Abstract
Intestinal parasitic infections have been suggested to cause persistent immune activation leading to an unbalanced immune state. Such a state has been proposed to be a major factor in the pathogenesis of AIDS in an African context. The present study investigated the effect of incidental parasitic infection and treatment on the profile of T cell differentiation and activation markers on CD4+ and CD8+ T cells from HIV-1 infected and uninfected adult Ethiopians. Cryopreserved PBMCs from 64 subjects (41 HIV-negative and 23 HIV-positive) with follow-up visits at 6-monthly intervals were used to compare the effect of incidental intestinal parasites and their treatment upon T cell subset profiles and activation status. The samples were stained with antibodies to various T cell differentiation and activation markers allowing naive, memory, effector, memory/effector, activated and resting CD4+ and CD8+ T cell subsets to be quantified by triple-colour FACScan. Incidental intestinal parasitic infections resulted in a significant increase in memory CD4+ T cell numbers both in HIV-negative and HIV-positive subjects (P < 0·05). There was also a significant increase in the percentage of CD8+ HLA-DR+ T cells (P < 0·05) in HIV-positive subjects co-infected with parasites. In HIV-negative subjects, a significant decline in activated cells and a significant increase in resting CD8+ T cells (P < 0·05) was observed after treatment for parasites. These data suggest that intestinal parasitic infections could result in the alteration of T cell subset counts and also in the up-regulation of T cell activation markers in peripheral blood. Treatment of parasitic infections showed a tendency to reduce the activation suggesting that, together with other community based intervention strategies, such treatment could be used to down-regulate immune activation and hence protect the host from being easily attacked by HIV.
Keywords: Ethiopia, immune activation, intestinal parasites, T cell subsets
Introduction
In areas of the world where the spread of AIDS is most dramatic, parasitic infections and HIV co-exist. This is more evident especially in developing countries such as Africa, where millions of individuals are infected with chronic infectious diseases of protozoal, helminthic, bacterial, viral and fungal origin [1–6]. Facilitated progression to AIDS [7] as well as shortened survival time after AIDS diagnosis are suggested to characterize African HIV infection [4]. Several co-factors have been implicated in the facilitated progression. One of these is an abnormal activation of the immune system resulting from persistent antigenic stimulation [3,8], which would thus lead to chronic activation of peripheral lymphocytes with consequent increased susceptibility of these lymphocytes to HIV infection and increased HIV replication [1,9]. Such a background of chronic infection was suggested to alter the host's ability to cope with subsequent infections [10], including HIV [1], which by itself activates multiple components of the immune system [11].
Intestinal parasitism occurs widely throughout Ethiopia [12]. Furthermore, the prevalence of HIV/AIDS is also increasing rapidly all over the country [13], making the country the third highest ranked in the world with regard to the total number of HIV-infected subjects [14]. However, there have been only a few studies on the interaction between intestinal parasitic infections and HIV in Ethiopia [15,16]. Previous studies on HIV-negative Ethiopian immigrant Jews to Israel, who were highly infected with intestinal parasitic infections, indicated an activated immune system and polarization of the immune response towards a dominant Th2 profile which promotes humoural responses [1, 9, 17–21]. These and a few other reports on Ethiopians indicated: lower CD4+ and higher CD8+ T lymphocyte counts; reduced proportions and absolute numbers of naive CD4+ and CD8+ T cells; increased proportions and absolute numbers of memory CD4+ and CD8+ T cells; and increased numbers of activated (HLA-DR+ CD38+) CD4+ and CD8+ T cells in HIV-negative Ethiopian subjects [18, 22–26]. We have recently confirmed some of the above observations in subjects residing in two geographically different localities within the country [27]. These immunological perturbations have been used commonly as indicators of persistent immune activation and ascribed to the increased load of environmental pathogens, especially intestinal parasites, in Ethiopia [18,25]. Therefore, this longitudinal study aimed to investigate the effect of incidental intestinal parasitic infection and therapy on the profile of CD4+ and CD8+ T cell subsets and activation status among adult Ethiopians.
Subjects and Methods
Study subjects and study design
The subjects included in this study are participants in a long-term cohort study on the incidence and progression of HIV type 1 infection in Ethiopia, performed by the Ethiopian–Netherlands AIDS Research Project (ENARP) at the Ethiopian Health and Nutrition Research Institute (EHNRI). They are factory workers in Akaki (a town about 15 km south-east of the Ethiopian capital Addis Ababa). A detailed description of the cohort studies has been reported previously [28]. The study has ethical approval from both EHNRI and the National Ethical Clearance Committee. Study participants were enrolled following pretest counselling and only after signing an informed consent form. Stool examination for parasites and a test for HIV antibodies were performed for each participant every 6 months. Sixty-four subjects, 41 HIV-negative and 23 HIV-positive, with incidental and/or cured parasitic infections, were included. Samples were selected if the subjects made at least two consecutive visits and were positive for intestinal parasites in one of their visits. Subjects found positive for intestinal parasites were given appropriate treatment. Those found negative for intestinal parasites 6 months after treatment were selected in order to study the effect of treatment. All the HIV-infected subjects were antiretroviral therapy naïve.
HIV screening and confirmatory assays
Whole blood was collected in a 10-ml vacutainer tubes containing ethylene diamine tetra-acetic acid (EDTA) as an anticoagulant between 8 : 30 and 11 : 30 a.m. and transported to ENARP laboratory from the cohort site within 6 h of collection. The presence of HIV antibodies was detected in plasma using HIVSPOT Rapid assay (Genelabs Diagnostics, Singapore) and the enzyme-linked immunosorbent assay (ELISA) (Vironostika HIV Uni-Form II plus O, Organon Teknika, Boxtel, the Netherlands). Positive and discrepant plasma samples were confirmed by Western blot analysis (HIVBLOT 2·2, Genelabs Diagnostics, Singapore).
Parasitological examination
Stool examinations for parasite infections were performed as part of the routine investigations on fresh stools at the study site on the same date as blood sample collection. Direct microscopy of samples in saline and iodine and the formalin–ether sedimentation concentration methods were employed [29]. The Baermann concentration method was also performed to detect Strongyloides stercoralis [30].
Haematological analysis
The absolute number of leucocytes per µl of whole blood was obtained using a Coulter counter T540 (Coulter Electronics, FL, USA). 4C PLUS Cell Control (Beckman Coulter, Miami, FL, USA) was used to monitor the performance of the Coulter machine.
Peripheral blood mononuclear cell separation and freezing
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by the Ficoll-Hypaque density gradient centrifugation method, frozen using a freezing machine (KRYO 10, Biomedical Series II, Cryotech, Shagen, the Netherlands) and stored in liquid nitrogen until analysed.
Thawing of peripheral blood mononuclear cells and preparation for FACScan analysis
Cryopreserved PBMCs were removed from liquid nitrogen and thawed in a 37°C water bath and washed in thawing and washing medium. The viability of cells was checked using trypan blue in a Burker–Turk counting chamber and found to be greater than 90%. The cell pellet was then resuspended in 500 µl of Isoton and this suspension was used for three-colour flow cytometric analysis.
Three-colour immunophenotyping of T cell subsets
CD45RA+ CD27+ (naive), CD45RA−CD27+ (memory), CD45RA+ CD27− (cytotoxic effector CD8; not yet described in CD4+ T cells) and CD45RA−CD27− (memory/effector) CD4+ and CD8+ T cells were quantified by three-colour flow cytometric analysis after staining with Perdinin-Chlorophyll-A Protein (PerCP)-conjugated CD4 or CD8 monoclonal antibodies (MoAbs) in combination with fluorescein isothiocyanate (FITC)-conjugated CD27 MoAbs and phycoerythrin (PE)-conjugated CD45RA (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) according to previous reports [31,32]. In vivo activated (HLA-DR+ CD38+) and resting (HLA-DR−CD38−) CD4+ and CD8+ T cells were also quantified by three-colour flow cytometric analysis after staining with PerCP-conjugated CD4 or CD8 MoAbs in combination with FITC-conjugated CD38 MoAb and PE-conjugated HLA-DR according to Giorgi et al. [33]. In brief, 250000 PBMCs in Isoton were mixed and incubated at room temperature with each combination of MoAbs (5 µl from each) for 30 min in separate tubes in the dark. Two ml of Isoton was added, vortexed and centrifuged at 1640 r.p.m. for 5 min. The supernatant was discarded leaving approximately 50 µl of residual fluid in the tube. Then 2 ml of Isoton was added to the cell pellet, mixed thoroughly, and centrifuged at the same speed and time interval. The supernatant was removed, the residue was re-suspended in 500 µl of Isoton. Events were acquired using a FACScan flow cytometer with Cellquest software (Becton Dickinson). For acquisition and storage, a gate was set on side scatter and PerCP fluorescence to stop acquiring when 2000 CD4+ or CD8+ T lymphocytes were collected. For analyses a gate was set for lymphocytes using forward versus side light scattering property of the cells. CD4+ and CD8+ cells were gated on side scatter and PerCP fluorescence in order to obtain a minimum of 1500 CD4+ or CD8+ T lymphocytes from the lymphocyte gate. The analysis gate for CD4 or CD8 subsets was set on CD4+ or CD8+ bright events to avoid contaminating CD3−CD4+ monocytes and CD3−CD8+ NK cells. The FACScan was calibrated with CaliBRITE fluorescent beads and FACScomp software (Becton Dickinson) weekly.
Statistical analysis
Data were entered and analysed using Dbase IV and stata programs, respectively. The distribution of T cell subsets and activation markers was compared between visits using the nonparametric Wilcoxon matched-pair signed-rank test. P-values < 0·05 were considered significant.
Results
Study subjects and types of intestinal parasites
The study involved 64 individuals (41 HIV negative and 23 HIV positive) with a median age of 37 years (range 23–47). Forty-nine per cent of the subjects were males while 51% were females. Forty-one subjects were infected with a single intestinal parasite (a helminth or a protozoan) while 23 were infected with more than one intestinal parasite. The types and frequency of the different intestinal parasites in the study subjects are depicted in Table 1.
Table 1.
Types and frequency of intestinal parasites in the study subjects by HIV status
| HIV status | ||
|---|---|---|
| Parasite | HIV− No. (%) | HIV+ No. (%) |
| Entamoeba histolytica/dispar | 19 (31) | 9 (31) |
| Ascaris lumbricoides | 18 (29) | 8 (28) |
| Trichuris trichiura | 6 (10) | 1 (3) |
| Strongyloides stercoralis | 10 (16) | 5 (17) |
| Giardia lamblia | 8 (13) | 4 (14) |
| Taenia saginata | 0 (0) | 2 (7) |
| Enterobius vermicualris | 1 (2) | 0 (0) |
| All helminths | 35 (56) | 16 (55) |
| All protozoa | 27 (44) | 13 (45) |
T cell subsets and activation markers in HIV-negative and HIV-positive subjects with incidental intestinal parasitic infection
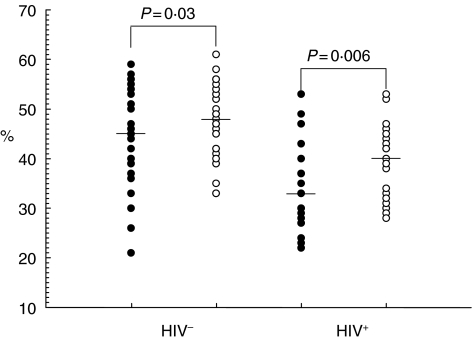
To investigate the effect of incidental intestinal parasitic infections on the profile of T cell subsets and activation status, 34 HIV-negative individuals who were negative for intestinal parasitic infection at their baseline visit but became positive in their second visit (6 months later) were included in this study. Intestinal parasitic infection did not bring any significant changes in median white blood cells (WBC), lymphocyte, CD4, CD8, CD4/CD8 ratios (Table 2) or CD4+ and CD8+ naive and effector subset values in both HIV-negative and HIV-positive subjects. Any changes seen in the absolute counts of leucocyte subsets in HIV-infected subjects with intestinal parasitosis (Table 2) were a reflection of the lower WBC counts rather than qualitative differences, as the percentages were not affected and the absolute subset counts are derivatives of three parameters (WBC, % lymphocyte and % T cell subset values). However, the percentage of memory CD4+ T cells increased significantly both in HIV-negatives and -positives (P = 0·03 and 0·006, respectively) (Fig. 1). Analysis of the expression of the activation markers HLA-DR and CD38 on CD4+ and CD8+ T cells showed no significant changes in expression on CD4 T cells in both HIV-negative and HIV-positive subjects. The same phenomenon was also observed in the CD8+ compartment, except that there was a significant (P = 0·02) increase in the median percentage of CD8+ HLA-DR+ T cells in HIV-positive subjects co-infected with intestinal parasites (75% versus 79%). Separate analysis of the data assessing infection by helminths indicated a significant increase only in memory CD4+ (P = 0·03) and memory CD8+ (P = 0·02) T cells, correlating with the incidence of helminth infection in HIV-negative subjects. This was accounted for mainly by incidental Ascaris lumbricoides infections. However, such a phenomenon was not observed when considering protozoans only.
Table 2.
Median values and 95th percentiles (in brackets) of white blood cells (WBC) subsets in HIV-negative and HIV-positive subjects with incidental parasitic infection
| Parameter | HIV-negative subjects | HIV-positive subjects | ||
|---|---|---|---|---|
| Baseline (n = 34) | With infection (n = 34) | Baseline (n = 21) | With infection (n = 21) | |
| WBC (per µl) | 5700 (3000–9800) | 5850 (3100–8700) | 5300(3200–9300) | 4900 (2700–8400) |
| Lymphocyte | 1854 (1373–4127)a | 1846 (1340–2741) | 1569 (828–2526) | 1414 (728–3385) |
| 34 (17–58)a | 33 (19–49) | 30 (20–55) | 31 (21–51) | |
| CD4+ | 603 (345–958) | 604 (346–801) | 221 (59–477) | 180 (64–472) |
| 31 (18–43) | 32 (19–46) | 15 (5–27) | 14 (5–27) | |
| CD8+ | 575 (226–2080) | 552 (219–1254) | 727 (285–1196) | 654 (392–1840) |
| 32 (15–56) | 31 (13–50) | 43 (29–61) | 45 (25–66) | |
| CD4/CD8 | 0·96 (0·32–2·59) | 1·11 (0·39–2·67) | 0·32 (0·09–0·65) | 0·32 (0·08–0·59) |
Top value = absolute count (cells/µl), bottom value = proportions.
Fig. 1.
Memory (CD45RA−CD27+) CD4+ T cells before (•) and following incidental intestinal parasitic infection (○) among adult HIV− and HIV+ subjects. Individual values are presented with the horizontal lines showing the median values.
T cell subsets and activation markers in HIV-negative and HIV-positive subjects after treatment for intestinal parasitic infection
To study the effect of treating intestinal parasites, 31 HIV-negative subjects who were positive for intestinal parasitic infection at one of their visits, treated for the infection and were parasite-free in their next visit (6 months later) were involved in this study. In HIV-negative subjects, a tendency for increased naïve CD4+ and decreased memory/effector CD4+ and a significant decrease in cytotoxic effector CD8+ T cells was seen after treatment for intestinal parasites (Table 3). However, in HIV-positive subjects, treatment did not result in a significant change in absolute counts and percentages of CD4+ and CD8+ T cells and their subsets, except for a significant increase in the absolute number of memory CD8+ T cells (Table 3).
Table 3.
Median valuesa and 95th percentiles (in brackets) of T cell subsets and activation markers in HIV-negative (n = 31) and HIV-positive subjects (n = 9) after treatment for intestinal parasites
| Parameter | With infection | After treatment |
|---|---|---|
| HIV-negative | ||
| CD4+ CD45RA+ CD27+ (naive) | 88 (35–241) | 95 (27–280) |
| CD4+ CD45RA-CD27+ (memory) | 265 (93–418) | 237 (120–483) |
| CD4+ CD45RA-CD27− (memory/effector) | 163 (51–273) | 155 (52–244) |
| CD8+ CD45RA+ CD27+ (naive) | 150 (55–247) | 126 (47–317) |
| CD8+ CD45RA-CD27+ (memory) | 56 (18–170) | 59 (16–202) |
| CD8+ CD45RA+ CD27−(cytotoxic effector) | 328 (81–819) | 267 (63–704)* |
| CD8+ HLA-DR+ | 254 (93–808) | 210 (77–592)* |
| CD8+ CD38+ | 123 (41–399) | 110 (46–337)* |
| HIV-positive | ||
| CD8+ CD45RA+ CD27+ (naive) | 146 (95–555) | 196 (79–453) |
| CD8+ CD45RA-CD27+ (memory) | 126 (43–230) | 149 (24–427)* |
Absolute counts per microlitre of whole blood
Wilcoxon matched-pair signed rank test, P < 0·05.
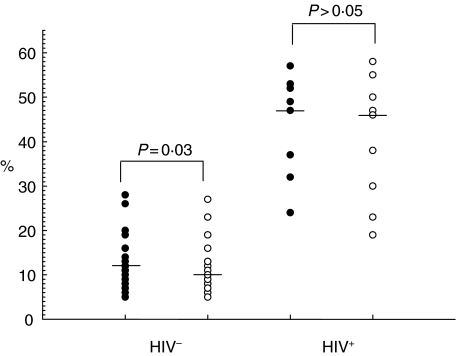
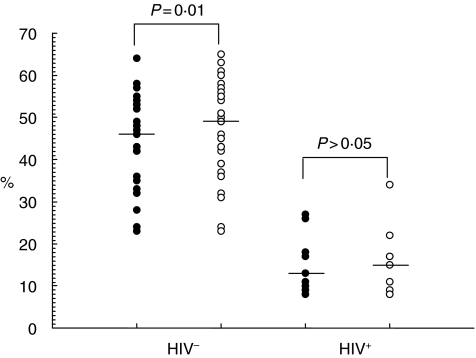
In terms of the effect of treatment on activation markers, no significant change in the expression of CD38 and HLA-DR on CD4+ T cells was observed in HIV-negative subjects. In the CD8 compartment of the HIV-negative subjects, however, treatment resulted in a significant decline in the percentages of activated cells (P = 0·03) (Fig. 2) and a significant increase in the percentage of resting cells (P = 0·01) (Fig. 3). Also, there was a significant decline in the counts of CD8+ HLA-DR+ cells (P = 0·04) and CD8+ CD38+ cells (P = 0·02) in HIV-negative subjects (Table 3). These observations remained true for helminthic infections but not for protozoans. In HIV-positive subjects treatment resulted in an increase in resting CD4+ (data not shown) and CD8+ T cell numbers (Fig. 3), although the difference was not statistically significant.
Fig. 2.
Activated (HLADR+ CD38+) CD8+ T cells with intestinal parasitic infections (•) and after treatment (○) for intestinal parasitic infection. Individual values are presented with horizontal lines showing median values.
Fig. 3.
Resting (HLA-DR-CD38-) CD8+ T cells with intestinal parasitic infection (•) and after treatment (○) in adult HIV− and HIV+ subjects. Individual values are presented with horizontal lines showing median values.
Thirty-one individuals (seven HIV-positive and 24 HIV-negative) were studied before, during and after treatment for intestinal parasitic infections. A significant increase in memory CD4+ T cells (P = 0·03) and memory/effector CD8+ T cells (P = 0·02) was observed in HIV-positive individuals with incidental intestinal parasitic infection. Treatment showed a statistically significant (P = 0·002) increase in the median absolute counts of CD4+ T cells (192 versus 279 cells/µl) in these subjects. In addition, a significant increase in resting CD8+ T cell numbers (P = 0·03) and a significant decrease in activated CD8+ T cell numbers (P = 0·02) was observed after treatment for intestinal parasites in the HIV-negative subjects, an observation similar to that of the 31 HIV-negative cohorts shown in Figs 2 and 3.
Discussion
Intestinal parasites are known to produce millions of eggs per day accompanied by equivalently copious amounts of secretory and excretory products in the host which would persistently stimulate the host's immune system [34]. Such exposure of the host to a multitude of parasitic products, besides other environmental antigens, could result in a Th2 polarized immune response, which has been suggested to be essential for increased susceptibility to HIV infection and its disease progression [1]. A study on HIV-negative Ethiopian immigrant Jews to Israel who were highly infected with intestinal parasites demonstrated substantial immune activation, which was associated with remarkable changes in the distribution and phenotype of peripheral blood T cell populations [18,35]. These observed phenotypic and functional cellular changes were reported to resemble those described in HIV-infected people. These investigators ascribed the observed immune dysregulation to the exposure of the subjects to intestinal parasites, specifically helminths.
In this study, too, intestinal parasitic infections resulted in a statistically significant increase in memory CD4+ T lymphocytes both in HIV-infected and -uninfected individuals. As such they avail more memory CD4+ T cells which largely express CCR5 [36] for the Ethiopian non-syncytium-inducing subtype C viruses [37]. Preferential infection of CD4+ memory cells by HIV-1 [38] and preferential replication of the virus in these subsets [39] has been reported. A similar effect of HIV on T cell activation is also indicated, as the observed increase in memory cells is lower in HIV-negative subjects compared to HIV-positives. Elevated expression of CD27 has been reported to occur whenever there is activation of the immune cells by antigens [40] and its down-regulation occurs through persistent antigenic stimulation [31, 40, 41]. Expression of CD27 antigen increased significantly on CD4+ T cells following intestinal parasite infection in HIV-seronegative subjects in this study, indicating the sole effect of parasites in bringing immune activation. The decline in resting cells and the increase in activated cells was noted both for CD4+ and CD8+ T cells in subjects with both intestinal parasitic infection and HIV. Furthermore, in agreement with the observation of Abdulkadir [42], an increase in HLA-DR expression on CD8+ T cells was observed for HIV-positive subjects with intestinal parasites in the present study. Other investigators have also noted an increase in activated CD8+ T cells in HIV-positive subjects [43–45], which indicates the expansion of these cells against the virus because the activated CD8+ T cells were reported to have higher HIV-specific cytotoxic activity [33].
In a study of helminth-infected Ethiopian immigrants to Israel, Kalinkovich et al. [18] demonstrated that eradication of the parasites was associated with a decrease in immune activation status which gradually returned to normal levels like those seen in parasite-negative Israelis. In our study, a decrease in cytotoxic effector cells was also seen in HIV-negatives after treatment for intestinal parasites. In contrast, an increase in these cells was seen in HIV-positives after treatment, further confirming the role of the virus in continuous activation of the CD8 compartment. It has been suggested that the increased number of cytotoxic effector cells over memory cells is associated with how successfully virus replication is contained [46].
Interestingly, the reversal of impaired cell-mediated immune response 3–6 months following the chemotherapeutic cure of intestinal parasitic infections has been reported [47–49]. In line with these observations, a recent study also showed an increase in T cell proliferative responses and gamma interferon production in purified protein derivative (PPD)-negative Ethiopians who were Bacille Calmette–Guerin (BCG) vaccinated after deworming compared to the placebo group [50]. Furthermore, an activated immune status due to chronic helminth infections has been shown to impair signal transduction and anergy, and the defective signalling responses have been reported to restore gradually following antihelminthic treatment [21].
In this study, too, a decline in CD4+ T cells expressing HLA-DR and CD38 and an increase in resting CD4+ T cells were seen after treatment both in HIV-positive and HIV-negative subjects, although the differences were not statistically significant. This is in agreement with the report by Kalinkovich et al. [18], where a decline in expression of HLA-DR was seen in Ethiopian immigrants to Israel who were treated for intestinal parasites. This report is reinforced by the present finding that treatment of intestinal parasites results in a statistically significant increase in resting CD8+ T cells and a significant decrease in activated cells in HIV-seronegative subjects. Furthermore, our determination that, in HIV-positive subjects, there is a tendency for an increase in resting cells and a decrease in activated cells (although the differences were not significant), observed after treating for intestinal parasites, substantiates the hypothesis of an immune interaction between intestinal parasites and HIV [20]. This, however, does not discount the fact that HIV by itself is potent enough to maintain the persistent activation of the immune system [11, 19, 51]. Recently Giorgi et al. [52] have reported that CD38 expression on CD8+ T cells is a strong predictive marker both at early and late stages of HIV infection. Thus, the effect of treating intestinal parasites might influence further up-regulation of the activation markers (HLA-DR and CD38) in these ART naive group of subjects as HIV infection progresses (there is a 6-month gap between visits). Taken together, this information is in line with the proposal of Bentwich and colleagues [1] that intestinal parasites, besides other endemic infections, cause chronic immune activation and their eradication is necessary to reverse the activation. This has a clear implication for HIV disease progression in Ethiopia, where the burden of intestinal parasite infection is very high.
In conclusion, the present study indicates that intestinal parasitic infections could result in the alteration of CD4+ and CD8+ T cell subsets, and also in the up-regulation of T cell activation markers in peripheral blood. Treating intestinal parasites tends to reduce activation of the T cell subsets. Hence treating intestinal parasites, in addition to other community based intervention strategies, could be considered as a possible strategy to bring about a state of decreased activation and so protect the host T cells from being easily attacked by HIV, the major cause of morbidity and mortality in the country and the developing world at large.
Acknowledgments
This study is part of the Ethiopian–Netherlands AIDS Research Project (ENARP), a collaborative effort of EHNRI, the Amsterdam Municipal Health Service (GGGD), the Department of Clinical Viro-Immunology, CLB and Laboratory for Experimental and Clinical Immunology of the University of Amsterdam and the Academic Medical Center of the University of Amsterdam (AMC). ENARP is a bilateral project financially supported by the Netherlands Ministry of Foreign Affairs and the Ethiopian Ministry of Health (MOH). We thank the study participants for their kind collaboration. We are also grateful to ENARP laboratory technicians for technical assistance.
References
- 1.Bentwich A, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–91. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 2.Chan MS. The global burden of intestinal nematode infections. Fifty years on. Parasitol Today. 1997;13:438–43. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 3.Lanzer M, Gross U, Moll H. Mechanisms of parasite persistence and immune evasion. Parasitol Today. 1997;13:1–3. [Google Scholar]
- 4.Grant AD, Djomand G, Decock KM. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS. 1997;11(Suppl. B):S43–54. [PubMed] [Google Scholar]
- 5.Tarantola D, Schwartlander B. HIV/AIDS epidemics in sub-Saharan Africa. dynamism, diversity and discrete declines. AIDS. 1997;11(Suppl. B):S5–21. [PubMed] [Google Scholar]
- 6.UNAIDS/WHO. Geneva, Switzerland: UNAIDS/WHO; 2000. Reports on global AIDS epidemic: December. [Google Scholar]
- 7.Gilks CF. The clinical challenge of HIV epidemic in the developing world. Lancet. 1993;342:1037–8. doi: 10.1016/0140-6736(93)92885-w. [DOI] [PubMed] [Google Scholar]
- 8.Miazeles RM, Bundy DA, Selkirk ME, Smith DF, Anderson RM. Immunological modulation and evasion by helminth parasites in human population. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- 9.Bentwich Z, Weisman Z, Grossman A, Galai N, Kalinkovich A. Pathogenesis of AIDS in Africa − lessons from the Ethiopian immigrants in Israel. Immunologist. 1997;5:21–6. [Google Scholar]
- 10.Christensen N, Nansen P, Fagbemi O, Monrad J. Heterologous antagonistic and synergistic interactions between helminths and protozoans in concurrent experimental infection of mammalian hosts. Parasitol Res. 1987;73:387–410. doi: 10.1007/BF00538196. [DOI] [PubMed] [Google Scholar]
- 11.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–8. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 12.Kloos H, Tesfa Yohanes TM. Intestinal parasites in Ethiopia. In: Kloos H, Zein AZ, editors. The ecology of health and disease in Ethiopia. Oxford: Westview Press; 1993. pp. 223–35. [Google Scholar]
- 13.Ministry of Health. AIDS in Ethiopia: background projections, impacts and interventions. 2. Addis Ababa: Ministry of Health; 2000. [Google Scholar]
- 14.UNAIDS/WHO. Geneva, Switzerland: 2000. Jun, Reports on global AIDS epidemic. UNAIDS/WHO. [Google Scholar]
- 15.Fontanet A, Woldemichael T, Sahlu T, et al. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol. 2000;94:145–55. [PubMed] [Google Scholar]
- 16.Fontanet A, Sahlu T, Rinke de Wit TF, et al. Epidemiology of infection with intestinal parasites and human immunodeficiency virus (HIV) among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol. 2000;94:269–78. doi: 10.1080/00034980050006456. [DOI] [PubMed] [Google Scholar]
- 17.Bentwich A, Weisman Z, Moroz C, Bar-Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminthic infections? Clin Exp Immunol. 1996;103:239–43. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalinkovich A, Weisman Z, Greenberg Z, et al. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–21. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentwich A, Kalinkovich A, Weisman Z, Grossman Z. Immune activation in the context of HIV infection. Clin Exp Immunol. 1998;111:1–2. doi: 10.1046/j.1365-2249.1998.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers AD. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol Today. 1999;20:485–7. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 21.Brokow G, Leng Q, Weisman Z, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–60. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollak S, Faud B, Etzioni A. CD4 T lymphocytopenia without opportunistic infections in HIV-seronegative Ethiopian immigrants to Israel. Lancet. 1994;342:50–1. doi: 10.1016/0140-6736(93)91912-6. [DOI] [PubMed] [Google Scholar]
- 23.Worku S, Christensson B, Bjorkman A, Dilara I. Higher proportions of CD8+ T cells in the blood in healthy adults from Ethiopia and Bangladesh compared with Sweden. Trans R Soc Trop Med Hyg. 1997;91:618–22. doi: 10.1016/s0035-9203(97)90051-1. [DOI] [PubMed] [Google Scholar]
- 24.Tsegaye A, Messele T, Tilahun T, et al. Immunohematological reference ranges for adult Ethiopians. Clin Diagn Lab Immunol. 1999;6:410–4. doi: 10.1128/cdli.6.3.410-414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messele T, Abdulkadir M, Fontanet AL, et al. Reduced naive and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin Exp Immunol. 1999;115:443–50. doi: 10.1046/j.1365-2249.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsegaye A, Hailu E, Tilahun T, et al. Immunohematological reference values for adult Ethiopians living at high and medium altitudes. Ethiop Med J. 2000;38:303–4. [Google Scholar]
- 27.Kassu A, Tsegaye A, Petros B, et al. Distribution of lymphocyte subsets in healthy human immunodeficiency virus negative adult Ethiopians from two geographical locales. Clin Diagn Lab Immunol. 2001;8:1171–6. doi: 10.1128/CDLI.8.6.1171-1176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahlu T, Kassa E, Agonafer T, et al. Sexual behaviours, perception of risk of HIV infection, and factors associated with attending HIV post-test counselling in Ethiopia. AIDS. 1999;13:1263–71. doi: 10.1097/00002030-199907090-00017. [DOI] [PubMed] [Google Scholar]
- 29.Beaver PC, Jung RC, Cupp EW. Examination of specimens for parasites. In: Beaver PC, Jung RC, Cupp EW, editors. Clinical parasitology. Philadelphia: Lea and Febiger; 1984. pp. 733–58. [Google Scholar]
- 30.Lima JP, Delgado G. Diagnosis of strongyloidiasis. Importance of Baermann's method. Am J Dig Dis. 1961;6:899–904. doi: 10.1007/BF02231086. [DOI] [PubMed] [Google Scholar]
- 31.Baars PA, Maurice MM, Rep M, Hooibrink B, Van Lier RAW. Heterogeneity of the circulating human CD4+ T cell population: further evidence that the CD4+CD45RA−CD27− T cell subset contains specialized primed T cells. J Immunol. 1995;154:17–25. [PubMed] [Google Scholar]
- 32.Hamann D, Baars PA, Rep MHG, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1–12. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgi JV, Ho HN, Hirji K, et al. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion. Development of HLA-DR+CD38-CD8+ cells is associated with subsequent stable CD4+ cell levels. J Infect Dis. 1994;170:775–81. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 34.Cox FEG. Immunology. In: Cox FEG, editor. Modern parasitology. 2nd edn. Oxford: Blackwell Scientific Publications; 1993. pp. 193–218. [Google Scholar]
- 35.Kalinkovich A, Weisman Z, Leng Q, et al. Increased CCR5 expression with decreased B chemokine secretion in Ethiopians: relevance to AIDS in Africa. J Hum Virol. 1999;2:283–9. [PubMed] [Google Scholar]
- 36.Helbert MR, Walter J, L’Age J, Bevereley PLC. HIV infection of CD45RA+ and CD45RO+ CD4+ T cells. Clin Exp Immunol. 1997;107:300–5. doi: 10.1111/j.1365-2249.1997.280-ce1170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abebe A, Demissie D, Goudsmit J, et al. HIV-1 subtype C syncytium and non-syncytium inducing phenotypes and co-receptor usage among Ethiopian patients with AIDS. AIDS. 1999;13:1305–11. doi: 10.1097/00002030-199907300-00006. [DOI] [PubMed] [Google Scholar]
- 38.Schnittman SM, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory cells by HIV-1. Evidence for a role in the selective T cell function defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6068–72. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spina CA, Prince HE, Richman DD. Preferential replication of HIV-1 in the CD45RO memory cell subsets of the primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–85. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamann D, Roos MT, Van Lier RAW. Faces and phases of human peripheral T cell differentiation. Immunol Today. 1999;20:177–80. doi: 10.1016/s0167-5699(99)01444-9. [DOI] [PubMed] [Google Scholar]
- 41.Hintzen RQ, de Jong R, Lens SM, Brouwer M, Baars P, Van Lier RA. Regulation of CD27 expression on subsets of mature T lymphocytes. J Immunol. 1993;151:2426–35. [PubMed] [Google Scholar]
- 42.Abdulkadir M. Assessment of the immune status of HIV positive and HIV negative individuals with and without intestinal parasitic infections. MSc thesis. Addis Ababa University. 1998 [Google Scholar]
- 43.Miedema F. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic Rev. 1992;3:73–193. [PubMed] [Google Scholar]
- 44.Ho HN, Hultin LE, Mitsuyasu RT, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–9. [PubMed] [Google Scholar]
- 45.Kestens L, Vanham G, Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altman JD, Moss PAH, Goulder PJR, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 47.Zwingenberger K, Irschick E, Siqueira G, et al. Release of interleukin 2 and gamma interferon by peripheral blood mononuclear cells in human Schistosoma mansoni infection normalizes after chemotherapy. Scand J Immunol. 1989;30:463–71. doi: 10.1111/j.1365-3083.1989.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 48.Roberts M, Butterworth A, Kimani G, et al. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–93. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grogan J, Kremsner P, Deelder A, Yazdanbkhsh M. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobium infection. Eur J Immunol. 1996;26:1365–70. doi: 10.1002/eji.1830260628. [DOI] [PubMed] [Google Scholar]
- 50.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth exposed individuals before and after Bacille Calmette–Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–25. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–8. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 52.Giorgi JJV, Lyles RH, Matud JL, et al. Predictive value of immunologic and virologic markers after long and short duration of HIV-1 infection. J AIDS. 2002;29:346–55. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]