Abstract
Pemphigus vulgaris (PV) is an antibody-mediated autoimmune disease of the skin and mucous membranes. Desmoglein-3 (dsg-3) expressed in the suprabasal layer of the skin serves as an autoantigen in PV. Passive transfer of sera, either from patients with PV or from experimental animals immunized with a recombinant human dsg3 (hdsg3) into neonatal BALB/c mice results in blister formation, suggesting strongly that there is significant cross-reactivity between the mouse dsg3 (mdsg3) and the hdsg3. However, efforts to induce disease in adult mice through active immunization using hdsg-3 have not been successful, suggesting that the epitopes required for the induction of pathogenic antibodies in adult mice might not be present in hdsg3. Therefore, in this study, we expressed a full-length mdsg3 in insect cells and compared its serological reactivity with that of the hdsg3 using species specific polyclonal sera and a panel of seven monoclonal antibodies (MoAbs) with unique binding specificities to hdsg3. Studies using sera demonstrated a considerable cross-reactivity, while studies using MoAbs exhibited specific epitope differences between the two proteins. Because of these differences, we reasoned that immunization with mdsg3 might induce disease in adult mice. Immunization of four strains of mice (i.e. BALB/c, DBA/1, HRS/J and SJL/J) with mdsg3 resulted in considerable antibody response, but failed to induce lesions. However, sera from immunized BALB/c mice induced acantholysis of neonatal mouse skin in vitro. These studies indicated that our inability to induce lesions in adult mice through active immunization is not due to differences in the ability of mouse and human dsg3 to induce acantholytic antibodies, but due probably to structural differences between adult and neonatal mouse skin. Alternatively, immunization with a combination of dsg3 protein along with other proteins might be necessary to induce pemphigus disease in adult mice. Nevertheless, our current studies show that molecular mechanisms leading to the production of acantholytic antibodies in mice can now be studied using homologous mdsg3.
Keywords: autoantibodies, autoimmunity, desmoglein-3, pemphigus vulgaris
Introduction
Pemphigus vulgaris (PV) is an autoimmune disease of skin and mucous membrane. Autoantibodies found in patients with PV bind to keratinocyte cell surface and cause loss of cell adhesion resulting in blister formation [1,2]. Previous studies have demonstrated that these pathogenic autoantibodies are directed against a desmosomal 130-kDa glycoprotein, desmoglein-3 (dsg3) [2,3]. The dsg3 is a transmembrane protein and belongs to the cadherin family of adhesion molecules [4,5]. Earlier, we and others have shown that recombinant extra-cellular domain of human dsg3 (hdsg3) was sufficient to neutralize blister-causing antibodies in sera from PV patients or experimental animals [6–8]. However, it remained unclear whether the extra-cellular domain of dsg3 was sufficient to induce blister-causing antibodies in experimental animals. Subsequently, we demonstrated that rabbits immunized with the full-length recombinant hdsg3, and not with the ectodomain, can produce pathogenic antibodies capable of causing blisters in neonatal mice and acantholysis of human skin in vitro [8,9]. More recently, we demonstrated further that only BALB/c mice immunized with a full-length hdsg3 could produce pathogenic antibodies capable of causing acantholysis of human foreskin in culture and blister in neonatal mice [10]. Recent studies using domain-swapped molecules between human dsg1 and dsg3, which are structurally similar but have distinct epitopes, showed that major epitopes for PV serum are located in the amino terminal residues 1–161 [11].
However, to date the pathogenesis of PV is not understood fully because of the lack of an animal model in which the lesions can be induced through active immunization. We reasoned that the failure to actively induce lesion in mice could be due to use of hdsg3 instead of a homologous mouse dsg3 (mdsg3). Therefore, we expressed a full-length mdsg3 protein in insect cells using a cDNA recently reported by Ishikawa et al. [12,13]. We compared the reactivity of mdsg3 with that of the hdsg3 and found that they are antigenically similar but exhibit considerable differences in specific epitopes. Subsequently, we used the purified protein along with 293 cells expressing mdsg3 on their cell surface to immunize different strains of mice. Although all strains of mice produced antibodies against the mdsg3, only sera from BALB/c mice could cause acantholysis of skin from neonatal mice in vitro.
Materials and Methods
Construction and subcloning of a full-length mdsg3
The 3120-bp full-length cDNA for mdsg3 was constructed from three partially overlapping cDNA fragments [12,13]. Clone 4 [1488 base pairs (bp)], clone 1 (533 bp) and clone 20 (1099 bp) were digested with Not I and Xho I, Xho I and Sac I and Sac I and EcoR I, respectively, and then ligated to obtain a full-length cDNA. The cDNA was inserted into the Not I–EcoR I sites of the baculovirus expression vector PVL 1392 (Pharmingen, San Diego, CA, USA) downstream of the polyhedrin promoter and used for generating recombinant virus.
Expression of full-length mdsg3 in insect cells
Expression of mdsg3 in insect cells was performed as described previously [14]. Briefly, 2 µg of PVL 1392 containing the full-length mdsg3 cDNA was mixed with 0·25 µg BaculoGold DNA (Pharmingen, San Diego, CA, USA) in a microcentrifuge tube containing 1 ml of transfection buffer B (Pharmingen). Culture medium from a monolayer of insect cells (Trichoplusia ni, TN-5) (Invitrogen, San Diego, CA, USA) was replaced with 1 ml of transfection buffer A. The above transfection buffer B containing DNAs was added dropwise on to the monolayer. Subsequently, the cells were supplemented with high-five serum-free expression medium (Invitrogen), and incubated at 27°C for 4 h. The attached cells were washed once with fresh high-five medium supplemented with serum, maintained in 3 ml of the same medium for 5 days and then harvested.
Purification and refolding of mdsg3
Culture supernatants and cells were analysed for protein production by sodium dodecyl sulphate-polyacrilamide gel electrophoresis (SDS-PAGE). A large proportion of the mdsg3 protein was cell associated and was found to be insoluble in buffers containing NP-40 detergent. To enrich for mdsg3, high-five cell pellet containing the recombinant protein was extracted sequentially with different buffers as described previously [14]. Briefly, the cells were suspended in lysis buffer (30 mm Tris-HCl, pH 7·5, containing 1% NP-40 and 150 mm NaCl) in a tube and vortexed. After incubating the cells at room temperature for 10 min, the insoluble mdsg3 along with nuclei were pelletted at 2300 g for 10 min, and then washed twice with PBS. The pellet was digested with nuclease buffer (10 mm Tris-HCl, pH 7·5, 10 mm NaCl) containing 500 µg/ml DNase I, 50 µg/ml RNase A and 50 U/ml RNase T1 for 1 h at room temperature, with occasional vortexing. The insoluble protein was pelleted by centrifugation at 2300 g for 10 min and the pellet was re-suspended in a high salt buffer (30 mm Tris-HCl, pH 7·5, containing 0·4 m (NH4)2SO4) and incubated for 15 min at room temperature, with vortexing. Final pellet was obtained after centrifugation at 2300 g for 10 min. The pellet was then solubilized in a buffer containing 50 mm Tris-HCl, pH 7·5 and 0·5 SDS. All buffers contained protease inhibitors; 0·5 µl of 0·1 m PMSF/ml, 5 µg/ml aprotinin and 5 µg/ml leupeptin. For gel purification of mdsg3, the protein sample was loaded onto an 8% preparative polyacrylamide gel flanked with broad range prestained molecular weight markers (Bio-Rad, Hercules, CA, USA). The protein was separated at 200 V for 5 h, and a 5-mm-wide band, corresponding to the 130-kDa prestained marker, was excised. The gel, containing mdsg3 was incubated for 2 h at 37°C in bicarbonate elution buffer (50 mm ammonium bicarbonate, 0·1% SDS). The eluted mdsg3 was lyophilized and repeatedly washed with cold 80% acetone to remove the SDS. This gel pure protein was used for immunizations. For renaturation of mdsg3, 1 mg of gel pure protein was solubilized in 40 ml of 6 m guanidine-HCl, and then neutralized with 20 ml of 1 m Tris-HCl, pH 7·5, and diluted to 160 ml with water containing equimolar concentrations of cystine and cysteine (final concentrations of 1 mm each). The solution was kept at 4°C overnight and then dialysed against 4 l of 0·1% ammonium bicarbonate, with three changes. The dialysed solution was lyophilized and resuspended in 100 mm Tris-HCl, pH 7·5 containing 2 mm CaCl2.
Western blot analysis
Proteins were separated in a 10% polyacrylamide gel and stained with 0·05% Coomassie brilliant blue R250 in 50% methanol and 10% acetic acid. For immunoblotting, the proteins were electrophoretically transferred to nitrocellulose membranes. Blots were treated for 1 h with a blocking buffer (5% non-fat dry milk in 200 mm NaCl with 0·1% Tween-20), and then incubated for 1 h each with the following reagents in succession with washing between each step: 1 : 2000 diluted rabbit anti-hdsg3 antibody [9], and 1 : 3000 diluted HRP-conjugated goat antirabbit IgG (Caltag Laboratories, San Francisco, CA, USA). The blots were developed using 4-chloro-1-naphthol as substrate.
Expression of mdsg3 on 293 cell surfaces
The 293 cells were transfected with pSRα neo vector containing cDNA encoding mdsg3 in serum free Dulbecco's modified Eagle' medium (DMEM). The pSRα vector [15] consists of a Simian virus (SV40) early promoter and part of the R-U5 segment of the long-terminal repeat (LTR) from human T cell leukaemia virus type I. The 293 cells cells were transfected with the plasmids using Lipofectamine (Life Technologies, Gaithersburg, MD, USA) following the manufacturer's protocol and cultured in DMEM containing 10% fetal bovine serum, 10 mm sodium pyruvate and 2 mm l-glutamine. Transfected cells (293 mdsg3 cells) were selected for neomycin resistance. Expression of mdsg3 was confirmed by flow cytometry using a polyclonal antibody against the hdsg3.
Immunization of different strains of mice with 293 cells expressing mdsg3
Six- to 8-week-old female BALB/c, SJL/J, HRS/J and DBA/1 (10 mice per group) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). They were immunized nine times as per the following schedule: mice were primed twice (s.c.) on days 0 and 14 with purified, refolded mdsg3 (50 µg/mouse) emulsified in Freund's complete adjuvant and then challenged with mitomycin C treated 2 × 107, 293 mdsg3 cells, along with cholera toxin B (i.p.) on days 44, 62, 92, 122, 152, 182 and 212. Control groups were similarly immunized twice with CFA, followed by immunization with 2 × 107 293 cells along with CTB. Mice were primed initially with purified mdsg3 protein to activate mdsg3-specific T cells that can then recognize the mdsg3 protein on 293 cells. To compare immune responses against mdsg3 and hdsg3, 6–8-week-old female BALB/c mice were immunized (i.p.), once with 20 µg/mouse of either mdsg3 or hdsg3 in Freund's complete adjuvant, followed by three additional inoculations, on days 10, 20 and 30, with the same amount of protein in Freund's incomplete adjuvant. On day 44, mice were bled to obtain sera, which were tested for antibody response. All animal studies were carried out according to the principles and procedures detailed in the institutional guideline for the care and use of laboratory animals.
Titration of antibodies against hdsg3 and mdsg3
Antibody titres against both mdsg3 and hdsg3 were determined using an enzyme-linked immunosorbent assay (ELISA) [16]. Briefly, polystyrene Immulon 2 microtitre plates (Dynatech Laboratories, Chantilly, VA, USA) were coated with 50 ng/well of either pure hdsg3 or mdsg3 using 100 µl of carbonate/bicarbonate buffer (pH 9·6) and incubated overnight at 4°C. The plates were washed three times using PBS containing 0·05% Tween 20 in a microplate washer (Titertek, Huntsville, AL, USA). Then the following reagents were added in succession with washing between each step: 100 µl of appropriately diluted sera for 1 h at room temperature; 100 µl of HRP-conjugated goat antimouse IgG diluted 1 : 3000 (Caltag) for 1 h at room temperature; and 100 µl of substrate solution containing 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (Sigma, St Louis, MO, USA) and 0·03% of 30% hydrogen peroxide in 0·1 m citrate buffer (pH 4·2). Then the plates were incubated at room temperature for 10 min. The reaction was stopped by adding 100 µl of 1% SDS to each well. Substrate conversion was measured at 405 nm using a model 550 microplate reader (Bio-Rad).
Competitive inhibition assay
The specificity of binding of sera to recombinant hdsg3 and mdsg3 was assessed by ELISA. Increasing concentrations of recombinant hdsg3 or mdsg3 were mixed with 1 : 200 dilutions of sera from mice immunized with either hdsg3 or mdsg3 and incubated for 1 h at room temperature. The mixture was then assayed using an ELISA as described above.
Generation of monoclonal antibodies
Six- to 8-week-old female BALB/c mice were purchased from the Jackson Laboratory. Two mice were immunized three times with 20 µg/mouse of purified hdsg3 protein. Splenocytes from these mice were fused with myeloma cells P3 × 63-Ag8·653 at a 10 : 1 ratio using 50% polyethylene glycol, as described previously [17]. After fusion, cells were plated into 96-well plates at 2 × 105 cells in 100 µl growth medium/well. Growth medium consisted of DMEM containing 20% fetal bovine serum, 25 mm HEPES buffer, 10 mm sodium pyruvate, 2 mm l-glutamine and 20 µg/ml gentamycin sulphate. After 24 h, 100 µl growth medium containing hypoxanthine, aminopterin and thymidine was added. When cells showed good growth, culture supernatants were tested for anti-hdsg3 antibodies using an ELISA. Cells from positive wells were transferred into 24-well plates and cloned subsequently by seeding single cell per well into 96-well plates. Culture supernatants were screened again for anti-hdsg3 and positive hybridomas were identified. Subclass specificities of MoAbs were determined using 100 µl of culture supernatants and peroxidase conjugated goat antimouse IgG1, IgG2a, IgG2b or IgG3 (Caltag Laboratories).
Induction of mouse skin acantholysis in vitro
Neonatal mouse skin was cut into 2 × 2-mm pieces and floated dermal side down in tissue culture dishes containing RPMI-1640 medium. We then added 200 µl of mouse serum diluted 1 : 4, and incubated at 37°C in a humidified CO2 incubator for 12 h. The skin explant specimens were processed for routine histological examination. Haemotoxylin–eosin-stained sections were examined for the presence of acantholytic cells and suprabasilar cleft formation.
Results
Expression of recombinant full-length mdsg3 in insect cells
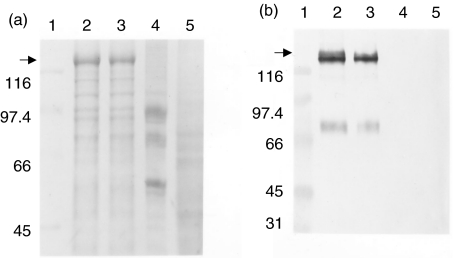
High-five insect cells infected with recombinant baculovirus containing a cDNA for mdsg3 were analysed by SDS-PAGE for the production of recombinant protein and found that the protein was expressed at a high level (not shown). Because the recombinant mdsg3 protein was insoluble in NP-40, we could obtain highly enriched mdsg3 protein readily in the cell pellet. The protein was enriched further using sequential extraction with various buffers. Figure 1 shows Coomassie blue staining of enriched recombinant mdsg3 protein. The protein band at approximately 130 kilo daltons (kDa) present in recombinant virus-infected cell extracts (lane 2) most probably represents msdg3. As a positive control we used insect cells infected with a recombinant virus that encodes hdsg3 (lane 3). As a negative control, we used cells expressing thyrotrophin receptor (TSHR) and uninfected cells (lanes 4 and 5, respectively). To confirm the identity of the recombinant proteins, proteins were transferred to a nitrocellulose membrane and the blot was stained using an hdsg3 specific antibody. As shown in Fig. 1b, the antibodies specifically reacted with the proteins in lanes 2 and 3 showing that the recombinant proteins in fact represent mdsg3 and hdsg3, respectively.
Fig. 1.
SDS-PAGE and Western blot of recombinant mdsg3. (a) Sequentially extracted recombinant virus-infected insect cells (high-five) proteins were separated on a 7·5% gel and stained with Coomassie blue. (b) Western blot prepared from a gel similar to that in (a) and reacted with rabbit antibody to recombinant hdsg3. (a) and (b) Lane 1, molecular weight marker proteins; lanes 2 and 3, proteins obtained from cells infected with recombinant virus-encoding mdsg3 and hdsg3, respectively; lane 4, TSHR (control), lane 5, normal high-five cells.
Reactivity of sera against dsg3 proteins
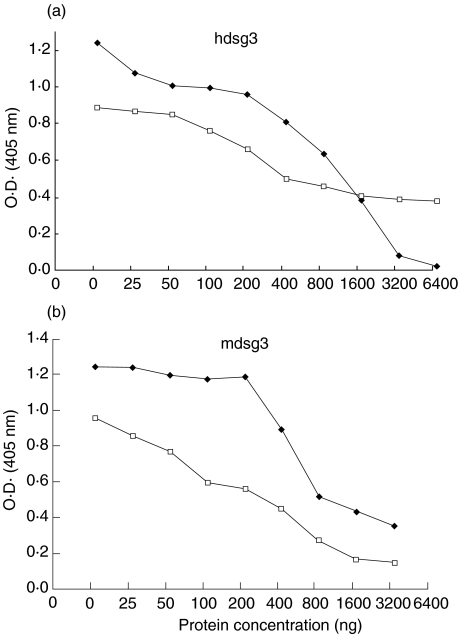
BALB/c mice were immunized with recombinant mdsg3 or hdsg3 proteins. To determine whether there were quantitative differences in their responses against different dsg3s and to evaluate cross-reactivity between the two proteins, we tested sera from these mice in an ELISA. As shown in Fig. 2a,c, sera from all four mice immunized with hdsg3 exhibited higher titres to hdsg3 relative to their titres against mdsg3. Similarly, sera from mice immunized with mdsg3 showed higher levels of antibodies against mdsg3 relative to the levels found against the hdsg3 (Fig. 2b,d).
Fig. 2.
Reactivity of sera from mice immunized with human and mouse dsg3 against dsg3 proteins. (a, c) Reactivity of mouse anti-hdsg3 antibodies with human and mouse dsg3. (b, d) Reactivity of mouse anti-mdsg3 antibodies with human and mouse dsg3. (a, c) •, Mouse 1: hdsg3; ⋄, mouse 2: hdsg3; □, mouse 3, hdsg3; ○, mouse 4: hdsg3. (b, d) •, Mouse 1: mdsg3; ⋄, mouse 2: mdsg3; □, mouse 3, mdsg3; ○, mouse 4: mdsg3.
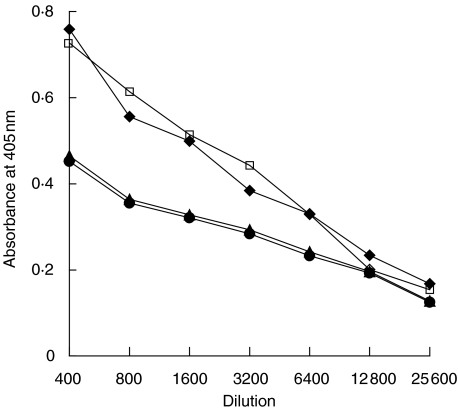
In competitive inhibition assays, 1 : 200 diluted pooled sera were preincubated with different concentrations of either hdsg3 or mdsg3 protein, and then their reactivity with hdsg3 and mdsg3 were assessed using ELISA. Figure 3a shows the reactivity of sera from mice immunized either with mdsg3 or hdsg3 protein that was preincubated with hdsg3. The reactivity of anti-hdsg3 sera against hdsg3 was completely inhibited, while the reactivity of anti-mdsg3 sera was only partially inhibited by hdsg3. Figure 3b shows the reactivity of sera from mice immunized with either mdsg3 or hdsg3 against mdsg3. The reactivity of anti-mdsg3 sera was almost completely inhibited by mdsg3, whereas hdsg3 only partially inhibited the binding. Collectively, our results showed that although there is considerable cross-reactivity between the proteins, antigenically they are not identical.
Fig. 3.
Specificity of binding of sera to recombinant hdsg3 and mdsg3. Increasing concentrations of recombinant hdsg3 or mdsg3 were mixed with 1 : 200 diluted of sera from mice immunized with hdsg3 or mdsg3 protein and incubated for 1 h at room temperature. The mixture was then assayed using an ELISA. (a) Reactivities of anti-mdsg3 and anti-hdsg3 antibodies against hdsg3 after preincubating with increasing concentrations of hdsg3. (b) Reactivities of anti-mdsd3 and anti-hdsg3 antibodies against mdsg3 after preincubating with increasing concentrations of mdsg3. □, Mouse mdsg3 sera; ⋄, mouse hdsg3 sera.
Reactivity of monoclonal antibodies
We generated monoclonal antibodies from mice immunized with recombinant hdsg3 and tested for their reactivity against hdsg3 using subclass specific antibodies. As shown in Table 1, most hybridomas produced IgG1 while two produced IgG2a antibodies. Three of the 11 antibodies (i.e. 4G12, Ic8 and IIId7) reacted with the extracellular domain of hdsg3 (EPVA), while others failed to react with EPVA.
Table 1.
Monoclonal antibodies to human dsg3
| Reactivity with | |||
|---|---|---|---|
| MoAbs | PVA O.D.a | EPVA O.D.a | Subclassb |
| 1A7 | 0·73 | – | IgG1 |
| 2C5 | 0·68 | – | IgG1 |
| 2H8 | 0·70 | – | IgG1 |
| 3A3 | 0·33 | – | IgG1 |
| 4G12 | 1·18 | 1·11 | IgG1 |
| 5C4 | 0·72 | – | IgG1 |
| 5E7 | 0·72 | – | IgG1 |
| 5G11 | 0·80 | – | IgG1 |
| Ic8 | 1·40 | 0·34 | IgG2a |
| IIIc10 | 1·80 | – | IgG1 |
| IIId7 | 0·64 | 0·83 | IgG2a |
Optical density at 405 nm when tested against human pemphigus vulgaris antigen (PVA) or extracellular domain of human pemphigus vulgaris antigen (EPVA).
Subclass was determined using subclass-specific HRP conjugated antibodies. – Represents optical density of less than 0·1 at 405 nm.
To identify regions of the protein with which MoAbs reacted, we tested a panel of 11 MoAbs against 46 synthetic peptides spanning the entire hdsg3 [10]. As shown in Table 2, MoAbs IA7, 2C5, 2H8, 5C4, 5E7 and 5G11 reacted very strongly with peptide 43 (911–931 aa). Monoclonal antibodies 3A3 and IIIc10 reacted with peptides 42 (890–910 aa) and 38 (806–826 aa), respectively. Similarly, MoAbs 4G12, Ic8 and IIId7 reacted with peptides 24 (484–504 aa), 12 (232–252 aa) and 28 (568–592 aa), respectively. These latter three peptides are derived from the extracellular domain of the hdsg3 and thus confirmed our earlier results, which had shown that MoAbs 4G12, Ic8 and IIId7 could react with EPVA. MoAb Ic8 reacted strongly with PVA and peptide 12, but weakly with EPVA. This could be due to the expected differences in the folding of PVA and EPVA, that might have prevented exposure of amino acids represented by peptide 12 on EPVA. Next, we selected seven different MoAbs with unique binding specificities and tested them for their ability to react with mdsg3. These results showed that only one of seven hdsg3-specific MoAbs (Ic8), with specificity for the extracellular domain (peptide 12; 232–252 aa) could react with mdsg3 (Table 3).
Table 2.
Reactivity of MoAbs with peptides
| Peptides | ||||||
|---|---|---|---|---|---|---|
| MoAbs | 12 | 24 | 28 | 38 | 42 | 43 |
| 1A7 | – | – | – | – | – | 1·14 |
| 2C5 | – | – | – | – | – | 1·28 |
| 2H8 | – | – | – | – | – | 1·33 |
| 3A3 | – | – | – | – | 0·64 | – |
| 4G12 | – | 1·18 | – | – | – | – |
| 5C4 | – | – | – | – | – | 1·45 |
| 5E7 | – | – | – | – | – | 1·33 |
| 5G11 | – | – | – | – | – | 1·34 |
| Ic8 | 1·69 | – | – | – | – | – |
| IIIc10 | – | – | – | 0·40 | – | – |
| IIId7 | – | – | 0·81 | – | – | – |
Reactivity was measured in an ELISA against 46 synthetic peptides, and only the peptides with which they reacted are listed here. Values represent the optical density at 405 nm. – Represents optical density less than 0·1 at 405 nm.
Table 3.
Sequence comparison of MoAbs reactive dsg3 peptides
| Reactivity with | ||||
|---|---|---|---|---|
| MoAbs | hdsg3 | mdsg3 | Residue numbers1 | Amino acid sequence2 |
| 1A7 | + | – | 911–931 | TETYSASGLVQPSTAGFDPL TETYSTSGSFAQPTTVFTDPH |
| 3A3 | + | – | 890–910 | SGSVQPAVSIPDPLQHGNYLV SGSVHPAVAIPDPLQLGNYLL |
| IIIc10 | + | – | 806–826 | GSVGCCSFIADDLDDSFLDSL – TLSSCSIFGDDLDDNFLDSL |
| IIId7 | + | – | 568–592 | NRGICGTSYPTTSPGTRYGRPHSGR DRSMCRAPIPSREPNT-YGE-SSWR |
| 4G12 | + | – | 484–504 | PSVVVSARTLNNRYTGPYTFA PSVTLSVRTLDRGKYTGPYTVS |
| Ic8 | + | + | 232–252 | IKVKDVNDNFPMFRDSQYSAR IKIKDVNDNFPVLRESQYSAR |
+ Optical density more than 0·2 at 405 nm; – optical density less than 0·1 at 405 nm
residue numbers based on human sequence
upper and lower sequences represent human and mouse dsg3, respectively.
Immune response against 293 cells expressing mdsg3
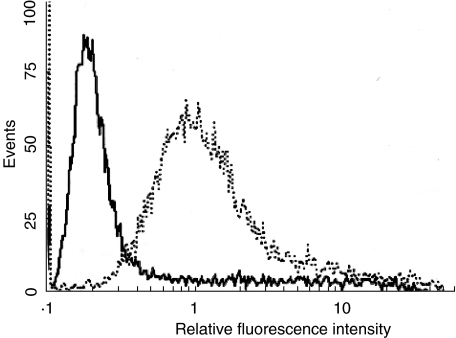
We developed 293 cells permanently expressing full-length mdsg3 on their surface (Fig. 4). These cells were used as a source of mdsg3 with native conformation. Mice were first primed with the insect cell derived mdsg3 protein followed by immunization with 293 cells expressing mdsg3. All strains of mice mounted antibody responses against the mdsg3, albeit to different levels as measured by ELISA (Fig. 5). BALB/c and SJL/J had higher antibody levels than DBA/1 and HRS/J. In spite of relatively high levels of antibodies against mdsg3, none of the adult mice exhibited skin lesions.
Fig. 4.
Reactivity of anti-hdsg3 Abs with cells expressing mdsg3. The 293 cells were transfected with pSRα neo vector containing full-length mdsg3 cDNA, and stable cell lines were established (293 mdsg3). Cells were analysed by flow cytometry for the expression of mdsg3 by staining with rabbit anti-hdsg3 antibody (1 : 100), followed by FITC- conjugated goat antirabbit IgG (1 : 100). Propidium iodide was used to distinguish live from dead cells. —, 293 cells; ···, 293 mdsg3 cells.
Fig. 5.
Titration of sera from mice immunized with mouse dsg3. Pooled sera from each of the four different strains of mice were assayed against mouse dsg3 in an ELISA as described under Materials and Methods. ⋄, BALB/c; □, SJL/J; •, HRS/J; ▴;, DBA/1.
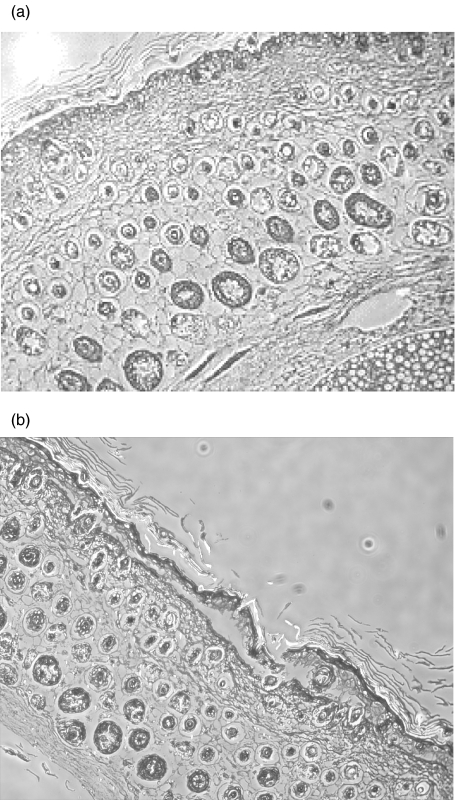
Next, skin from neonatal mice was incubated with sera from both immune and control mice and processed for histopathological examination. Only sera from BALB/c mice immunized with mdsg3 caused acantholysis (Fig. 6), whereas sera from other three strains of immune mice or the corresponding controls had no effect. The lesion was characterized by suprabasilar separation of keratinocytes, which is characteristic of PV.
Fig. 6.
Histopathology of skin treated with antibodies. Skin from neonatal mice incubated with (a) normal mouse sera and (b) sera from mdsg3 immunized mice. Please note normal architecture of the skin in (a) and suprabasalar blistering within the epidermis in (b).
Discussion
Understanding of the pathogenesis of PV is hindered by a lack of an animal model in which the disease could be induced by active immunization. Earlier studies have shown clearly that the disease can be transferred passively to neonatal mice using sera from either patients with PV or animals that have been immunized with hdsg3 [3, 4, 8–10, 18, 19]. Although we were able to produce antibodies that are acantholytic to neonatal mice by immunizing adult mice or rabbits with hdsg3, the immunized animals themselves did not develop any lesion. This, we reasoned, could be due to use of hdsg3 rather than a homologous mouse protein. There is 86% homology between mdsg3 and hdsg3 cDNA sequences [13], with a 74·6% overall identity at the amino acid level. These differences in the primary structure could have significant impact on the three dimensional structure of the protein, which largely determines the B cell epitopes. Considering that this is an antibody-mediated disease, we felt that it might be prudent to use a homologous protein for immunization of mice. Therefore, in the current study we expressed successfully mdsg3 protein in insect and 293 cells.
We used the recombinant mdsg3 and hdsg3 to immunize mice and to compare their responses against mdsg3 and hdsg3 [10]. Mice immunized with hdsg3 had higher titres than mice that were immunized with mdsg3. This was due most probably to expected higher immunogenicity of the heterologous hdsg3 protein relative to homologous mdsg3. Competitive inhibition studies, using a combination of both hdsg3 and mdsg3, and antibodies raised against those proteins, showed that hdsg3 could not adsorb all of the mouse anti-mdsg3 reactivity. Similarly, mdsg3 could not out-compete all the anti-hdsg3 antibodies. These studies showed clearly that in spite of significant similarity between the two proteins, they are not identical antigenically.
To define further the antigenic epitopes, we developed a panel of monoclonal antibodies against the hdsg3. Analyses of their binding specificities showed that only one (i.e. Ic8) of the seven MoAbs with unique binding specificities was able to cross-react with mdsg3. These studies demonstrated further that there are significant differences in the fine specificity of hdsg3 and mdsg3 which could affect the three-dimensional structures leading to a display of different arrays of antibody epitopes. This raised the possibility that acantholytic epitopes relevant for disease induction might reside in the non-homologous regions and are unique to mdsg3.
Upon testing these antibodies against a large panel of peptides that spanned the entire hdsg3, we were able to show that Ic8 reacted with peptide 12 [10], which is part of the ectodomain and consists of amino acids 232–252 of hdsg3. The relevance of this and other similar cross-reactivities for the induction of blisters in neonatal mice by human sera needs to be established.
Previously we used either hdsg3 or its ectodomain, produced in insect cells, to immunize mice. These mice failed to develop pathogenic antibodies. This was due primarily to lack of native conformation resulting from improper refolding of the protein, as refolded protein was able to induce pathogenic antibodies [8–10]. Therefore, in this study we developed permanently transfected 293 cells expressing mdsg3 protein on their surface. The expression was confirmed by flow cytometry and these cells were used as a source of protein with native conformation to immunize mice. All strains of immunized mice produced significant amounts of antibodies against mdsg3, but failed to develop lesions. To confirm that these mice in fact had produced acantholytic antibodies, we tested their sera for their ability to induce acantholysis of skin from neonatal mice. Only sera from BALB/c mice contained antibodies capable of inducing acantholysis. Although these results were somewhat surprising they are consistent with our earlier observations, in which we had shown that anti-hdsg3 from only BALB/c mice was capable of inducing blisters in neonatal mice [10]. These and our earlier observations [10] suggest that either hdsg3 or mdsg3 protein can be used to elicit antidsg3 antibodies in BALB/C mice, and these mice have the necessary immunogenetic background required for the production of acantholytic antibodies. Moreover, these results show that in spite of differences in the primary structure of mdsg3 and hdsg3, they are distinct antigenically, yet similar in that they are both able to elicit antibody responses that are acantholytic to neonatal mouse skin. As many of the B cell epitopes are represented by hydrophilic amino acids and hydrophilic index of a protein is often used to determine the most likely B cell epitopes, we compared the hydropathy plots of the two proteins. This analysis revealed that hdsg3 and mdsg3 are antigenically very similar (not shown).
Inability to induce active disease in adult mice, in spite of the presence of acantholytic antibodies, suggests strongly that the architecture of adult mouse skin, unlike that of adult human skin, might preclude development of lesion. Therefore, full resolution of this would require screening additional strains of mice that have a propensity to develop skin disorders. In addition, recent studies indicate that antigens other than dsg3 could be involved in the pathogenesis of the disease [20,21]. This suggests that immunization with a combination of more than one protein might be necessary to induce the disease in adult mice. Nevertheless, this report shows for the first time that acantholytic antibodies to dsg3 can be induced readily using a homologous protein. Availability of this mouse model would facilitate studies aimed at understanding the mechanism of induction of acantholytic antibodies against dsg3 with implications for understanding the human disease.
References
- 1.Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous disease due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- 2.Stanley JR, Yaar M, Hawley-Nelson P, Katz SI. Pemphigus antibodies identify a cell surface glycoprotein synthesized by human and mouse keratinocytes. J Clin Invest. 1982;70:281–8. doi: 10.1172/JCI110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amagai M, Karpati S, Prussick R, Klaus-Kovtun V, Stanley JR. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. J Clin Invest. 1992;90:919–26. doi: 10.1172/JCI115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adherin. Cell. 1991;67:869–77. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 5.Buxton RS, Cowin P, Franke WW, et al. Nomenclature of the desmosomal cadherins. J Cell Biol. 1993;121:481–7. doi: 10.1083/jcb.121.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amagai M, Hashimoto T, Shimizu N, Nishikawa T. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg 3) produced by baculovirus. J Clin Invest. 1994;94:59–66. doi: 10.1172/JCI117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto T, Ogawa MM, Konohana A, Nishikawa T. Detection of pemphigus vulgaris and pemphigus foliaceus antigens by immunoblot analysis using different antigen sources. J Invest Dermatol. 1990;94:327–34. doi: 10.1111/1523-1747.ep12874456. [DOI] [PubMed] [Google Scholar]
- 8.Memar OM, Rajaraman S, Thotakura R, et al. Recombinant desmoglein 3 has the necessary epitopes to adsorb and induce blister-causing antibodies. J Invest Dermatol. 1996;106:261–8. doi: 10.1111/1523-1747.ep12340663. [DOI] [PubMed] [Google Scholar]
- 9.Memar OM, Christensen B, Rajaraman S, et al. Induction of blister-causing antibodies by a recombinant full-length, but not the extracellular, domain of the pemphigus vulgaris antigen (desmoglein 3) J Immunol. 1996;157:3171–8. [PubMed] [Google Scholar]
- 10.Fan JL, Memar OM, McCormick DJ, Prabhakar BS. BALB/c mice produce blister-causing antibodies upon immunization with a recombinant human desmoglein 3. J Immunol. 1999;163:6228–35. [PubMed] [Google Scholar]
- 11.Futei Y, Amagai M, Sekiguchi M, Nishifuji K, Fujii Y, Nishikawa T. Use of domain-swapped molecules for conformational epitope mapping of desmoglein 3 in pemphigus vulgaris. J Invest Dermatol. 2000;115:829–34. doi: 10.1046/j.1523-1747.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa H, Silos SA, Tamai K, et al. cDNA cloning and chromosomal assignment of the mouse gene for desmoglein 3 (dsg 3), the pemphigus vulgaris antigen. Mammalian Genome. 1994;5:803–4. doi: 10.1007/BF00292018. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa H, Li K, Tamai K, Sawamura D, Uitto J. Cloning of the mouse desmoglein 3 gene (dsg3): interspecies conservation within the cadherin superfamily. Exp Dermatol. 2000;9:229–39. doi: 10.1034/j.1600-0625.2000.009004229.x. [DOI] [PubMed] [Google Scholar]
- 14.Fan JL, Patibandla SA, Kimura S, et al. Purification and characterization of a recombinant human thyroid peroxidase expressed in insect cells. J Autoimmunity. 1996;5:529–36. doi: 10.1006/jaut.1996.0071. [DOI] [PubMed] [Google Scholar]
- 15.Yutaka T, Motoharu S, Juni-ichi F, et al. SRα promoter an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type-I long terminal repeat. Mol Cell Biol. 1981;8:466. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan JL, Peterson JW, Prabhakar BS. Adjuvant effects of cholera toxin b subunit on immune response to recombinant thyrotropin receptor in mice. J Autoimmunity. 2000;14:43–52. doi: 10.1006/jaut.1999.0336. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakar BS, Haspel WV, Notkins AL. Monoclonal antibody techniques applied to viruses. In: Maramorosch K, Koprowski H, editors. Methods in virology, ch. 7. Orlando: Academic Press; 1984. pp. 1–19. [Google Scholar]
- 18.Bohl K, Natrajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci USA. 1995;92:5239–43. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–96. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 20.Grando SA. Autoimmunity to keratinocyte acetylcholine receptors in pemphigus. Dermatology. 2000;201:290–5. doi: 10.1159/000051540. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen VT, Ndoye A, Shultz LD, Pittelkow MR, Grando SA. Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris-like lesions. J Clin Invest. 2000;106:1467–79. doi: 10.1172/JCI10305. [DOI] [PMC free article] [PubMed] [Google Scholar]