Abstract
The objective of this study was to study immune system status in long-term asymptomatic (LTA) HIV-1-infected children. A cross-sectional study was used, involving HIV-1-infected children over 7 years of age who were rated into two groups according to their clinical and immunological classification: (a) LTA: 7 asymptomatic HIV-1-infected children in A1; (b) Rapid progressor (RP): 14 age-matched C3 HIV-1-infected children. The control group consisted of 17 age-matched uninfected children. The characterization of CD4+ T-cell subsets was determined by three-colour flow cytometry. The proliferative response and cytokine production by activated peripheral blood T-cells were also measured. IL-7 levels were measured in serum. Thymic production of T-cells was quantified by TCR rearrangement excision circles (TRECs). The LTA children showed similar proliferative responses to PHA, PWM and anti-CD3+ anti-CD28, but lower responses to tetanus toxoid and streptokinase, in comparison with the controls but always higher responses in comparison with the RP group. The production of TNF-α and IFN-γ was similar in the LTA and control groups, and both were higher than the levels in the RP group. The LTA group showed a lower percentage of memory CD4+ T-cells (CD4+ CD45RO+, CD4+ CD45RA-CD62L+) than the control and RP groups. The LTA group also showed lower percentages of CD4+ CD7- cells than the controls. As for naïve CD4+ T-cells (CD4+ CD45RA+ CD62L+), CD4+ CD45RA+ and CD4+ CD62L+ cells, the LTA group showed higher values than the control and RP groups. The LTA group showed higher percentages of CD4+ HLA-DR+ CD38+ than the controls, but lower values than the RP group. In contrast, the LTA group had percentages of CD4+ HLA-DR-CD38+ T-cells higher than both the control and RP groups, whereas CD4+ CD38+ levels were only higher in the LTA group in comparison with the controls. CD4+ HLA-DR+ CD38- and CD4+ HLA-DR+ cell numbers were lower in the LTA group in comparison with the RP group. We found almost normal values of TRECs and IL-7 in the LTA group, but lower values in the RP group. Moreover, we found an inverse relation between TREC levels and IL-7 in plasma from HIV-infected children. Asymptomatic HIV-1 infected children have a well preserved immune system similar to that of control uninfected children in spite of HIV-infection for more than 7 years. Moreover, our results identified new markers of HIV disease, such as TRECs and IL-7, that could be used to monitor disease.
Keywords: HIV-1, children, subsets CD4+, proliferation, cytokine, TRECs, IL-7
Introduction
Human immunodeficiency virus type 1 (HIV-1) infection causes a progressive and severe immunodeficiency [1]. In infected children, this disease has distinct patterns of disease progression and is known for its heterogeneous manifestations [2,3]. Impairment of T-cell function occurs early in HIV infection, when CD4 T-cells are still within normal ranges, and further decreases as disease progresses [4,5]. Early immunological signs of HIV immunodeficiency include decreased T-cell proliferation to recall antigens and allogeneic HLA responses (mixed lymphocyte reaction (MLR)), as well as impaired antigen specific IL-2 and IFN-γ production by memory CD4+ T-cells (CD45RO+) [4, 6, 7]. Also, decreased lymphocyte proliferation and production of cytokines in response to mitogens such as phytohemagglutinin (PHA), pokeweed (PWM) and anti-CD3 plus anti-CD28 [4, 8, 9], all of them more pronounced in advanced disease, have been described. Although initial T-cell defects may be accounted for by the selective loss of memory cells, the function of both naïve CD45RA+ and memory CD45RO+ cells is affected in later stages of HIV infection [7]. The CD4+ CD45RO+ memory T-cell subset derives from a post-thymic maturation process from CD4+ CD45RA+ naïve T-cells [10,11]. T-cells also recycle between blood and lymphoid tissues and then back to blood [12,13] in a process thought to be dependent on l-selectin (CD62L) expression [12,13]. During HIV infection, a subset of CD4+ T-cells, characterized by a lack of CD7 cell surface expression, expand in numbers. This CD4+ CD7- T-cell expansion correlates with disease progression and is associated with in vivo activation of these cells and an impaired profile of cytokine production [14]. CD4+ CD7- T-cells reflect a separate and stable differentiation state within CD45R0+ CD45RA- memory cells occurring late in the immune response [15].
Moreover, HIV disease is characterized by state of chronic activation, driven by HIV antigen as well as by cytokines released in antigen independent ways [16]. This activation is associated with CD4+ T-cell depletion and disease progression [17]. In adults, immune activation correlates with an increase in T-cells coexpressing the activation markers CD38 and HLA-DR [18,19]. However, in children, the CD38+ marker is a maturation rather than an activation marker [20] and its expression on T-cells decreases over the years. This fact may lead to a misinterpretation of the meaning of this marker in children, since these cells can be either immature and/or activated [21–23]. Increased expression of HLA-DR on T-cells has also been proposed as a progression marker of HIV infection both in adults [24] and children [25].
The decrease in CD4+ T-cells during HIV infection is thought to be the result of both peripheral destruction caused by the virus and inadequate replacement of destroyed T cells [26]. The thymus, the organ responsible for the production of new T cells, would allow for replacement of lost cells. It may play a more prominent role in T cell homeostasis in paediatric than in adult HIV infection, since children have not yet suffered the natural thymic involution associated with age. However, several studies have shown that HIV can infect and destroy thymocytes thus affecting the thymic function [27] and the production of new T-cells [28]. During T cell receptor rearrangement in newly developed T cells, the excised DNA persists episomally as T cell receptor (TCR) excision circles (TRECs), and TREC+ T-cells are recent thymic emigrants. Thus, the level of TRECs in peripheral blood T-cells is an excellent measure of thymic function in general [28]. TRECs predominate in CD45RA+ T-cells, confirming the recent emigration or lack of extensive post-thymic division of the CD45RA+ T-cell subset [28]. It has also been demonstrated, in HIV-infected patients that high levels of IL-7 in plasma are associated with low CD4+ T-cells counts, high viraemia and disease progression [29]. IL-7 is known to be involved in T-cell development, regeneration and function [30].
Long-term asymptomatic (LTA) adult HIV-infected patients, remain healthy and they are characterized by a relatively stable HIV-specific effector repertoire, undetectable viral load (VL), normal CD4+ T-cell counts, a strong neutralizing antibody response, and by vigorous inhibitory CD8+ T-cells [31–33]. However, similar studies have not been performed in children. Thus, the aim of this study was to analyse the immune status, determining the number and functional activity of T-cells, CD4+ subsets, proliferation in response to antigens and mitogens, and levels of cytokine production in a group of vertically LTA HIV-1-infected children characterized by 7 or more years of documented HIV infection. The children were not suffering any HIV-related disease, were not under antiretroviral therapy with protease inhibitor and most importantly had T-cell CD4+ counts ≥600 cell/mm3.
Patients And Methods
Study population
Twenty-one infants born to HIV-1-infected mothers were studied at the Unidad de Inmuno-Paediatría del Hospital General Universitario ‘Gregorio Marañón’ in Madrid, Spain. All infants were diagnosed as HIV-1-infected on the basis of positive results in both DNA-PCR and virus culture assays, as previously described [34]. A cross-sectional study was performed, consisting of the characterization of CD8+ T-cell subsets in HIV-infected children over 7 years of age. Children were separated into two groups according to their clinical and immunological classification: (a) LTA; Asymptomatic HIV-1-infected children with seven or more years of documented HIV infection, no HIV-related disease, stable CD4+ T-cell counts of ≥600 cell/mm3; none were receiving antiretroviral therapy with protease inhibitor. (b) RP: Age-matched symptomatic HIV-1-infected children with CD4+ T-cell counts <250 cell/mm3, CDC Class 3C disease, and treatment failures. We also studied 17 age-matched uninfected children as controls.
Drugs were prescribed by the attending paediatrician according to CDCP guidelines [35] upon obtaining written informed consent from legal guardians. Clinical and immunological classification was based on the 1994 revised guidelines of the Centers for Disease Control and Prevention (CDCP) [36] and were measured after obtaining written informed consent from parents or legal guardians. The study was conducted according to the declaration of Helsinki and approved by the Ethical Committee of our hospital.
Quantitative HIV-1 RNA assay
Blood samples were collected in EDTA tubes, separated within 4 h and the plasma stored at −70°C. VL was measured in 200 µl plasma using a quantitative reverse transcriptase PCR (RT-PCR) assay (Amplicor monitor, Roche Diagnostic Systems, Brandenburg, NJ, USA).
Proliferative assays and cytokine production
Peripheral blood mononuclear cells (PBMC) were seeded in 96-well flat-bottom microtiter plates (2 × 105/200 µl per well) in RPMI-1640 medium supplemented with 10% of fetal calf serum. PBMC were stimulated with 1 µg/ml of PHA (Murex Biotech Limited, Dartford, UK), or 1 µg/ml of monoclonal anti-CD3 antibody (SPV3Tb) plus 1 µg/ml of monoclonal anti-CD28 antibody (L-293, Becton-Dickinson Immunocytometry Systems, San José, CA, USA), or 4 µg/ml of PWM (Sigma Chemical Co, St Louis, MO, USA). In addition, we studied proliferation in response to the following antigens: 1 µg/ml of recombinant p24 HIV-1 (Protein Science Corporation, Meriden, CT, USA), 100 IU/ml of streptokinase (SK) (Kabikinase® Pharmacia & Upjohn, S.A., Sant Cugat del Valles, Barcelona, Spain), and 0·5 Lf/ml of tetanus toxoid (TT) (NIBSC, Mill Hill, UK). We also studied the proliferative responses of PBMC to a mixture of mitomycin-treated PBMCs from 6 unrelated donors (MLR, ratio responder: target 1 : 1).
The cultures were incubated at 37°C and maintained in an humidified atmosphere containing 5% CO2. After 3 days of culture with a mitogenic stimulus, and 6 days of culture with an antigenic stimulus, 50% of the culture supernatants were harvested and stored at −70°C and the wells were replenished with an equivalent volume of fresh medium with 1 µCi[3H]-Thymidine (Amershan, Buckinghamshire, UK). Cell proliferation was estimated by incorporation of [3H]-Thymidine into DNA during the last 6 h of culture. The cells were harvested in glass fibre filters in an automatic cell harvester (Titertek Cell Harvester, Skatron, A.S., Flow Laboratories, Lier, Norway) and radioactive incorporation was measured in a liquid scintillation spectrometer (1450 Microbeta Trilux, Wallac, Turku, Finland). The assay was carried out in quadruplicate. The results were expressed as ‘lymphocyte stimulation index’ (see statistical analysis).
Cytokine production was quantified in the supernatants of the cultures of PBMC stimulated with PHA. We have used commercially available ELISA assays, according to the manufacturer's instructions: IL-5 (Bio Source International, Camarillo, CA, USA), TNF-α, and IFN-γ (Bender Medical Systems Diagnostics, Vienna, Austria). Concentrations were assayed in triplicate.
Quantification in plasma samples of IL-7 levels was done using commercially available ELISA assays and according to the manufacturer's instructions (Quantikine HS human IL-7 kit; R & D Systems, Abingdon, UK). Samples were assayed in triplicate. All values were inside the range of the standard curve (0–16 pg/ml).
Quantification of CD4+ T lymphocyte subsets
Total counts and percentage of CD4+ and CD8+ T-cells were analysed by TRUCOUNT™ (Becton-Dickinson Immunocytometry Systems) in whole blood, whereby cells were selected by means of an SSC gate against anti-CD45 [37], following the manufacturer's instructions. The acquisition was carried out in a FACSCalibur cytometer (Becton-Dickinson) using the CELLQuest (Becton-Dickinson) acquisition program immediately after cell staining. TRUCOUNT™ Control Beads were routinely used as a quality control.
The monoclonal antibodies used for the analysis of CD4+ T-cell subsets were conjugated with Fluorescein-isotyocianate (FITC) (anti-IgG1, anti-HLA-DR, anti-CD45RA, anti-CD38, anti-CD7), Phycoeritrin (PE) (anti-IgG1, anti-CD45RO, anti-CD62L, anti-HLA-DR, anti-CD28), and Peridinin Chloropyll protein (PerCP) (anti-CD4). The monoclonal antibodies were obtained from Becton-Dickinson Immunocytometry Systems, except anti-CD38 (Immunotech, Marseille, France). Three-colour phenotypic characterizations of lymphocytes were performed by flow cytometry, using whole, lysed and washed blood [38]. Naïve CD4+ T-cells were defined as CD62L+ and CD45RA bright T cells (CD4+ CD45RAhi/CD62L+). Memory cells were defined as CD4+ CD45RO+, CD4+ CD7-, or CD4+ CD45RA-CD62L+ T-cells. Activated T-cells were defined as CD4+ HLA-DR+ and CD4+ HLA-DR+ CD38+ 0. Memory-activated CD4+ were defined as CD4+ CD45RO+ HLA-DR+ 0. CD4+ CD38+ are mostly naïve CD4+ T-cells [20]. Acquisition was performed in a FACScan (Becton-Dickinson) cytometer using the Lysis II software (Becton-Dickinson) within 2 h of cell staining. The optimal parameters for acquisition (detector sensitivity, detector amplification and compensation) were determined periodically using Calibrate (Becton-Dickinson) and the AutoComp (Becton-Dickinson) programs. Five thousand (5000) events were compiled using a collection gate for CD4+ T lymphocytes. The gate was defined using the low SSC and high expression of CD4 [21,37]. Data was analysed using the Lysis II analysis program (Becton-Dickinson). Appropriate isotypic controls (IgG1-FITC; IgG1-PE) were used to evaluate nonspecific staining, which was deducted from the obtained results.
Quantification of TCR rearrangement excision circles
Thymic function was studied by quantifying a highly specific marker for T cells recently produced by the thymus, named TCR rearrangement excision circles (TRECs). The TREC values were determined in PBMC by real-time quantitative PCR in a LightCycler system (Roche Molecular Biochemicals) as previously described [39]. Samples were analysed in duplicate or triplicate, never varied by more than 10% from each other, and the result were averaged. A β-globin control PCR was performed to verify that all the samples had the same DNA content. All samples from each child were measured in the same assay to avoid interassay variations.
Statistical analysis
In all analyses, VL was transformed to log10-scale in order to normalize their distribution. Cytokine production of PBMC stimulated by PHA were corrected subtracting the values of cytokines of unstimulated PBMC. Proliferation of PBMC is expressed as adjusted stimulation indexes (SI)
Differences in characteristics between infants were analysed using non-parametric tests because of the small sample size or the free distribution of data (the data did not come from normalized populations and with the same variances). The Mann–Whitney ‘U’ test was used to compare differences between groups studied.
Results
Characteristics of the HIV-infected children
Twenty-one HIV-infected children were studied in a cross-sectional study. Children were rated into two groups (see study population). The immunological, virological and clinical characteristics of the different groups at entry to the study are described in Table 1. The HIV-children of LTA group had higher values of CD4+ T-cell percentages and counts, and lower CD8+ T-cell levels than those in the RP group. Only the CD4+ T-cell count in the LTA group was similar to that of the control group. Most (85·7%) of the HIV-children in the LTA group had undetectable VL, in contrast to 28·6% with undetectable levels in the RP group. All the HIV-children of the RP group were on HAART.
Table 1.
Immunological and virological characteristics of the HIV-1-infected infants
| Control | LTA | RP | ||||
|---|---|---|---|---|---|---|
| No. HIV-1 children | 17 | 7 | 14 | |||
| Age (years), (mean ± s.e.m. (range)) | 12·5 ± 0·6 | (7·2; 16·0) | 10·9 ± 1·4 | (7·5; 16·9) | 11·6 ± 0·8 | (7·7; 17·4) |
| Immunological characteristics, (mean ± s.e.m. (range)) | ||||||
| % CD4+ | 44.0 ± 1·5 | (32·4; 51·1) | 34·5 ± 1·7 | (26·7; 38·6)** | 9·1 ± 1·8 | (1·2; 21·7)**‡ |
| CD4+ T/mm3 | 1072.0 ± 138 | (557; 2097) | 935.0 ± 80·6 | (657; 1298) | 133.0 ± 17·8 | (9; 225)**‡ |
| % CD8+ | 24·1 ± 1·3 | (19·5; 34·7) | 36·9 ± 2·5 | (28·7; 45·4)** | 61·2 ± 4·2 | (37·6; 86·5)**‡ |
| CD8+ T/mm3 | 582.0 ± 86 | (262; 1198) | 991.0 ± 102 | (760; 1370)* | 1129.0 ± 215 | (356; 2791)** |
| Virological characteristics | ||||||
| Log10 VL (copies/ml), (mean ± s.e.m. (range)) | – | 2·48 ± 0·08 | (2·30; 2·88) | 3·83 ± 0·35 | (2·30; 5·40) | |
| Undetectable VL (<400 copies/ml) | 6 (85·7%) | 4 (28·6%)† | ||||
| Anti-retroviral therapies | ||||||
| NT | – | 2 | – | |||
| MT | – | – | – | |||
| CT | – | 5 | – | |||
| HAART | – | – | 14 | |||
VL: viral load. HIV-1: Human Immunodeficiency virus type-1. NT: Not treated; MT: Monotherapy; CT: Combination therapy; HAART: highly active antiretroviral therapy.
Differences with control group (P < 0·05)
Differences with control group (P < 0·01).
Differences significant with LTA group (P < 0·05)
Differences significant with LTA group (P < 0·01).
Functional activity of T-cells
To analyse the functional status of T-cells, we tested proliferation and cytokine production of PBMC in response to mitogens and antigens. PBMC from LTA children showed similar proliferative responses to mitogenic stimuli as compared with those from the controls, as measured by stimulation indexes to PHA, PWM and anti-CD3+ anti-CD28. However, the LTA proliferative responses were higher than those of the RP group (Table 2). In the same way, LTA children showed similar MLR to the controls, but higher responses than the RP group. In contrast, PBMC from LTA children showed lower proliferative responses to specific antigenic challenge (SK and TT) than the controls, although those responses were still higher than those of the RP group.
Table 2.
Summary of proliferation and cytokine production by PBMC of HIV-1-infected children
| *P-value for difference between | ||||||
|---|---|---|---|---|---|---|
| control | LTA | RP | LTA and control | RP and control | LTA and RP | |
| s.i. of mitogenic stimulus | ||||||
| PHA | 130·6 ± 20·3 | 103·6 ± 25·9 | 52·3 ± 17·2 | 0·299 | 0·003 | 0·030 |
| PWM | 27·9 ± 6·2 | 26·5 ± 8·5 | 12·3 ± 3·1 | 0·681 | 0·007 | 0·115 |
| aCD3+ aCD28 | 111·4 ± 13·8 | 114·0 ± 28·7 | 67·6 ± 19·8 | 0·758 | 0·014 | 0·067 |
| s.i. of antigenic stimulus | ||||||
| TT | 46·5 ± 14·7 | 11·8 ± 4·4 | 0·82 ± 0·15 | 0·051 | 0·000 | 0·005 |
| SK | 12·7 ± 1·9 | 3·4 ± 1·9 | 0·89 ± 0·17 | 0·026 | 0·001 | 0·366 |
| p24 Ag | – | 2·0 ± 0·87 | 0·85 ± 0·19 | – | – | 0·299 |
| MLR | 51·4 ± 9·9 | 42·0 ± 10·4 | 13·9 ± 2·82 | 0·699 | 0·009 | 0·041 |
| Cytokine production† | ||||||
| IL-5 | 113.0 ± 48 | 123.0 ± 113 | 52.0 ± 31 | 0·456 | 0·489 | 0·689 |
| TNF-α | 1590.0 ± 371 | 1310.0 ± 267 | 335.0 ± 130 | 0·864 | 0·003 | 0·008 |
| IFN-γ | 4018.0 ± 992 | 3379.0 ± 1076 | 1057.0 ± 670 | 0·864 | 0·006 | 0·012 |
PHA, Phytohemagglutinin; PWM, Pokeweed; aCD3, anti-CD3.; aCD28, anti-CD28. TT, tetanus toxin; SK, streptokinase; p24 Ag, p24 antigen, MLR, mixed lymphocyte response.
After PHA stimulation. LTA, long-term asymptomatic HIV-infected children. RP, rapid progressors HIV-infected children. Control, age-matched HIV-negative healthy children. Values are expressed as mean ± s.e.m.
Difference between LTA, RP, and Control (level of significance).
Cytokine production in the LTA and RP groups, in the same cultures, was differentially affected. Thus, the production of TNF-α and IFN-γ in LTA samples was higher than RP, but similar to that of the controls (Table 2. Summary of proliferation and cytokine production by PBMC of HIV-1-infected children.).
CD4+ T-cell subsets
Next, we evaluated naïve, memory and activated T-cells. The LTA group showed lower values of memory CD4+ T-cells (CD4+ CD45RO+, CD4+ CD45RA-CD62L+) than the controls, except for the CD4+ CD45RO+ HLA-DR+ subset whose levels were similar between the two groups. On the other hand, the RP group had a higher percentage of all 3 subsets, CD4+ CD45RO+, CD4+ CD45RO+ HLA-DR+ and CD4+ CD45RA-CD62L+ T-cells than both the LTA children and the controls. The LTA group showed lower percentages of CD4+ CD7- than both the control and RP groups but the LTA group had similar values of CD4+ CD28-CD7- T-cells in comparison with the controls. Regarding naïve CD4+ T-cells (CD4+ CD45RA+ CD62L+), CD4+ CD45RA+, and CD4+ CD62L+ the LTA group showed higher values than both the control and RP groups (Table 3).
Table 3.
Percentages of CD4+ T-cell subsets in HIV-infected children
| *P-value for difference between | ||||||
|---|---|---|---|---|---|---|
| Phenotype | control | LTA | RP | LTA and control | RP and control | LTA and RP |
| Memory/naive | ||||||
| CD4+ CD45RO+ HLA-DR+ | 3·4 ± 0·4 | 3·2 ± 0·4 | 32·2 ± 5·9 | 0·962 | 0·000 | 0·000 |
| CD4+ CD45RO+ | 50·9 ± 3·9 | 33·7 ± 3·3 | 71·9 ± 6·4 | 0·005 | 0·042 | 0·001 |
| CD4+ CD45RA-CD62L+ | 35·4 ± 1·3 | 28·9 ± 2·2 | 47·8 ± 3·2 | 0·019 | 0·005 | 0·000 |
| CD4+ CD7- | 11·2 ± 1·3 | 6·1 ± 1·0 | 24·3 ± 7·0 | 0·018 | 0·371 | 0·005 |
| CD4+ CD28- | 1·8 ± 0·9 | 0·8 ± 0·3 | 11·9 ± 6·5 | 0·432 | 0·371 | 0·070 |
| CD4+ CD28-CD7- | 1·0 ± 0·6 | 0·6 ± 0·3 | 11·3 ± 6·4 | 0·530 | 0·310 | 0·109 |
| CD4+ CD45RA+ CD62L+ | 46·3 ± 2·9 | 65·1 ± 2·9 | 28·3 ± 6·5 | 0·001 | 0·212 | 0·000 |
| CD4+ CD28+ | 98·3 ± 0·9 | 99·2 ± 0·3 | 88·1 ± 6·5 | 0·432 | 0·371 | 0·070 |
| CD4+ CD45RA+ | 47·0 ± 2·9 | 65·4 ± 2·9 | 29·3 ± 6·5 | 0·001 | 0·192 | 0·000 |
| CD4+ CD62L+ | 81·7 ± 2·1 | 94·0 ± 0·9 | 76·2 ± 4·6 | 0·000 | 0·752 | 0·000 |
| Activated | ||||||
| CD4+ HLA-DR+ CD38+ | 1·5 ± 0·2 | 3·5 ± 1·0 | 28·1 ± 5·4 | 0·005 | 0·000 | 0·000 |
| CD4+ HLA-DR-CD38+ | 60·8 ± 2·6 | 75·7 ± 1·7 | 42·6 ± 5·8 | 0·001 | 0·074 | 0·000 |
| CD4+ HLA-DR+ CD38- | 2·5 ± 0·3 | 1·9 ± 0·2 | 12·1 ± 2·4 | 0·230 | 0·000 | 0·000 |
| CD4+ HLA-DR+ | 3·9 ± 0·4 | 5·4 ± 1·2 | 40·2 ± 6·0 | 0·230 | 0·000 | 0·000 |
| CD4+ CD38+ | 62·3 ± 2·7 | 79·1 ± 1·8 | 70·6 ± 4·9 | 0·001 | 0·084 | 0·585 |
LTA, long-term asymptomatic HIV-infected children. RP, rapid progressors HIV-infected children. Control, age-matched HIV-negative healthy children. RFI, relative fluorescence intensities. Values are expressed as mean ± s.e.m.
Difference between LTA, RP and Control (level of significance).
The LTA group showed higher percentages of circulating activated CD4+ HLA-DR+ CD38+ cells than the controls, and lower values than the RP group. In contrast, the LTA group had values of CD4+ HLA-DR-CD38+ T-cells higher than both the control and RP groups, whereas the percentages of CD4+ CD38+ cells were higher in LTA children than the controls. Both subpopulations of activated cells (CD4+ HLA-DR+ CD38- and CD4+ HLA-DR+) were lower in LTA than RP children (Table 3).
Thymic function and regulation of lymphopoiesis
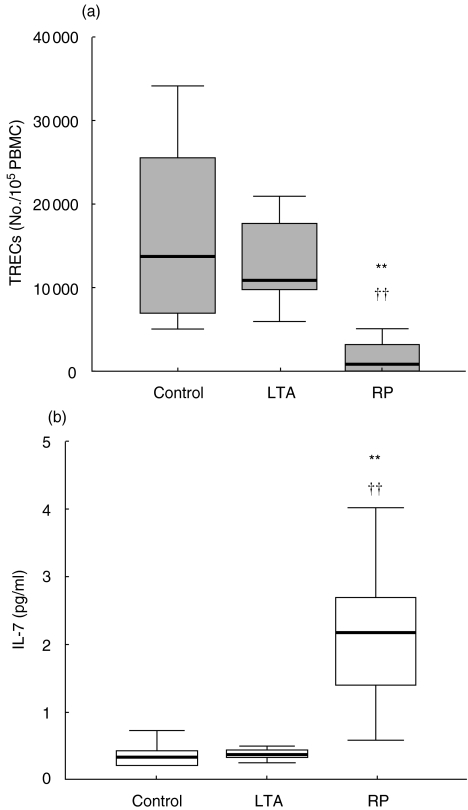
To assess the effect of HIV-1 infection on the thymus, it is important to compare thymic function in early and late stages of HIV-1 infection with age-matched controls. In such analysis, the LTA group showed values of TRECs (median: 10804) not significantly different from the controls (median: 13720) but much higher than the RP group (median: 783) (Fig. 1). On the other hand, plasma levels of the haematopoietic cytokine IL-7, were much higher in the RP group than in uninfected controls. Interestingly, LTA had low values similar to those of the controls (Fig. 1).
Fig. 1.
(a) TREC values and (b) IL-7 concentrations in the HIV-infected children. LTA: long-term asymptomatic HIV-infected children. RP: rapid progressor HIV-infected children. Control: age-matched HIV-negative healthy children. Differences with Control group (*P < 0·05; **P < 0·01) by U-test of Mann–Whitney. Differences with RP (†P < 0·05; ††P < 0·01).
Discussion
Disease non-progression has been observed in a small percentage of children with vertically transmitted HIV-infection [2,3]. Here, we have analysed the immune status of a group of vertically HIV-infected children with 7 or more years of documented HIV-infection, stable CD4+ T-cells, no HIV-related disease, and none under antiretroviral therapy with protease inhibitor. This type of study is necessary because it may help to define the pathogenesis and evolution of the HIV infection in children, essential since HIV-1 infection in children shows several characteristics distinct from those in adults [2,3].
Among the first abnormalities noted in HIV-infection function is a loss of proliferative CD4+ T-cell responses to common recall antigens such as TT, associated with decreased IL-2 production. This is followed by defects in T-cell proliferative responses to alloantigens. Subsequently, with the continued decline in CD4+ T cells, defects in response to mitogenic stimulation as PHA, or PWM occur [4]. The LTA children conserved T-cell proliferative responses to alloantigens, but not to common recall antigens. Thus, these results can be interpreted as indicating that LTA children remain stabilized in the early stages of HIV-1 disease. On the other hand, the RP group had high values of memory and activated CD4+ T-cell subsets that are highly differentiated and show low proliferative capacity [40]. However, the LTA and RP groups did not display significant differences in the specific response to HIV-antigen. The almost normal proliferative response pattern to mitogenic and antigenic stimulation by the LTA group may be due to the almost normal numbers of naïve, memory, and activated CD4+ T-cell subsets in these children.
The dramatic decrease of CD4+ T cell numbers during HIV infection may be ascribed not only to the peripheral CD4+ T cell depletion caused by the virus, but also to an inadequate replacement of the destroyed T cells [26]. T cells may be replaced by newly produced naive T cells from the thymus or by the peripheral expansion of naive and memory T cells [41]. HIV can infect and destroy human thymocytes [42,43], and HIV infected individuals have a number of thymic abnormalities including atrophy, reduced mass [44] and severe depletion of thymocytes [4], thus jeopardising the capacity for regeneration of new T cells. Such effects will be more easily detected in HIV-infected children, because due to their age they maintain a complete thymic functionality as compared to adults, and therefore, the differences between infected and uninfected children are expected to be greater. The dramatic different between the RP and the control groups with respect to TREC levels further supports this. Interestingly, LTA children had TREC values very close to those of the controls indicating a normal thymic function. This may explain why all other immunological parameters are well preserved. In addition, we have found similar low levels of IL-7 in the plasma of both the LTA and control groups. In contrast, the RP group had much higher circulating IL-7 levels. Similar high levels have been previously described in HIV-1 infected symptomatic children and adults [29, 45, 46]. Such elevated levels in HIV-1-infected patients have been interpreted as being part of a homeostatic mechanism in response to T-cell depletion [29], since IL-7 is required for T-cell differentiation in the thymus. Our results are in agreement with this idea since in LTA children there was no CD4+ depletion and IL-7 levels were normal. More interestingly, our data show for the first time an inverse relation between TREC levels and IL-7 plasma levels in HIV-infected children. Thus, despite HIV-1 infection, the presence of normal thymic function is associated with a lack of IL-7 elevation.
Although it is clear that the Type 1 limb of cellular immune responses is impaired during the course of HIV infection [47,48], controversy surrounds the proposed dominance of Type 2-like responses during progression of HIV disease [48]. We have found similar values of IL-5 (a type 2 cytokine), TNF-α, and IFN-γ (type 1 cytokines) production by PBMC from LTA and control PBMC. Thus, the LTA group had conserved both Type 1 and Type 2-responses. In contrast, PBMC from the RP group showed a diminished production of TNF-α and IFN-γ. Although, this can be explained by the lower proliferative ability of PBMC from the RP group, the production of IL-5 was not depressed to the same extent as proliferation. This somewhat supports the skewed type 2 response in HIV-1 infection [49].
In addition to functional alterations, T-cells from HIV-infected individuals manifest a variety of phenotypic abnormalities. Among those, the percentage of activated CD4+ CD28+ T-cells is reduced compared with uninfected individuals [50]. CD28- T-cells do not proliferate to activation signals, including anti-CD3 monoclonal antibodies or mitogens, and express markers of terminal activation (HLA-DR, CD38, and CD45RO) [51]. Children of the RP group had higher numbers of activated and memory CD4+ T-cells which correlates with the lower proliferative responses observed in those children. During chronic HIV-infection, an increase in CD45RO expressing memory T-cells and a loss of naïve, CD45RA-expressing cells has been observed [52]. Children of the LTA group had similar naïve CD4+ T-cell subset levels as those seen in the controls. This may indicate a control of viral replication that means naïve T-cells can avoid becoming activated and/or memory CD4+ T-cells [53]. By contrast, the lower naïve CD4+ T-cell percentages observed in the RP group could either be ascribed to the preferential destruction of naïve T-lymphocytes infected by HIV-1 as thymic precursors [27,54], or to the lack of generation of new cells as a consequence of thymic atrophy secondary to HIV-1 infection [43]. However, the fact that the RP group had a greatly reduced TREC numbers indicates that T-cell generation in the thymus is severely impaired in those children.
HIV-infected patients have a high proportion of T-cells that express CD38 and HLA-DR [22,25] which is associated with worse prognosis, decreased CD4+ T-cell numbers and increased VL [22]. The LTA group had almost similar values of activated CD4+ T-cells (HLA-DR+ CD38+ and HLA-DR+) to those of the controls, possibly due to a low level of viral replication. However, in children, high CD38 expression on CD4+ T-cells is not a good prognostic marker [25,55] being a marker of immaturity expressed on cells with a naïve phenotype instead [56]. It could be used as a marker of preserved immune status rather than activation, since its decrease is associated with immunodeficiency [21,25]. In this regard, the LTA group had percentages of CD4+ CD38+ and CD4+ HLA-DR-CD38+ T-cells higher than those of the controls, although the absolute counts of these T-cells subsets remained similar to those of the controls (data not shown). In contrast, the persistence of high percentages of activated CD4+ T-cells is a marker of viral replication and activation of the immune system in HIV-infected children of the RP type [21].
In summary, long- term asymptomatic, HIV-infected children, who have stable CD4+ T-cell counts and no HIV disease progression despite several years of HIV infection, have low VL that ensures the thymic production of naïve CD4+ T-cells is preserved. Furthermore, T cell function is very close to normal, with low levels of activation, and plasma IL-7 levels in the normal range. Moreover, besides the classic markers of HIV-disease (CD4, VL, CD45RO, CD45RA, HLA-DR, cytokines, etc.), other markers such as TRECs and IL-7 could be used as indicators of disease progression according to our results.
Acknowledgments
We thank Dr Dolores Gurbindo (Sección de Inmunopaediatría, Hospital Gregorio Marañón, Madrid, Spain) for her participation in the selection and control of patients. We also want to thank Dolores García Alonso and Consuelo Muñoz for their excellent technical assistance. This work was supported by grants of the Fundación para la Investigación y la Prevención del SIDA en España (FIPSE 3008/99), Fondo de Investigacion Sanitaria (00/0207) Programa Nacional de Salud (SAF 99–0022), Comunidad de Madrid (08·5/0034/2001), and Bristol-Myers, S.A. (Grupo Bristol-Myers Squibb). S. Resino is supported by a grant from Comionidad de Madrid.
References
- 1.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–18. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 2.Italian Register. HIVc Features of children perinatally infected with HIV-1 surviving longer than 5 years. Lancet. 1994;343:191–5. [PubMed] [Google Scholar]
- 3.Nielsen K, McSherry G, Petru A, et al. A descriptive survey of pediatric human immunodeficiency virus-infected long-term survivors. Pediatrics. 1997;99:E4. doi: 10.1542/peds.99.4.e4. [DOI] [PubMed] [Google Scholar]
- 4.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass HZ, Fahey JL, Nishanian P, Detels R, Cumberland W, Kemeny M, Plaeger S. Relation of impaired lymphocyte proliferative function to other major human immunodeficiency virus type 1-induced immunological changes. Clin Diagn Laboratory Immunol. 1997;4:64–9. doi: 10.1128/cdli.4.1.64-69.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roilides E, Clerici M, DePalma L, Rubin M, Pizzo PA, Shearer GM. Helper T-cell responses in children infected with human immunodeficiency virus type 1. J Pediatr. 1991;118:724–30. doi: 10.1016/s0022-3476(05)80033-2. [DOI] [PubMed] [Google Scholar]
- 7.Meyaard L, Otto SA, Hooibrink B, Miedema F. Quantitative analysis of CD4+ T cell function in the course of human immunodeficiency virus infection. Gradual decline of both naive and memory alloreactive T cells. J Clin Invest. 1994;94:1947–52. doi: 10.1172/JCI117545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roos MT, Prins M, Koot M, de Wolf F, Bakker M, Coutinho RA, Miedema F, Schellekens PT. Low T-cell responses to CD3 plus CD28 monoclonal antibodies are predictive of development of AIDS. AIDS. 1998;12:1745–51. doi: 10.1097/00002030-199814000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR. Cytokine patterns during the progression to AIDS. Science. 1994;265:193–4. doi: 10.1126/science.8023139. [DOI] [PubMed] [Google Scholar]
- 10.Clement LT, Yamashita N, Martin AM. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post- thymic differentiation. J Immunol. 1988;141:1464–70. [PubMed] [Google Scholar]
- 11.Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8. [PubMed] [Google Scholar]
- 12.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LW. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor l-selection on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–21. [PubMed] [Google Scholar]
- 13.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 14.Autran B, Legac E, Blanc C, Debre P. A Th0/Th2-like function of CD4+CD7- T helper cells from normal donors and HIV-infected patients. J Immunol. 1995;154:1408–17. [PubMed] [Google Scholar]
- 15.Reinhold U, Abken H. CD4+ CD7- T cells. a separate subpopulation of memory T cells? J Clin Immunol. 1997;17:265–71. doi: 10.1023/a:1027318530127. [DOI] [PubMed] [Google Scholar]
- 16.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–9. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 17.Cohen OJ, Kinter A, Fauci AS. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 18.Levacher M, Hulstaert F, Tallet S, Ullery S, Pocidalo JJ, Bach BA. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–82. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips AN, Sabin CA, Elford J, Bofill M, Lee CA, Janossy G. CD8 lymphocyte counts and serum immunoglobulin A levels early in HIV infection as predictors of CD4 lymphocyte depletion during 8 years of follow-up. AIDS. 1993;7:975–80. doi: 10.1097/00002030-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–15. [PubMed] [Google Scholar]
- 21.Resino S, Navarro J, Bellón JM, Gurbindo D, León JA, Muñoz-Fernández MA. Naïve and memory CD4+ T-cells and T-cell activation markers in HIV-1 infected children on HAART. Clin Exp Immunol. 2001;125:266–73. doi: 10.1046/j.1365-2249.2001.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocroft A, Bofill M, Lipman M, et al. CD8+, CD38+ lymphocyte percent: a useful immunological marker for monitoring HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:158–62. doi: 10.1097/00042560-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Plaeger-Marshall S, Isacescu V, O'Rourke S, Bertolli J, Bryson YJ, Stiehm ER. T cell activation in pediatric AIDS pathogenesis: three-color immunophenotyping. Clin Immunol Immunopathol. 1994;71:27–32. doi: 10.1006/clin.1994.1046. [DOI] [PubMed] [Google Scholar]
- 24.Kestens L, Vanham G, Vereecken C, Vandenbruaene M, Vercauteren G, Colebunders RL, Gigase PL. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Martino M, Rossi ME, Azzari C, Gelli MG, Galli L, Vierucci A. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–8. doi: 10.1203/00006450-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Rowland-Jones S. HIV infection: where have all the T cells gone? Lancet. 1999;354:5–7. doi: 10.1016/S0140-6736(99)90017-X. [DOI] [PubMed] [Google Scholar]
- 27.Bonyhadi ML, Rabin L, Salimi S, Brown DA, Kosek J, McCune JM, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–32. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 28.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 29.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 30.Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7. physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 31.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–16. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 33.Propato A, Schiaffella E, Vicenzi E, et al. Spreading of HIV-specific CD8(+) T-cell repertoire in long-term nonprogressors and its role in the control of viral load and disease activity. Hum Immunol. 2001;62:561–76. doi: 10.1016/s0198-8859(01)00245-2. [DOI] [PubMed] [Google Scholar]
- 34.Munoz-Fernandez MA, Obregon E, Navarro J, Borner C, Gurbindo MD, Sampelayo TH, Fernandez-Cruz E. Relationship of virologic, immunologic, and clinical parameters in infants with vertically acquired human immunodeficiency virus type 1 infection. Pediatr Res. 1996;40:597–602. doi: 10.1203/00006450-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Center for Diseases Control Prevention. Guidelines for use of antiretroviral agents in pediatric HIV, infection. MMWR. 1998;47:RR–4. [PubMed] [Google Scholar]
- 36.Center for Diseases Control Prevention. Revised classification system for HIV-1 infection in children less than 13 years of age. MMWR. 1994;43:1–13. [Google Scholar]
- 37.Nicholson JK, Hubbard M, Jones BM. Use of CD45 fluorescence and side-scatter characteristics for gating lymphocytes when using the whole blood lysis procedure and flow cytometry. Cytometry. 1996;26:16–21. doi: 10.1002/(SICI)1097-0320(19960315)26:1<16::AID-CYTO3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson J, Kidd P, Mandy F, Livnat D, Kagan J. Three-color supplement to the NIAID DAIDS guideline for flow cytometric immunophenotyping. Cytometry. 1996;26:227–30. doi: 10.1002/(SICI)1097-0320(19960915)26:3<227::AID-CYTO8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 39.Correa R, Muñoz-Fernández MA. Viral phenotype affects the thymical production of new T-cells in HIV-1 infected children. AIDS. 2001;15:1959–63. doi: 10.1097/00002030-200110190-00007. [DOI] [PubMed] [Google Scholar]
- 40.Brinchmann JE, Dobloug JH, Heger BH, Haaheim LL, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–8. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 41.Mackall CL, Hakim FT, Gress RE. T-cell regeneration: all repertoires are not created equal. Immunol Today. 1997;18:245–51. doi: 10.1016/s0167-5699(97)81664-7. [DOI] [PubMed] [Google Scholar]
- 42.Burke A, Anderson D, Benson W, et al. Localization of human immunodeficiency virus 1 RNA in thymic tissues from asymptomatic drug addicts. Arch Pathol Laboratory Med. 1995;119:36–41. [PubMed] [Google Scholar]
- 43.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 44.Wolthers KC, Schuitemaker H, Miedema F. Rapid CD4+ T-cell turnover in HIV-1 infection: a paradigm revisted. Immunol Today. 1998;19:44–8. doi: 10.1016/s0167-5699(97)01188-2. [DOI] [PubMed] [Google Scholar]
- 45.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 46.Darcissac EC, Vidal V, De La Tribonniere X, Mouton Y, Bahr GM. Variations in serum IL-7 and 90K/Mac-2 binding protein (Mac−2 BP) levels analysed in cohorts of HIV-1 patients and correlated with clinical changes following antiretroviral therapy. Clin Exp Immunol. 2001;126:287–94. doi: 10.1046/j.1365-2249.2001.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Resino S, Bellon JM, Gurbindo D, Munoz-Fernandez MA. Dysruption in cytokine and chemokine production by T cells in vertically HIV-1 infected children. Acta Paediatrica. 2001;90:989–97. doi: 10.1080/080352501316978057. [DOI] [PubMed] [Google Scholar]
- 48.Clerici M, Hakim FT, Venzon DJ, Blatt S, Hendrix CW, Wynn TA, Shearer GM. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759–65. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clerici M, Shearer GM. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 50.Choremi-Papadopoulou H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Down-regulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J AIDS. 1994;7:245–53. [PubMed] [Google Scholar]
- 51.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. Clin Immunol Immunopathol. 1994;71:2–7. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Benito JM, Zabay JM, Gil J, Bermejo M, Escudero A, Sanchez E, Fernandez-Cruz E. Quantitative alterations of the functionally distinct subsets of CD4 and CD8 T lymphocytes in asymptomatic HIV infection: changes in the expression of CD45RO, CD45RA, CD11b, CD38, HLA-DR, and CD25 antigens. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:128–35. doi: 10.1097/00042560-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 53.Brinchmann JE. Differential responses of T cell subsets: possible role in the immunopathogenesis of AIDS. AIDS. 2000;14:1689–700. doi: 10.1097/00002030-200008180-00002. [DOI] [PubMed] [Google Scholar]
- 54.Stanley SK, McCune JM, Kaneshima H, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Infect Dis. 1993;168:810–7. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlesinger M, Peters V, Jiang JD, Roboz JP, Bekesi JG. Increased expression of activation markers on CD8 lymphocytes in children with human immunodeficiency virus-1 infection. Pediatr Res. 1995;38:390–6. doi: 10.1203/00006450-199509000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Dianzani U, Funaro A, DiFranco D, et al. Interaction between endothelium and CD4+, CD45RA:+ lymphocytes. Role of the human CD38 molecule. J Immunol. 1994;153:952–9. [PubMed] [Google Scholar]