Abstract
Anti-C1q autoantibodies are present in the serum of patients with different autoimmune diseases such as systemic lupus erythematosus (SLE). The occurrence of these autoantibodies correlates with renal involvement. In the present study we examined whether injection of rabbit antimouse C1q antibodies in mice leads to deposition in kidneys. Injection of healthy mice with a single dose of rabbit IgG antimouse C1q antibodies resulted in deposition of both C1q and IgG anti-C1q in glomeruli. The pattern of deposition observed in the glomeruli of mice injected with antimouse C1q antibodies both at 24 h and 2 weeks was both glomerular basement membrane (GBM)-associated and mesangial. Injection of control IgG did not have a detectable effect on circulating C1q levels, and no deposition of either C1q or rabbit IgG was seen at 24 h. The deposition of rabbit antimouse C1q and C1q in glomeruli resulted in complement activation, as assessed by C3 deposition, and influx of leucocytes associated with albuminuria in some, but not all mice. In none of the control mice was albuminuria observed. This report is the first to show that anti-C1q antibodies deposit in the healthy glomerulus together with autologous C1q. This deposition is stable for at least 2 weeks, causes complement activation, leucocyte influx and can lead to mild albuminuria.
Keywords: autoantibody, C1q, kidney, mouse, SLE
Introduction
The complement system plays a crucial role in innate defence [1,2]. In addition the complement system plays a role in the generation of an acquired immune response [3]. Under normal circumstances the contribution of complement is beneficial to the host. However, it may also amplify tissue injury. Deficiency of certain complement components predisposes to autoimmunity [4]. In autoimmune individuals, complement components can even be the target of an autoantibody response [5]. Activation of the classical pathway of complement is mediated via activation of C1 leading to enzymatic cleavage of C4, C2 and C3, finally resulting in the activation of the terminal pathway. C1 is composed of the subunits C1q, C1r and C1s.
Anti-C1q autoantibodies can be found in the serum of patients with different systemic and renal diseases such as rheumatoid arthritis (RA), rheumatoid vasculitis (RV), systemic lupus erythematosus (SLE), hypocomplementaemic urticarial vasculitis syndrome (HUVS), membrano-proliferative glomerulonephritis (MPGN) and anti-glomerular basement membrane (GBM) nephritis [6,7]. The percentage of anti-C1q positive individuals varies per disease with 35% of the SLE patients and up to 100% of the HUVS patients being positive [7]. Low titre anti-C1q autoantibodies have also been demonstrated in some healthy individuals [8]. The autoantibodies against C1q tend to react stronger with solid-phase C1q than with fluid phase C1q [9] and are predominantly of the IgG and IgA isotype [6]. In SLE patients there is a correlation between the occurrence of anti-C1q autoantibodies, hypocomplementaemia and nephritis [10,11]. Indeed, elution of glomeruli from patients with SLE and nephritis has demonstrated the presence of anti-C1q antibodies in the kidney [12]. It has been suggested that a rise in the titre of anti-C1q autoantibodies can be used as a predictive marker for a flare in nephritis [13,14]. Absence of anti-C1q autoantibodies is a very strong indicator for the absence of nephritis [15]. Despite these correlations there is no insight into how anti-C1q antibodies deposit in the kidney and contribute to disease.
Previous studies have demonstrated that administration of cationized immune complexes, containing human C1q, followed by human anti-C1q autoantibodies resulted in deposition of these autoantibodies in glomerular immune deposits [16]. Further work showed that human C1q when injected in mice has a transient interaction with the GBM and that rabbit antihuman C1q antibodies were able to stabilize this interaction [17]. Both studies used high concentrations of human C1q and antihuman C1q antibodies in a mouse.
In the present study we investigated whether rabbit antimouse C1q antibodies when injected into mice have a pathogenic effect on the kidney. We observed that injection of anti-C1q antibodies leads to their glomerular deposition in both a linear and mesangial pattern and depletion of circulating C1q. However, renal disease was observed only in a limited number of mice, suggesting the need for additional pathogenic factors.
Materials And Methods
Purification of mouse C1q
Mouse C1q was isolated following a procedure described earlier for human C1q [18]. For this purpose 200 ml mouse serum (Harlan, Horst, the Netherlands), was adjusted to 10 mm EDTA, and applied to a rabbit IgG Sepharose column. After extensive washing with PBS containing 10 mm EDTA, bound C1q was eluted using 1 m NaCl containing 10 mm EDTA. C1q in the fractions was identified by ELISA, pooled and dialysed against PBS, concentrated to 0·5 mg/ml and stored in aliquots at – 80°C.
Detection of mouse C1q
For the detection of mouse C1q, Microlon F-shape plates (Greiner bio-one, Alphen aan de Rijn, the Netherlands) were coated with 10 µg/ml rabbit antimouse C1q in coating buffer (100 mm Na2CO3/NaHCO3, pH 9·6), for 1 h at 37°C. After incubation, plates were washed three times with PBS containing 0·05% Tween 20 (PBS-T). Residual binding sites were blocked by incubation with PBS containing 1% BSA (PBS-BSA), for 1 h at 37°C. After washing, sera diluted 1 : 400 in PBS-T-BSA containing 1 m NaCl were incubated, for 1 h at 37°C. After washing, bound C1q was detected using rabbit antimouse C1q conjugated to digoxigenin (Dig) (Boehringer Mannheim, Mannheim, Germany), in PBS-T-BSA, for 1 h at 37°C. Detection of binding of the Dig-conjugated antibodies was performed by sheep Fab anti-Dig antibodies (Boehringer Mannheim) in PBS-T-BSA for 1 h at 37°C. Enzyme activity of HRP was developed using 2,2′-azino-bis(3-ethyl benzathioline-6-sulphonic acid) (Sigma-Aldrich, Zwijndrecht, the Netherlands). The O.D. at 415 nm was measured using a microplate biokinetics reader (EL312e; Biotek Instruments, Winooski, VT, USA). A calibration line was produced using purified mouse C1q with a known concentration. Data are expressed as C1q concentration in µg/ml. All ELISAs were performed using the same protocol as the above-mentioned C1q detection ELISA, unless stated otherwise.
Detection of serum C1q by Western blot: serum of mice injected with anti-C1q or control rabbit IgG together with a positive control, normal mouse serum (NMS) and a negative control, C1q-deficient mouse serum, was separated by 10% SDS-PAGE under reducing conditions; proteins were transferred to nitrocellulose membrane and stained with rabbit antimouse C1q conjugated to Dig and anti-Dig-HRP, followed by chemiluminescence (Supersignal, Pierce, Rockford, IL, USA).
Preparation of rabbit antimouse C1q
Polyclonal antiserum against mouse C1q was obtained by immunization of male New Zealand White rabbits (Harlan) with purified mouse C1q. Injection of 30 µg C1q in 100 µl complete Freund's adjuvant (Difco, Detroit, MI, USA) subcutaneously was followed by three boosts with 30 µg mouse C1q in 100 µl incomplete Freund's adjuvant (Difco) at 2-week intervals. Rabbit immune serum was heat-inactivated at 56°C for 30 min and centrifuged for 20 min at 3000 r.p.m. Gamma globulin precipitation was performed using (NH4)2SO4 at 40% saturation and the precipitate was purified further by anion exchange chromatoghraphy using DEAE-Sephacel (Pharmacia, Uppsala, Sweden). Fractions were tested for the presence of antimouse C1q reactivity by ELISA. Microtitre plates were coated with purified mouse C1q at 2·5 µg/ml and serial dilutions of the fractions tested in PBS-T-BSA containing 1 m NaCl. The binding of rabbit IgG was demonstrated using goat antirabbit IgG conjugated to HRP (Jackson Immuno Research Laboratory Inc.,West Grove, PA, USA). C1q reactive fractions were pooled, concentrated and dialysed against 0·15 m NaCl. Cross-reactivity towards other mouse serum components was removed by absorbing the antiserum using an affinity column onto which total serum proteins of C1q deficient mice (C1q -/-) was coupled [19]. For control experiments, non-immune rabbit IgG was purified using the same protocol.
To determine the specificity of the rabbit IgG antimouse C1q two methods were used. For Western blot analysis normal mouse serum (NMS) or C1q-deficient mouse serum was separated by 10% SDS-PAGE under reducing conditions; proteins were transferred onto nitrocellulose membrane and stained with rabbit antimouse C1q conjugated to Dig and anti-Dig-HRP, followed by chemiluminescence (Supersignal, Pierce, Rockford, IL, USA). For ELISA, microtitre plates were coated with 3 µg/ml purified mouse C1q, washed, blocked and incubated with increasing concentrations of rabbit IgG antimouse C1q or control rabbit IgG starting at 5 µg/ml. After washing, rabbit IgG binding was detected using goat antirabbit IgG conjugated to HRP (Jackson) in PBS-T-BSA containing 1 m NaCl.
Animals
Female BALB/C mice (Harlan), 6 weeks of age, had free access to water and standard chow. Animal care and experimental procedures were performed in accordance with the National Institutes of Health Guidelines for the care and use of laboratory animals.
Experimental protocol
Groups of 10 BALB/C mice were injected with 5 mg rabbit antimouse C1q or 5 mg rabbit IgG intraperitoneally in 500 µl 0·15 m NaCl. Groups of five mice were sacrificed at 24 h or at 2 weeks after injection. Urine was collected by putting the mice into metabolic cages for 18 h and blood was collected by tail cut at day 0, day 1 and 2 weeks. Mice were anaesthetized with urethane (Sigma) and sacrificed by heart puncture. At sacrifice, one kidney was frozen for immunohistochemistry, and the other kidney fixed for histology. Urinary albumin was measured using an autoanalyser (Hitachi-911, Hitachi, Tokyo, Japan) and is expressed as µg albumin per 24 h.
Detection of circulating rabbit IgG and mouse antirabbit IgG
Circulating rabbit IgG was detected using a sandwich ELISA. Plates were coated with goat antirabbit IgG at 10 µg/ml (DAKO, Glostrup, Denmark). Sera were diluted 1 : 1000 in PBS-T-BSA and rabbit IgG was detected using goat antirabbit IgG conjugated to HRP (Jackson). A calibration line was produced using purified rabbit IgG with a known concentration. Data were expressed as rabbit IgG concentration in µg/ml.
Mouse antirabbit IgG antibodies were detected using ELISA. Plates were coated with rabbit IgG, sera were diluted 1 : 400 and mouse IgG was detected using goat-anti mouse IgG conjugated to HRP (Dako). Data are expressed relative to a mouse antirabbit IgG immune serum, set arbitrarily at 1000 units/ml.
Histological studies
For light microscopy, renal tissue was fixed in methyl Carnoy's solution, embedded in paraffin and 3 µm sections were stained with haematoxylin–eosin (H&E) or periodic acid-Schiff (PAS).
Histological changes were scored by a pathologist (H.B.), who was blinded to the code of the sections. Sections were graded for glomerular proliferation, hyaline deposits and glomerular sclerosis using an arbitrary scale as (–) absent, (+) present in less than 25% of the glomeruli, (+ +) 25–50%, or (+ + +) more than 50% of the glomeruli. For statistical analysis these scores were assigned values of 0–3.
For immunofluorescence one kidney was snap-frozen in precooled isobutanol and stored at − 150°C. Cryostat sections of 3 µm were fixed using acetone and stained for the deposition of rabbit IgG, mouse C1q, mouse C3 and mouse IgG. All antibodies were incubated in PBS containing 1% BSA in a humid incubator for 1 h at room temperature. Sections were washed three times for 5 min using PBS.
Rabbit IgG was detected using goat antirabbit IgG conjugated to FITC (Nordic, Tilburg, the Netherlands), mouse C1q was detected using rabbit antimouse C1q Dig and sheep Fab anti-Dig conjugated to FITC (Boehringer Mannheim). Mouse C3 was detected using FITC conjugated goat antimouse C3 (Nordic, Tilburg, the Netherlands). Mouse IgG was detected using goat antimouse IgG conjugated to Oregon Green (Molecular Probes, Leiden, the Netherlands).
Fluorescence intensity was arbitrarily scored as – (negative), + (positive), + + (strongly positive) and + + + (brightly positive). For statistical analysis these scores were assigned values of 0–3.
Infiltrating leucocytes were visualized using rat antimouse CD45 (BD PharMingen, Woerden, the Netherlands). Rat IgG was detected using goat antirat IgG conjugated to HRP (Dako), which was preincubated with 10% NMS to absorb antimouse IgG cross-reactivity. Nova RED (Vector, Brunschwig, Amsterdam, the Netherlands) was used as a substrate. Infiltrating CD45-positive cells were counted, using a microscope, in at least 10 glomeruli by two observers who were blinded to the code of the sections. Data were expressed as absolute numbers of CD45-positive cells per glomerulus.
Statistics
Statistical analysis was performed using SPSS software. Mann–Whitney tests were performed for the evaluation of differences between rabbit anti-C1q injected mice and IgG injected mice. P-values were considered statistically significant when P < 0·05.
Results
Generation of rabbit antimouse C1q antibodies
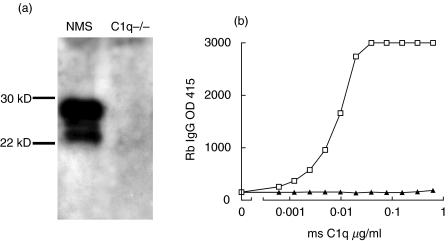
By immunization of rabbits with purified mouse C1q, an immune serum was obtained from which rabbit IgG was purified. Rabbit antimouse C1q antibodies were tested using Western blot and ELISA. Under reducing conditions C1q falls apart in its three chains of 24, 25 and 28 kDa, of which all three bands are recognized by the polyclonal antibody on Western blot. Only reactivity with C1q-sufficient serum and not C1q-deficient serum was seen (Fig. 1a).
Fig. 1.
Characterization of rabbit antimouse C1q antibodies. (a) Western blot analysis of normal mouse serum (NMS) and serum of a C1q knock out mouse (C1q -/-). Total serum was separated on a 10% SDS-PAGE gel under reducing conditions, blotted to nitrocellulose and stained with Dig-conjugated rabbit antimouse C1q followed by goat anti-Dig-HRP. (b) ELISA reactivity of rabbit antimouse C1q and control non-immune rabbit IgG with increasing doses of plate-bound C1q as indicated; the experiment was performed in 1 m NaCl buffer conditions, and bound antibody was detected by goat antirabbit IgG HRP. □, Rb anti-C1q; ▴, Rb IgG.
For ELISA, wells were coated with 2·5 µg/ml mouse C1q, followed by rabbit antimouse C1q or control rabbit IgG under 1 m NaCl buffer conditions to prevent Fc-mediated interaction of IgG with C1q [20,21]. While purified immune IgG exhibited a dose-dependent interaction with C1q, no reactivity of control rabbit IgG with C1q was observed (Fig. 1b).
Glomerular deposition of rabbit antimouse C1q and C1q 24 h after injection
Groups of mice were injected with rabbit antimouse C1q or control non-immune rabbit IgG and assessed for renal deposition of rabbit IgG, mouse C1q, mouse C3 and mouse IgG at 24 h after injection. Injection of rabbit antimouse C1q antibodies resulted in both a GBM-associated and mesangial pattern of rabbit IgG and mouse C1q in the glomeruli of these mice at 24 h after injection (Fig. 2). This deposition is associated with the presence of mouse C3. Mice receiving control rabbit IgG did not exhibit detectable deposition of rabbit IgG, or C3. C1q deposition was marginal and not different from normal, non-injected mice.
Fig. 2.
Immunofluorescence analysis of renal sections obtained 24 h after injection.Analysis of deposition of rabbit IgG (Rb IgG), mouse C1q (Ms C1q), mouse C3 (Ms C3) and mouse IgG (Ms IgG) in kidney sections of mice injected with rabbit antimouse C1q (top row) or rabbit IgG (bottom row) and sacrificed at 24 h after injection.
The deposition of rabbit IgG and C1q in mice injected with rabbit antimouse C1q is associated with depletion of circulating C1q (Fig. 3a). This depletion of circulating C1q was confirmed by Western blot analysis using denaturing and reducing conditions, showing no C1q in the anti-C1q injected mice and a normal C1q level in the rabbit IgG-injected mice. (Fig. 3b)
Fig. 3.
Analysis of serum levels of mouse C1q and rabbit IgG at 24 h after injection. Assessment of serum levels of C1q (a) and rabbit IgG (c) of control mice and in sera of mice sacrificed 24 h after injection with anti-C1q or IgG. The mean and standard deviation are shown (n = 5 mice per group). (b) Western blot analysis of mouse sera, obtained 24 h after injection, of mice injected with anti-C1q or control rabbit IgG with NMS as a positive and C1q -/- as a negative control. Total serum was separated on a 10% SDS-PAGE gel under reducing conditions, blotted onto nitrocellulose and stained with Dig-conjugated rabbit antimouse C1q followed by goat anti-Dig-HRP. □, Anti-C1q;  , control IgG.
, control IgG.
The deposition in the glomerulus and the depletion of C1q are not the result of differences in the amount of injected rabbit IgG because at 24 h there is even less circulating rabbit IgG in the anti-C1q injected mice than in the control rabbit IgG injected group (Fig. 3c).
Mouse antirabbit IgG antibodies enhance the effect of rabbit antimouse C1q
At 2 weeks after injection there was still a clear glomerular deposition in both a GBM-associated and mesangial pattern of rabbit IgG, mouse C1q and mouse C3 in the mice receiving anti-C1q antibodies (Fig. 4). Interestingly, mice receiving control IgG exhibited limited mesangial deposition of rabbit IgG and mouse C1q, however, without significant C3 deposition. While at 24 h no detectable mouse IgG was present, at 2 weeks rabbit IgG deposition is associated with deposition of mouse IgG.
Fig. 4.
Immunofluorescence analysis of renal sections obtained 2 weeks after injection. Analysis of deposition of rabbit IgG (Rb IgG), mouse C1q (Ms C1q), mouse C3 (Ms C3) and mouse IgG (Ms IgG) in kidney sections of mice injected with rabbit antimouse C1q (top row) or rabbit IgG (bottom row) and sacrificed at 2 weeks after injection.
ELISA analysis confirmed a mouse antibody response against rabbit IgG that was equally strong in both groups (Fig. 5a). In both conditions circulating rabbit IgG disappeared from the circulation (Fig. 5b). Circulating C1q levels were lower in both groups compared to those in control normal mouse serum (Fig. 5b compared to Fig. 3a), indicating that the depletion of C1q seen at 24 h is transient and that at 2 weeks C1q is partially consumed, probably by immune complexes between mouse antirabbit IgG and rabbit IgG.
Fig. 5.
Analysis of serum levels of mouse antirabbit IgG, mouse C1q and rabbit IgG at 2 weeks after injection. Serum levels of mouse antirabbit IgG (a) of control mice (t = 0) and of mice injected with rabbit antimouse C1q or rabbit IgG were assessed at 24 h and 2 weeks after injection. Serum levels of mouse C1q and rabbit IgG (c) of mice injected with rabbit IgG antimouse C1q or rabbit IgG and sacrificed at 2 weeks after injection. The mean and standard deviation are shown (n = 5 mice per group). (a) □, anti-C1q; ▴, Control IgG. (b) □, Anti-C1q;  , control IgG.
, control IgG.
Consequences of the deposition of rabbit antimouse C1q and C1q in glomeruli
In order to evaluate the possible pathogenic effect of renal deposition of C1q and anti-C1q antibodies, we monitored renal depositions and histological changes, quantified infiltrating cells, and determined urinary albumin loss as a measure of renal damage. Table 1 lists the results of the different analyses for all the individual mice. Glomerular deposition of rabbit IgG, mouse C1q, mouse C3 and mouse IgG were determined using immunofluorescence. Fluorescence intensities were scored semiquantitatively and are depicted for all mice in Table 1. Cryostat sections were stained for the presence of cells positive for CD45. This revealed that only at 2 weeks there were significantly more CD45-positive cells present in the glomeruli of the mice injected with rabbit antimouse C1q compared to the mice injected with rabbit IgG (P = 0·046).
Table 1.
Summary of results of various parameters of mice injected with rabbit anti mouse C1q or control IgG
| Experimental | Control | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Average | 1 | 2 | 3 | 4 | 5 | Average | p value | |
| 24 h | |||||||||||||
| Rb IgG deposition | + + | + + | + + | + + | + + | + + | – | – | – | – | – | – | 0·03* |
| Ms C1q deposition | + + | + + | + + | + + | + + | + + | – | – | – | – | – | – | 0·03* |
| Ms C3 deposition | + + | + + | + + | + + | + + | + + | – | – | – | – | – | – | 0·03* |
| Ms IgG deposition | – | – | – | – | – | – | – | – | – | – | – | – | – |
| CD 45 pos cells | 0·7 | 0·2 | 2 | 0·5 | 0·85 | 0·85 | 0·7 | 0·6 | 0·7 | 0·6 | 0·6 | 0·64 | 0·748 |
| Glom. proliferation | – | – | – | – | – | – | – | + | – | – | – | – | 0·317 |
| Hyaline deposits | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Glom. sclerosis | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Albuminuria | 30 | 26·1 | 29·8 | 19·1 | 42·1 | 29·42 | 34·6 | 16·2 | 40·9 | 18·4 | 5·3 | 23·08 | 0·347 |
| Mouse no. | 6 | 7 | 8 | 9 | 10 | Average | 6 | 7 | 8 | 9 | 10 | Average | P-value |
| 2 weeks | |||||||||||||
| Rb IgG deposition | + + + | + + + | + + + | + + + | + + + | + + + | + | + | + | + | + | + | 0·03* |
| Ms C1q deposition | + + + | + + + | + + + | + + + | + + + | + + + | + | + | + | + | + | + | 0·03* |
| Ms C3 deposition | + | + | + | + | + | + | – | – | – | – | – | – | 0·03* |
| Ms IgG deposition | + + | + + | + + | + + | + + | + + | + | + | + | + | + | + | 0·03* |
| CD 45 pos cells | 3·7 | 1·9 | 2·2 | 2·1 | 2·5 | 2·48 | 1·9 | 2·2 | 1·2 | 0·7 | 1·3 | 1·46 | 0·046* |
| Glom. proliferation | + | + | + | + | + | + | + | + | + | + | + | + | – |
| Hyaline deposits | – | + | + | – | – | – | – | – | – | – | – | – | 0·134 |
| Glom. sclerosis | – | – | + | – | – | – | – | – | – | – | – | – | 0·317 |
| Albuminuria | 4·8 | 12·6 | 364·8 | 30·7 | 86·1 | 99·8 | 59·8 | 7·9 | 15·3 | 32·8 | 7·5 | 24·66 | 0·602 |
Immunofluorescence intensities were scored as – (absent), + (positive), + + (strongly positive), + + + (brightly positive) (for statistical analysis scored as 0, 1, 2, 3).CD45-positive cells, as a marker for leucocytes, were counted in at least 10 glomeruli and expressed as absolute numbers of CD45-positive cells per glomerulus.Glomerular cell proliferation, hyaline deposits and glomerular sclerosis were scored as (–), absent,(+) present in less than 25% of the glomeruli,(+ +), 25–50% or (+ + +), more than 50% of the glomeruli (for statistical analysis scored as 0, 1, 2, 3). Renal injury was quantified by measuring 24 h urinary albumin excretion, data expressed as ug albumin per 24 h. Statistical analysis was performed by testing mice injected with rabbit antimouse C1q against those that received rabbit IgG in a Mann–Whitney test.
Significant difference between groups.
Paraffin sections stained with H/E and PAS were scored by a pathologist (H.B.) who was blinded to the code of the sections, At 24 h there were no histological changes but at 2 weeks there were some changes in the anti-C1q injected group (Table 1). Some mice showed glomerular cell proliferation and CD45 positive cell influx. One mouse showed both hyaline deposits (thrombi) and some glomerular sclerosis. In this case (anti-C1q mouse no. 8) histological alterations were associated with significant albuminuria, which was not found in the other mice.
Discussion
In SLE there is a correlation between the presence of anti-C1q autoantibodies, hypocomplementaemia and the development of glomerulonephritis [11,22]. However, this does not provide proof for pathogenicity of anti-C1q autoantibodies. Our goal in the present study was to determine the effect of the administration of anti-C1q antibodies in relation to the deposition of C1q and anti-C1q antibodies in the kidneys of healthy mice. This study demonstrates that injection of healthy mice with rabbit antimouse C1q antibodies resulted in reduced levels of circulating C1q and deposition of anti-C1q antibodies and complement components in the glomerulus.
Different experimental approaches have been used previously to assess pathogenicity of anti-C1q autoantibodies. First, human immune complexes containing human C1q were injected into a mouse [16]. These complexes deposited in the glomerulus and a subsequent injection of human anti-C1q autoantibodies resulted in the binding of these autoantibodies to the deposited C1q. This demonstrated that anti-C1q autoantibodies can deposit in the glomerulus onto immune complexes that are already present in the glomerulus. In another study, injection of mice with human C1q resulted in a transient interaction between human C1q and mouse GBM and subsequent injection of rabbit antihuman C1q antibodies resulted in stabilization of this C1q–GBM interaction, suggesting that autoantibodies to C1q can also deposit in the glomerulus onto ‘non-immune complex-associated’-C1q, bound transiently to the GBM [17]. Both studies used relatively high concentrations of C1q and anti-C1q to achieve binding and assess pathogenicity.
Administration of anti-C1q antibodies resulted in the deposition of both IgG anti-C1q and C1q both along the GBM and in the mesangium 24 h after injection. Regarding the two models proposed for the deposition of anti-C1q in the glomerulus, the present study indicates that in a healthy mouse, anti-C1q is targeted to the glomerulus by the interaction of anti-C1q with both solid phase C1q on the GBM, based on the GBM-like pattern of deposition, and with fluid phase C1q, based on the mesangial type of deposition. In SLE, with many immune complexes in the circulation that deposit in the glomerulus, C1q in these complexes will be an additional target for anti-C1q autoantibodies. After 2 weeks the pattern of deposition remains the same in the anti-C1q-injected mice whereas at this time point only there is some deposition of rabbit IgG in the glomeruli of the rabbit IgG-injected mice. This deposition is only mesangial and probably resulted from the mouse antirabbit IgG response by which complexes of mouse IgG and rabbit IgG were trapped in the mesangium.
After injection of anti-C1q antibodies there was a time-dependent depletion of circulating C1q, as demonstrated by both ELISA and Western blot analysis. This is a condition which may also be found in SLE patients during a flare [23]. C1q depletion can be the result of antibody-specific interactions with C1q or consumption by rabbit IgG aggregates. To ensure that no aggregates were injected the antibodies were centrifuged at high speed and only the supernatant was used. Furthermore, mice were injected intraperitoneally, which ensures slow release of the antibodies and prevents large complexes from entering the blood compartment. Injecting the antibodies in this way may explain the difference in the amount of circulating rabbit IgG at 24 h. At 2 weeks a mouse antirabbit IgG response was observed in all mice. At this time-point C1q levels were reduced to approximately 50% in both groups, which is probably the result of C1q consumption by mouse antirabbit IgG–rabbit IgG complexes.
Anti-C1q autoantibodies of SLE patients have been reported to react predominantly with the collagen-like region of solid phase C1q [9]. Our polyclonal antibody was generated by immunizing a rabbit with total mouse C1q, resulting in reactivity with both solid phase and fluid phase C1q. The interaction with fluid phase C1q results in complex formation and deposition in the mesangium, while the interaction with GBM-associated C1q leads to binding of anti-C1q on the GBM, followed by the C3 deposition.
Although there was clear deposition of rabbit IgG and mouse C1q, there was only limited C3 deposition. Membrane-bound complement regulatory proteins (mCRP) inhibit complement activation and are present throughout the glomerulus [24]. The importance of mCRP is shown clearly in both a model of passive and a model of accelerated anti-GBM nephritis [25,26]. Mice genetically deficient for decay-accelerating factor (DAF) showed abundant C3 deposition and albuminuria, whereas wild-type control mice treated with the same dose of nephrotoxic antibody did not. Apparently, complement activation by C1q anti-C1q complexes at the given dose is not sufficient to overcome complement regulation and to result in robust complement activation.
Complement activation results in the generation of anaphylatoxins C3a and C5a that may attract leucocytes to the site of complement activation. Staining for CD45 showed that there are significantly more infiltrating cells in the anti-C1q injected group at 2 weeks.
In conclusion, despite the deposition of C1q and anti-C1q along the GBM and in the mesangial area, the local complement deposition, the presence of CD45 positive cells and some histological changes, there was only mild albuminuria in some of the mice. This suggests that anti-C1q when injected as a single dose of 5 mg is not sufficient to result in robust albuminuria. Repeated injection might result in overt disease, but the development of a mouse antirabbit IgG response hampers experiments lasting longer than 2 weeks [27]. It has also been demonstrated for other autoantibodies [27] that autoantibodies for one antigen alone are not sufficient to induce a full-blown renal disease, suggesting strongly that autoantibodies with multiple specificities are required to induce overt disease. To overcome the problems of a heterologous system we have developed mouse antimouse C1q monoclonal antibodies and are in progress to test their potential.
Acknowledgments
This work was supported by a grant from the Dutch Kidney Foundation (grant number C98·1763). We thank Dr A. Roos (Department Nephrology, LUMC, Leiden, the Netherlands) for critical reading of the manuscript.
References
- 1.Walport MJ. Complement. Part 1. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Part 2. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 4.Walport MJ, Davies KA, Morley BJ, Botto M. Complement deficiency and autoimmunity. Ann NY Acad Sci. 1997;815:267–81. doi: 10.1111/j.1749-6632.1997.tb52069.x. [DOI] [PubMed] [Google Scholar]
- 5.Trouw LA, Roos A, Daha MR. Autoantibodies to complement components. Mol Immunol. 2001;38:199–206. doi: 10.1016/s0161-5890(01)00043-8. [DOI] [PubMed] [Google Scholar]
- 6.Siegert CE, Daha MR, van Halma C, d V, Breedveld FC. IgG and IgA autoantibodies to C1q in systemic and renal diseases. Clin Exp Rheumatol. 1992;10:19–23. [PubMed] [Google Scholar]
- 7.Wisnieski JJ, Jones SM. IgG autoantibody to the collagen-like region of Clq in hypocomplementemic urticarial vasculitis syndrome, systemic lupus erythematosus, and 6 other musculoskeletal or rheumatic diseases. J Rheumatol. 1992;19:884–8. [PubMed] [Google Scholar]
- 8.Siegert CE, Daha MR, Swaak AJ, van der Voort EA, Breedveld FC. The relationship between serum titers of autoantibodies to C1q and age in the general population and in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1993;67:204–9. doi: 10.1006/clin.1993.1066. [DOI] [PubMed] [Google Scholar]
- 9.Antes U, Heinz HP, Loos M. Evidence for the presence of autoantibodies to the collagen-like portion of C1q in systemic lupus erythematosus. Arthritis Rheum. 1988;31:457–64. doi: 10.1002/art.1780310401. [DOI] [PubMed] [Google Scholar]
- 10.Fremeaux-Bacchi V, Weiss L, Demouchy C, Blouin J, Kazatchkine MD. Autoantibodies to the collagen-like region of C1q are strongly associated with classical pathway-mediated hypocomplementemia in systemic lupus erythematosus. Lupus. 1996;5:216–20. doi: 10.1177/096120339600500309. [DOI] [PubMed] [Google Scholar]
- 11.Siegert C, Daha M, van Westedt ML, van der Voort EA, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230–4. [PubMed] [Google Scholar]
- 12.Mannik M, Wener MH. Deposition of antibodies to the collagen-like region of C1q in renal glomeruli of patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 1997;40:1504–11. doi: 10.1002/art.1780400819. [DOI] [PubMed] [Google Scholar]
- 13.Coremans IE, Spronk PE, Bootsma H, et al. Changes in antibodies to C1q predict renal relapses in systemic lupus erythematosus. Am J Kidney Dis. 1995;26:595–601. doi: 10.1016/0272-6386(95)90595-2. [DOI] [PubMed] [Google Scholar]
- 14.Moroni G, Trendelenburg M, Del Papa N, et al. Anti-C1q antibodies may help in diagnosing a renal flare in lupus nephritis. Am J Kidney Dis. 2001;37:490–8. doi: 10.1053/ajkd.2001.22071. [DOI] [PubMed] [Google Scholar]
- 15.Trendelenburg M, Marfurt J, Gerber I, Tyndall A, Schifferli JA. Lack of occurrence of severe lupus nephritis among anti-C1q autoantibody-negative patients. Arthritis Rheum. 1999;42:187–8. doi: 10.1002/1529-0131(199901)42:1<187::AID-ANR24>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Uwatoko S, Gauthier VJ, Mannik M. Autoantibodies to the collagen-like region of C1Q deposit in glomeruli via C1Q in immune deposits. Clin Immunol Immunopathol. 1991;61:268–73. doi: 10.1016/s0090-1229(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 17.Coremans IE, Bruijn JA, de Heer E, van der Voort EAM, Breedveld FC, Daha M. Stabilization of glomerular deposits of C1q by antibodies against C1q in mice. J Clin Lab Immunol. 1995;44:47–61. [Google Scholar]
- 18.Wing MG, Seilly DJ, Bridgman DJ, Harrison RA. Rapid isolation and biochemical characterization of rat C1 and C1q. Mol Immunol. 1993;30:433–40. doi: 10.1016/0161-5890(93)90111-n. [DOI] [PubMed] [Google Scholar]
- 19.Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 20.Kohro-Kawata J, Wener MH, Mannik M. The effect of high salt concentration on detection of serum immune complexes and autoantibodies to C1q in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:84–9. [PubMed] [Google Scholar]
- 21.Siegert CE, Daha MR, van der Voort EA, Breedveld FC. IgG and IgA antibodies to the collagen-like region of C1q in rheumatoid vasculitis. Arthritis Rheum. 1990;33:1646–54. doi: 10.1002/art.1780331107. [DOI] [PubMed] [Google Scholar]
- 22.Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4(Suppl. 3):S279–S293. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtsson A, Nezlin R, Shoenfeld Y, Sturfelt G. DNA levels in circulating immune complexes decrease at severe SLE flares-correlation with complement component C1q. J Autoimmun. 1999;13:111–9. doi: 10.1006/jaut.1999.0300. [DOI] [PubMed] [Google Scholar]
- 24.Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998;54:1419–28. doi: 10.1046/j.1523-1755.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin F, Emancipator SN, Salant DJ, Medof ME. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 2002;82:563–9. doi: 10.1038/labinvest.3780451. [DOI] [PubMed] [Google Scholar]
- 26.Sogabe H, Nangaku M, Ishibashi Y, et al. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001;167:2791–7. doi: 10.4049/jimmunol.167.5.2791. [DOI] [PubMed] [Google Scholar]
- 27.Kootstra JC, Veninga A, Baelde JJ, van Eendenburg J, De Heer E, Bruijn JA. Characterization of reactivity of monoclonal autoantibodies with renal antigens in experimental lupus nephritis. J Clin Lab Immunol. 1996;48:201–18. [PubMed] [Google Scholar]