Abstract
An increased frequency of antiviral CD8+ T cells is seen in chronic viral infections. During herpes virus infections the expanded CD8+ T cells are thought to control the reactivation of the latent infection. Because multiple sclerosis (MS), a presumed autoimmune disease of the central nervous system, has been associated with a late Epstein–Barr virus (EBV) infection, we wished to examine whether the CD8+ T cell response to EBV epitopes differed between MS patients and healthy controls. Here we report an increased frequency of CD8+ T cells responding to EBV epitopes from nuclear antigen 3 A (HLA-A2/CLG) and latent membrane protein 2 (HLA-B7/RPP) in MS patients. Noticeably, the altered CD8+ T cell response occurred to some but not all EBV epitopes and did not reach the high level seen during acute infection. The responses towards two immunodominant epitopes from human cytomegalovirus (HCMV) were similar in MS patients and normal controls. Together, our data demonstrate the presence of an increased frequency of CD8+ T cells reacting with two epitopes from EBV in patients with MS. The altered response to only two of the tested EBV epitopes would be consistent with the presence of cross-reactive epitopes.
Keywords: ELIspot, herpesvirus, HLA, lymphocytes, peptides
Introduction
The cellular immune system is critical in the control of human herpesvirus infections. In particular, CD8+ T cells are important for the host defence to an established infection by their antigen-specific recognition of viral peptide fragments of 8–10 amino acids presented by major histocompatibility complex (MHC) class I molecules. The recognition of viral peptides is restricted by many factors, including an enzymatic processing of the viral proteins into fragments that fit the anchor pockets of the MHC class I molecules [1]. The polymorphism of the MHC region permits the presentation of a range of peptides, which ensure the shaping of the immune defence by allowing the selection of the best and most efficient T cells from precursors with a diverse T cell receptor repertoire [2]. It has been estimated that only 0·05% of the presented peptides are immunodominant; that is, they induce a significant detectable CD8+ T cell response. A somewhat similar percentage of peptides are subdominant and give rise to a weak or barely detectable immune response [1].
During primary infection with Epstein–Barr virus (EBV), neutralizing antibodies develop and may limit the viral load. However, it is the CD8+ T cells that are responsible for controlling the expansion of the latently infected B cells. The CD8+ T cells are directed towards both latent and lytic antigens. Importantly, adoptive transfer of cytotoxic CD8+ T cells may resolve lymphomas, Hodgkin's disease and post-transplant lymphoproliferative disease in patients [3–5]. Because CD8+ T cell responses to EBV are critical in controlling the infection, the use of immunodominant epitopes as a mean of a T cell-based vaccine approach is being evaluated [6].
As known for other viral diseases, the CD8+ T cell response to EBV is focused on few epitopes [7]. The response to the individual epitopes is regulated dynamically and is therefore dependent on the course of infection [8–13]. In the case of HLA-B8+ individuals, the frequency of CD8+ T cells to an epitope within the BZLF1 gene may comprise up to 44% of the total CD8+ T cells during primary infection [14]. Even in healthy individuals, the response to this epitope may comprise up to 5% of the CD8+ T cells [15].
Viral infection has been associated with multiple sclerosis (MS), a presumed autoimmune disease of the central nervous system. First of all, the concordance rate among monozygotic twins implicate non-genetic factors in the disease [16]. Secondly, epidemiological observations on migration demonstrate that the risk of acquiring MS diminishes by moving before puberty to an area with low prevalence of MS [17]. This is consistent with a ubiquitous infectious agent that predisposes to MS in genetically susceptible individuals, if the infection occurs around puberty or later. Epidemiological and serological analysis indicate that EBV may fulfil these requirements (reviewed in [18]). Furthermore, all MS patients, but not all healthy controls, have antibodies to EBV [18], and a recent prospective study demonstrates that the level of antibodies to EBV correlates with the later development of MS [19]. This is in agreement with a register-based historic prospective study demonstrating an increased risk of MS (OR = 2·8) if the individuals had experienced infectious mononucleosis earlier in life [20]. Lastly, while a recent randomized, double-blind, placebo-controlled MRI study of antiherpes virus treatment of relapsing-remitting MS patients did not indicate a significant overall effect, a statistical significant reduction of new MRI lesions in patients with high disease activity was observed [21].
The potential association of MS with a herpesvirus infection suggests that an altered cellular immune response to EBV might be present in these patients. We therefore examined the frequency of T cells specific to previously identified HLA-A2 and -B7-associated immunogenic peptides from EBV. Our data indicate that MS patients have an increased frequency of CD8+ T cells to certain immunodominant epitopes from EBV.
Materials And Methods
HLA-A2 and HLA-B7 genes
Mononuclear cells (MNC) were isolated from blood using Ficoll. DNA extracts were prepared by resuspending 5 × 106 MNC in 0·5 ml of 0·1 × phosphate buffered saline (PBS), pH 7·4 for 5 min. Cells were boiled at 99°C for 10 min, cooled to 55°C and treated with × U/ml of proteinase K, and subsequently heated to 99°C to inactivate the enzyme. An equivalent of 10 000 cells (10 µl) was used in polymerase chain reactions (PCR). Primers for HLA-A2: 5′-GGA GCC CCG CTT CAT CGC A-3′ and 5′-CTC CCC GTC CCA ATA CTC CGG A-3′, identifying HLA-A*02011 to A*0232 N. Primers for HLA-B7: 5′-CAA GTG GGA GGC GGC CCG TGA-3′ and 5′-TGG TAC CAG CGC GCT CCA GCT-3′, identifying HLA-B*07021 to B*0713 and some rare HLA-B alleles: HLA-B*4015, B*4016, and B*4805. Samples were amplified by real-time PCR on a Light Cycler (Roche, Hvidovre, Denmark) and the PCR product verified further by 1% agarose gel electrophoreses with ethidium bromide [13]. Individuals from whom HLA-A2 or HLA-B7 can be amplified are also HLA-A2 or HLA-B7 when tested by a microcytotoxicity assay [13].
Patients
Consecutive MS patients and controls were tested by PCR for the presence of HLA-A2 and HLA-B7 alleles. Thereby 33 MS patients and 33 control individuals were selected for the antigen-specific T-cell frequency analysis. The average age ± s.e. for the MS patients was 47·2 ± 1·7 years versus 40·9 ± 2·3 years in the control group. The female–male ratio was 1·5 in the MS group versus 1·1 in the control group. Thus, the MS group was on average 6·3 years older than the control group. Similarly, the female–male ratios reflected the expected ratio for MS patients and healthy controls, respectively. The clinical phase of the MS patients, assigned by a neurologist specializing in MS, was performed independently of the laboratory data and concealed until all laboratory data were obtained. All subjects gave their informed consent for the blood drawing and the study was approved by the local ethical committee of Århus.
Peptides
Peptides (Schafer-N, Copenhagen, Denmark) were HPLC-purified to higher than 95% and checked by mass spectrometry analysis. Peptides from EBV, human cytomegalovirus (HCMV) and measles virus were selected [22–29] and the sequences and abbreviated names are as indicated in Table 1.
Table 1.
Viral peptides used in this study
| Virus | Protein | Peptide | Abbreviated name | MHC association | References |
|---|---|---|---|---|---|
| EBV | EBNA3A | SVRDRLARL | SVR | HLA-A2 | [22] |
| EBV | EBNA3C | LLDFVRFMGV | LLD | HLA-A2 | [23] |
| EBV | LMP2 | LLWTLVVLL | LLW | HLA-A2 | [24] |
| EBV | LMP2 | CLGGLLTMV | CLG | HLA-A2 | [24] |
| EBV | BMLF1 | GLCTLVAML | GLC | HLA-A2 | [25] |
| MVa | NPb | LLWSYAMGV | LLWS | HLA-A2 | [27] |
| HCMV | pp65 | NLVPMVATV | NLV | HLA-A2 | [28] |
| EBV | EBNA3A | RPPIFIRRL | RPP | HLA-B7 | [26] |
| HCMV | IE-1 | CRVLCCYVL | CRV | HLA-B7 | [29] |
MV, measles virus
NP, nucleoprotein.
ELIspot
Multiscreen IP 96-well plates (Millipore, Bedford, MA, USA) were coated overnight at 4°C with 3 µg/ml primary IFN-γ (R&D Research, Abingdon, UK) diluted in 0·1 m carbonate–bicarbonate coating buffer, pH 9·6. The nitrocellulose in the wells was blocked with 2% bovine serum albumin (BSA) in PBS for 30 min at room temperature (RT) and washed four times in PBS. MNC were added at 250 000, 125 000 and 62 500 cells per well together with 2 µm of synthetic peptide, as indicated. Peptides were purchased at Schafer-N, Copenhagen, Denmark and purified to higher than 95% by HPLC and subsequently tested by mass spectrometry. Cultures were incubated for 48 h at 37°C and 5% CO2 in a humidified incubator. Cells were then discarded and the plate was washed four times with PBS before incubation for 3 h at RT with secondary biotinylated anti-IFN-γ antibody (R&D systems, UK) at 0·3 µg/ml. The plate was incubated subsequently with streptavidine–alkaline phosphatase conjugate for 1 h at RT and washed five times in PBS. To visualize the spots, 5-bromo-4-chloro-3-indolyl phosphate and nitro-blue tretrazolium using an alkaline phosphatase–conjugate substrate kit (Bio-Rad, Richmond, CA, USA) was added for 30 min at RT and the development was stopped by washing in distilled water 2 × 5 min. The plate was left to dry before counting spots under a dissection microscope. MNC incubated without peptide was included as background value and subtracted from the values obtained with the specific peptides. The number of spots per 106 cells was calculated by extrapolating from the data obtained by ELIspot on twofold dilutions of the MNC. Extrapolation was performed using the smallest sum of the squares method as provided by the Tendency function in the Excel software. To decide whether the frequency of MS patients was increased Student's t-test was applied.
Cytokine ELISA
Flat-bottomed 96-well Maxi-Sorp™ plates (Nunc, Roskilde, Denmark) were coated with 4 µg/ml of anti-IL4 (MAB 604, R&D Systems, Abingdon, UK) or anti-IL-10 (MAB 217, R&D Systems) in a phosphate-buffered solution containing 15 mm Na2CO3, 35 mm NaHCO3 and 0·2% sodium azide. Plates were washed three times in PBS with 0·05% Tween 20 (Sigma, Bromma, Sweden) and non-specific binding blocked by incubating with TBS with 0·05% Tween-20 and 1% BSA (Sigma) for 2 h at 37°C. The plates were washed three times and incubated with 50 µl of the sample in duplicate overnight at 4°C. Following three washes, the plates were incubated with biotin-conjugated anti-IL-4 (BAF 204, R&D Systems) or biotin-conjugated anti-IL-10 (BAF 217, R&D Systems). Finally, the plates were washed three times and incubated with streptavidin-HRP (P0397, Dako, Glostrup, Denmark) for 20 min at RT followed by the addition of developing reagents (Bio-Rad, Hercules, CA, USA). Reactions were stopped by the addition of 1 m H2SO4 and analysed on an ELISA-reader at 450 nm.
Serology
Blood was collected and allowed to coagulate. Serum was recovered following centrifugation at 500 g for 10 min. IgG antibodies to EBV and HCMV were measured using an ELISA to EBV nuclear antigen (EBNA)1 and to two genetically engineered fusion proteins each combining two immunodominant regions from UL32 and UL44/UL32 of HCMV (Biotest, Germany).
Results
Expression of HLA-A2 and HLA-B7 alleles in MS patients
To examine the cellular immune response in HLA-A2 and HLA-B7 individuals, peripheral blood MNC of consecutive MS patients and controls were analysed by PCR amplification using primers for HLA-A2 and HLA-B7 [13]. Table 2 shows the distribution of these alleles among the MS patients and controls. The frequency of HLA-A2 was 0·53 in controls and 0·41 in MS patients (P = 0·10), whereas the frequency of HLA-B7 was 0·36 in controls and 0·41 in MS patients (P = 0·37).
Table 2.
Expression of HLA-A2 and HLA-B7 genes in MS patients and healthy controls
| HLA-A2 | HLA-A7 | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | % Positive | Positive | Negative | % Positive | |
| C | 35 | 31 | 53·0 | 24 | 42 | 36·4 |
| MS | 28 | 41 | 40·6 | 28 | 41 | 40·6 |
The difference in frequency of HLA-A2 and HLA-B7 between healthy controls (C) and MS patients did not reach statistical significance (P = 0·10 and P = 0·37, respectively, Fisher's exact test).
CD8+ T cell responses to EBV epitopes in MS patients
The CD8+ T cell recognition of EBV epitopes presented by HLA-A2 or HLA-B7 was investigated in MS patients and healthy controls. Serum from all individuals was tested initially by ELISA for the presence of EBNA1 and HCMV IgG, an indication of previous infection. Whereas 100% of the MS patients had EBNA1 antibodies, 13% of the healthy controls were excluded on the basis of a seronegative ELISA result (data not shown). Analysis of IgG antibodies to HCMV showed that 56% of MS patients and 41% of the controls were seropositive, consistent with a higher average age in the MS group.
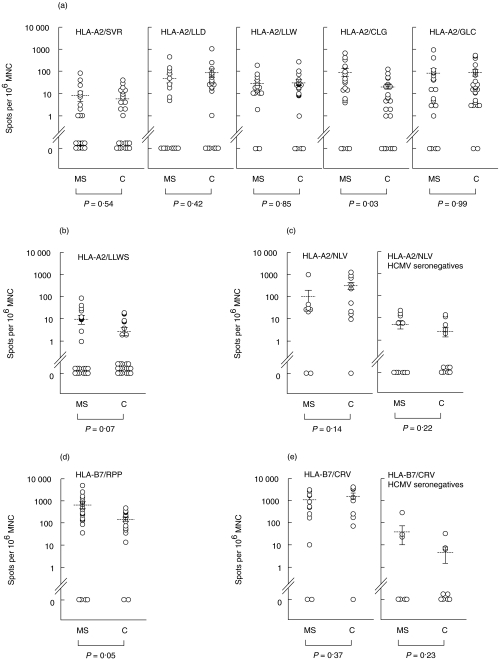
Peripheral blood MNC from EBV seropositive individuals expressing HLA-A2, HLA-B7, or both, were separated and incubated with the indicated peptides (Table 1). The frequency of CD8+ T cell responses to the epitopes was measured by an ELISpot assay detecting IFN-γ secreting cells (Fig. 1). While the response to most of the peptides did not differ significantly between MS patients and controls, the frequency of cells reacting to HLA-A2/CLG (P = 0·02) and HLA-B7/RPP (P = 0·05) were significantly higher among MS patients (Fig. 1a,d). In contrast, the responses to immunodominant peptides from HCMV (HLA-A2/NLV and HLA-B7/CRV) did not differ statistically between MS patients and controls (Fig. 1c,e). The response to an immunodominant epitope from the measles virus nucleoprotein (HLA-A2/LLWS) showed a tendency (P = 0·07) towards a more frequent response in MS patients when compared with controls (Fig. 1b), although these responses were generally of low frequency (compare Fig. 1b with the response to HCMV in seronegatives, Fig. 1c).
Fig. 1.
Frequency of peptide-specific CD8+ T cells in MS patients and healthy controls. (a) MNC from HLA-A2+ MS patients and EBV-seropositive healthy controls were seeded at 250 000, 125 000 and 62 500 cells per well and tested in ELIspot assays for the presence of IFN-γ-secreting cells (spots) to the indicated EBV peptides and expressed as spots per 106 MNC. The mean values with s.e. are indicated. Note that spots are presented on a log scale. The P-value for each comparison between MS patients and controls is indicated. (b) MNC from HLA-A2+ MS patients and EBV-seropositive healthy controls were tested for the response to the measles virus peptide LLWS by ELIspot assays as described in (a). (c) MNC from HCMV-seropositive and -seronegative HLA-A2+ MS patients and healthy controls were tested for the response to the HCMV peptide NLV by ELIspot assays as described in (a). (d) MNC from HLA-B7+ MS patients and EBV-seropositive healthy controls were tested for the response to the EBV peptide RPP by ELIspot assays as described in (a). (e) MNC from HCMV-seropositive and -seronegative HLA-B7+ MS patients and healthy controls were tested for the response to the HCMV peptide CRV by ELIspot assays as described in (a).
The vast majority of CD8+ T cells secrete IFN-γ upon activation. Nevertheless, a subset with Th2-like cytokine profile has been reported. The presence of Th2-like cytokines may potentially inhibit the Th1-like response. For three of the HLA-A2-associated peptides (LLD, CLG and GLC) and the HLA-B7-associated peptide (RPP), supernatants from the peptide-stimulated cultures were analysed for the presence of IL-4 and IL-10. While we were unable to detect IL-4 in the supernatant 24 h after peptide stimulation (data not shown), IL-10 was detected from most cultures at 100–600 ng of IL-10 per ml, but the amount of IL-10 did not differ between MS patients and controls (data not shown).
Correlation of the cellular response to EBV and clinical phase of MS
To examine whether the response of the MS patients differed depending on the clinical phase of the disease, the responses for the MS patients were divided according to their assignment as relapsing-remitting (RR), primary progressive (PP) or secondary progressive (SP). The clinical evaluation, which was blinded with respect to the laboratory results, was performed by an MS specialist. The separation into RR and SP patients indicated that the RR patients contributed more to the overall higher frequency in MS patients than did the SP MS patients (Fig. 2a,b), although neither RR patients (P = 0·15) nor SP patients (P = 0·64) were significantly different from the controls.
Fig. 2.
The dependency on disease phase of CD8+ T cell responses to EBV peptides in MS patients. (a) Comparison of the frequency of IFN-γ-secreting cells (spots) per 106 MNC in HLA-A2 + relapsing remitting (RR) and secondary progressive (SP) MS patients. Mean values with SE are indicated. (b) Frequency of HLA-B7+/RPP-specific CD8+ T cells in primary progressive (PP), RR and SP MS patients. The frequency of IFN-γ-secreting cells during stimulation with the EBV epitope RPP was determined by ELIspot assays. Results are shown as spots per 106 MNC. The results for controls (C), secondary progressive MS patients (SP), relapsing-remitting MS patients (RR) and all MS patients together are shown as mean values with s.e., whereas the primary progressive MS patients are shown with individual responses (PP1 to PP3). ▪, RR; □, SP.
Whereas the majority of patients belong to the RR or SP phase, three HLA-B7+ patients were PP. Surprisingly, all of the three HLA-B7 expressing PP MS patients had a higher frequency of RPP-reactive CD8+ T cells than did the SP and RR group of subjects (Fig. 2b).
Discussion
Based on an epidemiological and serological association of EBV infection with MS [18], we examined the CD8+ T cell responses in MS patients and controls to previously identified HLA-A2 or -B7-presented immunogenic epitopes encoded by EBV. Others have demonstrated a reduced frequency of HLA-A2 and an increased frequency of HLA-B7 in MS patients. The increase in HLA-B7 is explained by its linkage disequilibrium with HLA-DRB1*1501, whereas HLA-A2 reduces the risk of MS (OR = 0·52) in the context of either HLA-A3 or HLA-DRB1*1501 [30]. Our consecutive analysis of the HLA-A2 and -B7 alleles did not show a statistical significant difference between MS patients and controls, although a tendency was indicated.
We observed an increased frequency of CD8+ T cells specific to HLA-A2/CLG and HLA-B7/RPP in MS patients. The increased response to EBV epitopes in MS patients may be explained by an increased load of EBV, an increased expression of the latent genes, a general increase in the immune response due to impaired immunological suppression, or the presence of cross-reactive epitopes that maintain the memory pool of T cells at a higher frequency [31].
There is no evidence of an increased load of EBV in MS patients. Nevertheless, serological studies have shown an increased level of IgM and IgA antibodies to EBV early antigens in addition to the presence of EBV DNA in the serum of MS patients in an active phase of the disease in contrast to clinically stable patients [32], although this has not yet been confirmed by independent groups. In addition, a higher propensity of B cells from MS patients to spontaneously transform in vitro has been reported by two independent groups [33,34]. Nevertheless, there is no direct evidence for an altered regulation of EBV genes in MS patients.
The increased frequency may also, in part, represent a generally reduced suppression of the immune response. In mice, a critical role of the CD4+CD25+ subset for preventing autoimmunity has been demonstrated [35]. A CD4+CD25+ regulatory T cell has also been found in humans [36], although its significance for the development of autoimmunity is unknown.
Finally, an increased frequency of T cell response to selected EBV epitopes may be based on the maintenance of these memory T cells in MS patients by a cross-reacting epitope. Indeed, accumulating evidence suggest that previous infections of an individual influences the responses to immunodominant epitopes through heterologous, cross-reacting pathogens (reviewed in [37]). Thus, CD8+ T cells reacting with influenza virus epitopes may cross-react with rotavirus [38] and hepatitis C virus [39]. Importantly, analysis of the dynamics of the memory T cell pool has demonstrated that cytokine stimulation did not alter the number or the hierarchy of the antigen-specific T cells [40]. Homologous virus stimulation resulted in a dramatic increase in the frequency, but maintained more or less the immunodominant hierarchy, whereas heterologous virus stimulation increased the frequency approximately sevenfold to some but not other epitopes [40]. A moderate increase to some but not other epitopes may describe our finding of increased CD8+ T cell response to two EBV epitopes. Thus, it remains an intriguing possibility that the prior sequence of infections in MS patients may be different than in healthy controls. Alternatively, it may be speculated that a cross-reacting epitope may be a self-peptide. Cross-reacting CD8+ T cells may also explain the responses to HCMV peptides in HCMV seronegative individuals, although a seronegative HCMV infection is also possible.
Noticeably, a higher frequency of HLA-B7/RPP-response was found in all of the three HLA-B7+ patients with PP MS when compared with SP and RR MS. While we believe this is of interest, the low number of subjects makes this observation very preliminary. We plan to examine additional PP patients, but because this is a relatively rare manifestation of MS, and because the frequency of HLA-B7 in northern Europe is approximately 26% (that is, only one in four PP patients can be tested for the response to HLA-B7/RPP), this is a long-term project.
While our data demonstrate an association between the CD8+ T-cell response to EBV epitopes and MS, we do not know whether the association is causal. Speculation of a causal relationship may involve the possibility of cross-reacting epitopes to prime an autoreactive CD8+ T cell response against a myelin antigen [41]. Indeed, CD4+ T cells cross-reacting between an EBV epitope and myelin basic protein has been demonstrated [42].
Acknowledgments
This work was supported by grants from the Danish Multiple Sclerosis Society. We thank B. Bundgaard and M. Schjerven for excellent technical assistance.
References
- 1.Yewdell JT, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Ann Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Messaoudi I, Patiño JAG, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorfism, T cell avidity, and diversity in immune defence. Science. 2002;298:1797–800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 3.Rooney CM, Smith CA, Ng CYC, et al. Use of gene-modified virus-specific T-lymphocytes to control Epstein–Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 4.Roskrow MA, Suzuki N, Gan YJ, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease. Blood. 1998;91:2925–34. [PubMed] [Google Scholar]
- 5.Khanna R, Bell S, Sherritt M, et al. Activation and adoptive transfer of Epstein–Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci. 1999;96:10391–6. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharadwaj M, Moss DJ. Epstein–Barr virus vaccine: a cytotoxic T-cell-based approach. Expert Rev Vaccines. 2002;1:467–76. doi: 10.1586/14760584.1.4.467. [DOI] [PubMed] [Google Scholar]
- 7.Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein–Barr virus-associated diseases. Ann Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein–Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport MP, Fazou C, McMichael AJ, Callan MFC. Clonal selection, clonal senescence, and clonal succession: the evolution of the T cell response to infection with a persistent virus. J Immunol. 2002;168:3309–17. doi: 10.4049/jimmunol.168.7.3309. [DOI] [PubMed] [Google Scholar]
- 10.Catalina MD, Sullivan JL, Bak KR, Luzuriaga K. Differential evolution and stability of epitope-specific CD8+ T cell responses in EBV infection. J Immunol. 2001;167:4450–7. doi: 10.4049/jimmunol.167.8.4450. [DOI] [PubMed] [Google Scholar]
- 11.Annels NE, Callan MFC, Tan L, Rickinson AB. Changing patterns of dominant RCR usage with maturation of an EBV-specific cytotoxic T cell response. J Immunol. 2000;165:4831–41. doi: 10.4049/jimmunol.165.9.4831. [DOI] [PubMed] [Google Scholar]
- 12.Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Immediate early and early lytic cycle proteins are frequenct targets of the Epstein–Barr virus-induced cytotosic T cell response. J Exp Med. 1997;185:1605–17. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharadwaj M, Burrows SR, Burrows JM, Moss DJ, Catalina M, Khanna R. Longitudinal dynamics of antigen-specific CD8 (+) cytotoxic T lymphocytes following primary Epstein–Barr virus infection. Blood. 2001;98:2588–9. doi: 10.1182/blood.v98.8.2588. [DOI] [PubMed] [Google Scholar]
- 14.Callan MFC, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8 (+) T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan LC, Gudgeon N, Annels NE, et al. A re-evaluation of the frequency of CD8 (+) T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–35. [PubMed] [Google Scholar]
- 16.Dyment DA, Sadnovich AD, Ebers GC. Genetics of multiple sclerosis. Hum Mol Genet. 1997;6:1693–8. doi: 10.1093/hmg/6.10.1693. [DOI] [PubMed] [Google Scholar]
- 17.Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47:425–48. [PubMed] [Google Scholar]
- 18.Haahr S, Höllsberg P. The ability of candidate viruses to explain epidemiological findings in multiple sclerosis. In: Hommes OR, Wekerle H, Clanet M, editors. Genes and viruses in multiple sclerosis. Amsterdam: Elsevier Science BV; 2001. pp. 163–84. [Google Scholar]
- 19.Ascherio A, Munger KL, Lennette ET, et al. Epstein–Barr virus antibodies and risk of multiple sclerosis − a prospective study. JAMA. 2001;286:3083–8. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 20.Haahr S, Koch-Henriksen N, Møller-Larsen A, Eriksen LS, Andersen HMK. Increased risk of multiple sclerosis after late Epstein–Barr virus infection: a historical prospective study. Multiple Sclerosis. 1995;1:73–7. doi: 10.1177/135245859500100203. [DOI] [PubMed] [Google Scholar]
- 21.Bech E, Lycke J, Gadeberg P, et al. A randomized, double-blind, placebo-controlled MRI study of anti-herpes virus therapy in MS. Neurology. 2002;58:31–6. doi: 10.1212/wnl.58.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Burrows SR, Gardner J, Khanna R, et al. Five new cytotoxic T cell epitopes identified within Epstein–Barr virus nuclear antigen 3. J Gen Virol. 1993;75:2489–93. doi: 10.1099/0022-1317-75-9-2489. [DOI] [PubMed] [Google Scholar]
- 23.Kerr BM, Kienzle N, Burrows JM, et al. Identification of type B-specific and cross-reactive cytotoxic T-lymphocyte responses to Epstein–Barr virus. J Virol. 1996;70:8858–64. doi: 10.1128/jvi.70.12.8858-8864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SP, Thomas WA, Murray RJ, et al. HLA-A2.1-restricted cytotoxic T cells recognizing a range of Epstein–Barr virus isolates through a defined epitope in latent membrane protein LMP2. J Virol. 1993;67:7428–35. doi: 10.1128/jvi.67.12.7428-7435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scotet E, DavidAmeline J, Peyrat MA, et al. T cell response to Epstein–Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996;184:1791–800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill A, Worth A, Elliott S, et al. Characterization of two Epstein–Barr virus epitopes restricted by HLA-B7. Eur J Immunol. 1995;25:18–24. doi: 10.1002/eji.1830250105. [DOI] [PubMed] [Google Scholar]
- 27.Nanan R, Carstens C, Kreth HW. Demonstration of virus-specific CD8 (+) memory T cells in measles-seropositive individuals by in vitro peptide stimulation. Clin Exp Immunol. 1995;102:40–5. doi: 10.1111/j.1365-2249.1995.tb06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solache A, Morgan CL, Dodi AI, et al. Identification of three HLA-A*0201-restricted cytotosic T cell epitopes in the cytomegalovirus protein pp65 that are conserved between eight strains of the virus. J Immunol. 1999;163:5512–8. [PubMed] [Google Scholar]
- 29.Kern F, Surel IP, Faulhaber N, et al. Target structures of the CD8 (+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999;73:8179–84. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55:140–8. doi: 10.1034/j.1399-0039.2000.550205.x. [DOI] [PubMed] [Google Scholar]
- 31.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–34. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 32.Wandinger KP, Jabs W, Siekhaus A, et al. Association between clinical disease activity and Epstein–Barr virus reactivation in MS. Neurology. 2000;55:178–84. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- 33.Fraser KB, Haire M, Millar JHD, McCrea S. Increased tendency to spontaneous in vitro lymphocyte-transformation in clinically active multiple sclerosis. Lancet. 1979;2:715–7. [PubMed] [Google Scholar]
- 34.Munch M, Møller-Larsen A, Christensen T, Morling N, Hansen HJ, Haahr S. B-lymphoblastoid cell-lines from multiple sclerosis patients and a healthy control producing a putative new human retrovirus and Epstein–Barr virus. Multiple Sclerosis. 1995;1:78–81. doi: 10.1177/135245859500100204. [DOI] [PubMed] [Google Scholar]
- 35.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 36.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 (high) regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 37.Welsh RM, Selin LK. No one is naïve: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–26. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 38.Shimojo N, Maloy WL, Anderson RW, Biddison WE, Coligan JE. Specificity of peptide binding by the HLA-A2.1 molecule. J Immunol. 1989;143:2939–47. [PubMed] [Google Scholar]
- 39.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392–400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SK, Brehm MA, Welsh RM, Selin LK. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol. 2002;169:90–8. doi: 10.4049/jimmunol.169.1.90. [DOI] [PubMed] [Google Scholar]
- 41.Misko IS, Cross SM, Khanna R, et al. Crossreactive recognition of viral, self, and bacterial peptide ligands by human class I-restricted cytotoxic T lymphocyte clonotypes: implications for molecular mimicry in autoimmune disease. Proc Natl Acad Sci USA. 1999;96:2279–84. doi: 10.1073/pnas.96.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wucherpfennig KW, Strominger JL. Molecular mimicry in T-cell-mediated autoimmunity − viral peptides activate human T-cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]