol. 2001;118:277–85. doi: 10.1016/s0165-5728(01)00337-x. [DOI] [PubMed] [Google Scholar]
- 28.Zagon IS, McLaughlin PJ. Heroin prolongs survival time and retards tumor growth in mice with neuroblastoma. Brain Res Bull. 1981;7:25–32. doi: 10.1016/0361-9230(81)90094-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim MH, Hahm TS. Plasma levels of interleukin-6 and interleukin-10 are affected by ketorolac as an adjunct to patient-controlled morphine after abdominal hysterectomy. Clin J Pain. 2001;17:72–7. doi: 10.1097/00002508-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Nathan C. Natural resistance and nitric oxide. Cell. 1995;82:873–6. doi: 10.1016/0092-8674(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 31.Holáň V, Krulová M, Zajícová A, Pindjáková J. Nitric oxide as a regulatory and effector molecule in the immune system. Molec Immunol. 2002;38:989–95. doi: 10.1016/s0161-5890(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function. role of caspase-3, -8, and nitric oxide. J Leukoc Biol. 2001;70:527–36. [PubMed] [Google Scholar]
- 33.Fecho K, Maslonek KA, Coussons-Read ME, Dykstra LA, Lysle DT. Macrophage-derived nitric oxide is involved in the depressed concanavalin A responsiveness of splenic lymphocytes from rats administered morphine in vivo. J Immunol. 1994;152:1545–52. [PubMed] [Google Scholar]