Abstract
Lung cancer is strongly associated with cigarette smoking. More than 20 lung carcinogens have been identified in cigarette smoke and one of the most abundant is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). We hypothesized that NNK modulates alveolar macrophage (AM) mediator production, thus contributing to carcinogenesis. An AM cell line, NR8383, was treated with [3H]NNK and lipopolysaccharide (LPS), and NNK metabolites released in supernatants were analysed by high-performance liquid chromatography (HPLC). NNK was metabolized by carbonyl reduction to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butan-1-ol (NNAL) or activated by α-carbon hydroxylation. AMs were also treated with NNK (100–1000 µM), with and without LPS, for different periods of time (6–72 h), and mediators released in supernatants were quantified by enzyme-linked immunosorbent assay (ELISA) or the Griess reaction. NNK inhibited (in a concentration-dependent manner) AM production of tumour necrosis factor (TNF), macrophage inflammatory protein-1α (MIP-1α), interleukin (IL)-12 and nitric oxide (NO), whereas IL-10 production was increased. Cyclooxygenase inhibitors – NS-398 and indomethacin – and anti-prostaglandin E2 (anti-PGE2) antibody abrogated the NNK-inhibitory effect on MIP-1α production by AM. NNK stimulated the release of PGE2, and exogenous PGE2 inhibited AM MIP-1α production, suggesting that the NNK immunomodulatory effect may be mediated by PGE2 production. Thus, in addition to its carcinogenic effects, NNK may contribute to the lung immunosuppression observed in tobacco smokers.
Keywords: alveolar macrophages, cytokines, NNK metabolism, prostaglandin
INTRODUCTION
Lung cancer is the leading cause of cancer death among men and women [1]. More than 85% of all lung cancers are linked to tobacco smoke [2]. Tobacco smoke contains at least 20 substances known to induce lung cancers in laboratory animals [3]. Among those, the nicotine-derived 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, NNK, is one of the most abundant nitrosamines in the human environment and the most potent and specific carcinogen for lung tissues [4]. NNK is a procarcinogen and, as such, requires metabolic activation to generate electrophilic intermediates capable of damaging DNA [4]. This activation is mediated by P450 monooxygenases, cyclooxygenases (COX) and lypooxygenases [5,6]. Although the mechanism of NNK-induced carcinogenesis is well documented [7], its effects on the immune system, which may facilitate lung cancer pathogenesis, are poorly understood.
Alveolar macrophages (AMs) are located in the respiratory tract from the alveolus to the larynx and represent the most abundant cells in the airway lumen and alveolar space. AMs play a key role in the maintenance of immunological homeostasis in the respiratory tract [8]. They are the first cell type to encounter inhaled toxic particles and are responsible for the clearance of these particles by phagocytosis [8]. AMs produce several secretory mediators such as tumour necrosis factor (TNF) and nitric oxide (NO) that, in addition to being involved in the inflammatory process, are potentially toxic for tumour cells [9,10]. AMs are also important producers of macrophage inflammatory protein-1α (MIP-1α), involved in the recruitment of leucocytes, and of interleukin (IL)-12, known to activate the cytotoxic activity of macrophages, lymphocytes and natural killer (NK) cells [9,11]. Furthermore, AMs can produce anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGF-β), which inhibit or control inflammation [9]. These two cytokines have also been shown to suppress host anti-tumour immunity [12,13]. AMs are well known for their tumoricidal activity against numerous tumoral cells [10]. Furthermore, they may play an indirect role in the anti-tumour immune response through the recruitment and activation of leucocytes to the tumour site. Alterations in these functions of AMs may contribute to the increased incidence of lung cancer in tobacco smokers.
Chronic tobacco smoke exposure inhibits a wide range of immunological functions of AMs, including their capacity to phagocytose inert particles and to become activated for tumour-cell killing [14,15]. Furthermore, cigarette smoking has been shown to suppress the production of proinflammatory cytokines such as IL-1, IL-6 and TNF [16,17]. These cytokines play an important role in the immune response against pathogens, explaining, in part, the increased susceptibility of cigarette smokers to pulmonary infections [18].
Given the importance of AMs in modulating immune responses and the presence of NNK in tobacco smoke, we hypothesized that NNK modulates the production of mediators by AMs, thus contributing to the lung immunosuppression observed in tobacco smokers. For this study, we used the AM cell line, NR8383. We have already demonstrated that the modulation of mediator production is similar in NR8383 cells compared with freshly isolated human and rat AMs [19]. The aim of this study was to document the effects of NNK on AM production of various mediators likely to be implicated in carcinogenesis.
MATERIALS AND METHODS
Chemicals
NNK (99% pure by thin-layer chromatography) and [5-3H]NNK [1·89 Ci/mmol, 99% pure by high-performance liquid chromatography (HPLC)] were purchased from Chemsyn Science Laboratory (Lenexa, KS). NNK metabolites were synthesized, as previously described [20]. Indomethacin and prostaglandin E2 (PGE2) were purchased from Sigma Chemical Co. (St Louis, MO), and NS-398 and anti-PGE2 antibody were purchased from Cayman Chemical Company (Ann Arbor, MI).
Cell culture
NR8383 is an AM cell line initiated by lung lavage of a normal Sprague-Dawley rat. These cells represent a homogenous source of highly responsive AMs which have been previously used in vitro to study macrophage-related activities [19,21]. NR8383 cells were maintained in Ham's F-12 media (GIBCO BRL, Burlington, Ont., Canada), as previously described [21]. All experiments were carried out in RPMI-1640 containing 5% fetal bovine serum (FBS), 1% HEPES and 1% penicillin–streptomycin (GIBCO). After a 2-h adherence in 96-well plates (Falcon; Becton-Dickinson Labware, Lincoln Park, NJ) at 37°C, AMs were treated with increasing concentrations of NNK for different periods of time, with and without lipopolysaccharide (LPS) (10 ng/ml) (Salmonella enteritidis; Sigma Chemical Co.) or bacille Calmette–Guérin (BCG) [5 × 106 colony-forming units (CFU)/ml; Calbiochem, San Diego, CA]. At the end of the treatment, cell-free supernatants were recovered and stored at −70°C for future analysis. Cells were also treated with keto acid (15 µM), NS-398 (10 µM), indomethacin (10 µM), PGE2 (25 ng/ml) and anti-PGE2 (1 : 25 dilution) without LPS, and cell-free supernatants were analysed for MIP-1α content.
NNK metabolism
NR8383 cells (1 × 106/ml) were incubated with [3H]NNK (30 µCi/ml; 16 µM) and LPS (10 ng/ml) for 20 h at 37°C. RPMI containing [3H]NNK and LPS, without cells, was used as control. Cell-free supernatants were analysed for NNK and NNK metabolites using a reverse-phase HPLC system, as previously described [20]. Recovery of total radioactivity during HPLC analysis was 80%.
Mediator production
MIP-1α content in cell-free supernatants was measured using an enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 7 pg/ml [21]. Levels of TNF, IL-12, IL-10, TGF-β (Pharmingen, San Diego, CA) and PGE2 (Cayman Chemical Co.) were measured in cell-free supernatants using ELISAs with sensitivities of 4 pg/ml, 5 pg/ml, 5 pg/ml, 4 pg/ml, and 7 pg/ml, respectively.
Measurement of NO production
AMs were incubated with NNK (1–1000 µM) and LPS (10 ng/ml) for 48 or 72 h. Cell-free supernatants were assayed for NO2− using the Griess reaction, as previously described [21]. The NO2− concentration, proportional to the optical density at 540 nm, was determined using a Vmax. kinetic microplate reader (Thermo Max; Molecular Devices, Menlo Park, CA) with reference to a standard curve (NaNO2).
Reverse transcription–polymerase chain reaction (RT–PCR)
Given that mRNA is expressed before the release of protein, AMs (106 cells/ml) were treated with NNK (500 µM) for only 2 h followed by a further 2 h of incubation with or without LPS. Cells (3 × 106) were collected and total RNA extracted using TRIzol reagent (GIBCO BRL). Total RNA was quantified using the RiboGreen™ RNA quantification reagent (Molecular Probes Inc., Eugene, OR) and read on a Fluoroskan Ascent FL (Labsystems, Franklin, MA). For complementary DNA synthesis, 1 µg of total RNA was reverse transcribed by Moloney murine leukaemia virus reverse transcriptase enzyme (GIBCO RBL) using a Peltier Thermal Cycler 200 (MJ Research Inc., Watertown, MA) according to the manufacturer's protocol. PCR amplification was performed using the Qiagen Taq DNA polymerase protocol in a 20-µl final volume. The primers used were: rat β-actin, sense: 5′-ATG CCA TCC TGC GTC TGG ACC TGG C-3′, and antisense: 5′-AGC ATT TGC GGT GCA CGA TGG C-3′ (607 bp); and rat MIP-1α, sense: 5′-ATG AAG GTC TCC ACC ACT-3′, and antisense: 5′-TCA GGC ATT CAG TTC CAG-3′ (279 bp). Products were run on a 2% agarose gel and stained with ethidium bromide (5 mg/ml).
Statistical analysis
Analysis of variance combined with the Scheffe F-test or Student's t-test for paired data were used to compare treatments. Differences were considered significant when the P-value was < 0·05.
RESULTS
Metabolism of NNK by NR8383 cells
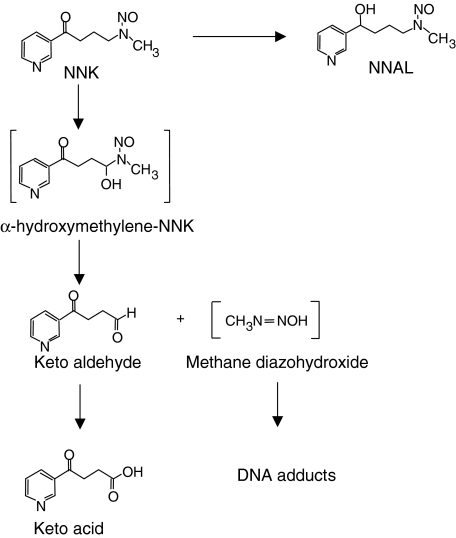
Before investigating the immunomodulatory effects of NNK on AMs, we determined how the rat AM cell line, NR8383, metabolizes NNK. [3H]NNK was incubated in RPMI, with or without AM. HPLC analyses of cell-free supernatants indicated that NR8383 cells metabolized NNK along two pathways: a carbonyl reduction producing 4-(methylnitrosamino)-1-(3-pyridyl)-1-butan-1-ol (NNAL); and a bioactivation involving hydroxylation of the carbon adjacent (α-carbon) to the N-nitroso group, producing a keto acid (Fig. 1). The major metabolite of NNK was NNAL (1009·4 ± 45·0 pmol/106 AM), but NNK was also converted, to a lesser extent, to keto acid (10·9 ± 0·6 pmol/106 AM) (Table 1). These metabolites represented approximately 56% and 1% of the initial amount of NNK, respectively.
Fig. 1.
Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by the rat alveolar macrophage cell line, NR8383. Structures in square brackets are hypothetical.
Table 1.
Metabolism of NNK by alveolar macrophages (AM)
| NNK (pmol/106 AM) | NNAL (pmol/106 AM) | Keto acid (pmol/106 AM) | |
|---|---|---|---|
| Without cells | 2104·9 ± 277·7 | 13·0 ± 7·1 | 0·8 ± 0·4 |
| With cells | 1880·0 ± 22·9 | 1009·4 ± 45·0† | 10·9 ± 0·6** |
[3H]NNK was incubated for 20 h with or without AM, and the cell-free supernatants were analysed by high-performance liquid chromatography (HPLC) to identify and quantify NNK metabolites
P < 0·01 and
P < 0·005.
NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butan-1-ol; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
Mean values ± standard error of the mean (s.e.m.) of three experiments are shown.
Inhibition of MIP-1α production
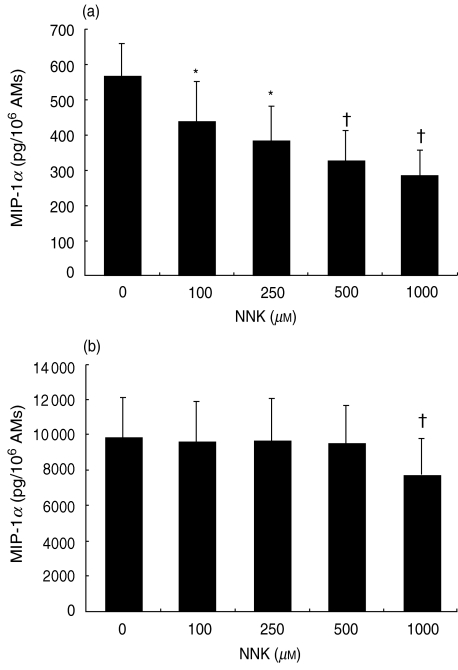
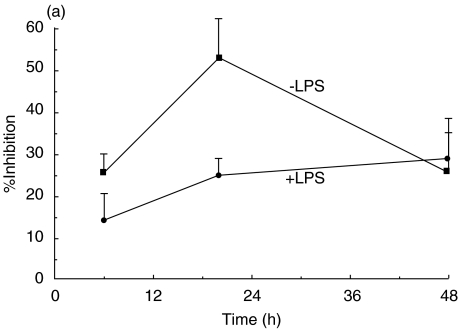
To investigate the modulatory effects of NNK on chemokine production by AM, NR8383 cells were treated with increasing concentrations of NNK (100–1000 µM) for different periods of time (6, 20 and 48 h) and MIP-1α release was measured in cell-free supernatants. These treatments had no detectable effect on cell viability, as determined by Trypan Blue exclusion. NNK treatment (20 h) significantly inhibited, in concentration-dependent manner (100–1000 µM), the production of MIP-1α by unstimulated AMs (Fig. 2a). However, MIP-1α production by LPS-stimulated AMs was significantly inhibited only at 1000 µM NNK (Fig. 2b). Maximum inhibition of MIP-1α production was observed after 48 h (29·2%) and 20 h (52·7%) of treatment with NNK in AMs treated with and without LPS, respectively (Fig. 3a).
Fig. 2.
Inhibition of alveolar macrophage (AM) production of macrophage inflammatory protein-1α (MIP-1α) by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). AMs were treated for 20 h with NNK (100–1000 µM), without (a) or with (b) lipopolysaccharide (LPS) (10 ng/ml) and cell-free supernatants were tested for MIP-1α. Spontaneous release of MIP-1α was significantly inhibited (*P < 0·05, †P < 0·005) in a concentration-dependent manner. Furthermore, NNK (1000 µM) significantly (†P < 0·005) inhibited LPS-stimulated MIP-1α release. The mean values ± standard error of the mean (s.e.m.) are shown of seven to 11 experiments.
Fig. 3.
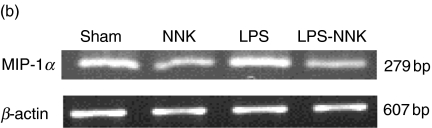
Time-course analysis of macrophage inflammatory protein-1α (MIP-1α) inhibition by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (a) and modulation of MIP-1α mRNA expression (b).(a) Alveolar macrophages (AMs) were treated with NNK (1000 µM), with or without lipopolysaccharide (LPS) (10 ng/ml), for increasing periods of time (6, 20 and 48 h). Maximum inhibition was observed at 48 h (29·2%) and 20 h (52·7%) when AMs were treated with or without LPS, respectively (*P < 0·05, †P < 0·005). Mean values ± standard error of the mean (s.e.m.) of four to 11 experiments are shown. (b) AMs were treated with 500 µM NNK for 2 h followed by a further 2 h of incubation with or without LPS (10 ng/ml), RNA was isolated and reverse transcription–polymerase chain reaction (RT–PCR) performed to assess mRNA expression of MIP-1α and β-actin (housekeeping gene). NNK inhibited mRNA expression in both LPS-stimulated and unstimulated AMs. One representative experiment out of three is shown.
To determine if MIP-1α release reflected an inhibition in the steady-state levels of MIP-1α mRNA, RT–PCR was performed on RNA isolated from sham- and NNK-treated cells (500 µM for 2 h) followed by 2 h of incubation with or without LPS (10 ng/ml). NNK inhibited MIP-1α mRNA expression in AMs (Fig. 3b). Figures 2 and 3 suggest that NNK inhibits the production of MIP-1α at both protein and mRNA levels. In order to determine if the NNK-inhibitory effect on MIP-1α production was NNK specific, AMs were treated with its final product, keto acid (15 µM). This concentration corresponded to the level of keto acid observed during the metabolism study. However, keto acid did not inhibit the production of MIP-1α by AM (data not shown), suggesting that NNK itself or its reactive intermediates are responsible for MIP-1α inhibition.
Modulation of inflammatory mediators
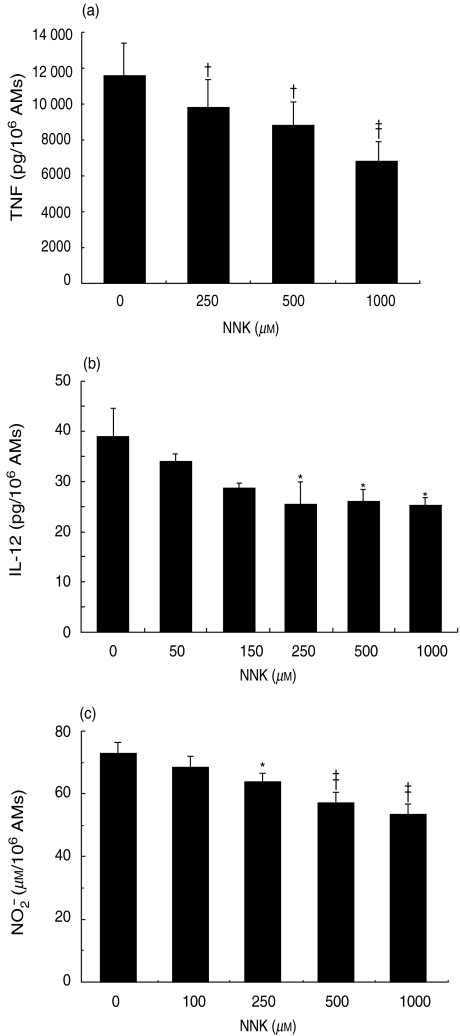
In view of the fact that TNF is an important proinflammatory and anti-tumoral cytokine produced by AMs, we investigated its modulation by NNK. AMs were treated with NNK (100–1000 µM), with or without LPS, for 20 h after which the TNF concentration was measured in cell-free supernatants. NNK treatment significantly inhibited TNF production by LPS-stimulated AMs (Fig. 4a). In the absence of LPS, NNK (1000 µM) inhibited TNF production (34·4 ± 8·9 pg/106 AM) by 71·0% compared to the control (116·5 ± 29·4 pg/106 AM). Time-course analysis demonstrated that 20 h of treatment with NNK resulted in the maximum inhibition of AM TNF production (data not shown).
Fig. 4.
Inhibition of alveolar macrophage (AM) mediator production by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). AMs were treated for 20 h with NNK in the presence of lipopolysaccharide (LPS) (10 ng/ml) for the release of tumour necrosis factor (TNF) (a) or in the presence of bacille Calmette–Guérin (BCG) for the release of interleukin-12 (IL-12) b). NNK significantly inhibited (†P < 0·005, ‡P < 0·001 and *P < 0·05) the production of tumour necrosis factor (TNF) and IL-12 by AMs. Mean values ± standard error of the mean (s.e.m.) of five to eight experiments are shown. (c) AMs were treated with NNK for 48 h in the presence of LPS (10 ng/ml) for the release of nitric oxide (NO). NNK significantly inhibited (*P < 0·05 and ‡P < 0·001) AM NO production in a concentration-dependent manner. Mean values ± standard error of the mean (s.e.m.) of 13 experiments are shown.
The level of IL-12 production by unstimulated AMs was at the limit of detection by ELISA (<5 pg/ml). Thus, the modulation of IL-12 release by NNK was investigated in BCG-stimulated AMs. Cells were treated with NNK (50–1000 µM) in the presence of BCG (5 × 106 CFU/ml) for 20 h, after which IL-12 was measured in cell-free supernatants. NNK significantly inhibited (35·4%) IL-12 release by AMs (Fig. 4b).
To investigate NNK modulation of NO production by AM, cells were treated with NNK (100–1000 µM) for 48 h in the presence of LPS (10 ng/ml). NNK inhibited (in a concentration-dependent manner) NO production by AM, with the maximum inhibition (20·1%) occurring at 1000 µM NNK (Fig. 4c). Similar results were observed after 72 h of treatment.
Modulation of anti-inflammatory cytokines
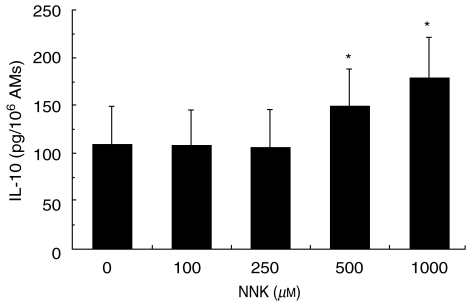
To determine the modulation of anti-inflammatory cytokine production by NNK, AMs were treated with NNK (100–1000 µM), with or without LPS, and IL-10 and TGF-β were measured in cell-free supernatants. The level of IL-10 production in the absence of LPS was at the limit of detection and this production was not modulated by NNK (data not shown). However, NNK significantly potentiated IL-10 production by AM in the presence of LPS (Fig. 5). Time-course analysis revealed that at least 20 h were needed to observe IL-10 stimulation by NNK (data not shown). In contrast, the production of another anti-inflammatory cytokine, TGF-β, was not modulated by NNK (data not shown).
Fig. 5.
Increase of alveolar macrophage (AM) interleukin-10 (IL-10) production by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). AMs were treated with NNK (100–1000 µM) in the presence of lipopolysaccharide (LPS) (10 ng/ml) for 20 h, and IL-10 was measured in cell-free supernatants. NNK significantly (*P < 0·05) increased IL-10 release by AMs. Mean values ± standard error of the mean (s.e.m.) of six experiments are shown.
Mechanism of the immunomodulatory effect of NNK
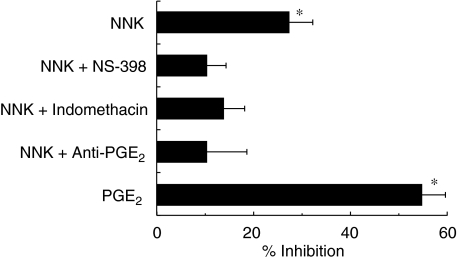
To further characterize the immunomodulatory effects of NNK, unstimulated AMs were treated with NNK (500 µM), with or without NS-398 (10 µM), a specific COX-2 inhibitor, or indomethacin (10 µM), a COX-1 and -2 inhibitor, for 20 h, and MIP-1α release was measured. NS-398 and indomethacin abrogated the inhibitory effect of NNK on the production of MIP-1α by AM (Fig. 6). To determine the role of PGE2 in the inhibition of AM MIP-1α production by NNK, AMs were treated for 20 h with NNK and anti-PGE2 (diluted 1 : 25), or with exogenous PGE2 (25 ng/ml), and MIP-1α production was measured. Anti-PGE2 abrogated the NNK effect, whereas exogenous PGE2 significantly inhibited (54·7%) the production of MIP-1α (Fig. 6).
Fig. 6.
Mediator implicated in the inhibition of alveolar macrophage (AM) macrophage inflammatory protein-1α (MIP-1α) production by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). AMs were treated with NNK (500 µM), alone or together with NS-398 (10 µM), indomethacin (10 µM) or anti-prostaglandin E2 (anti-PGE2) antibody (dilution 1 : 25), or with PGE2 (25 ng/ml) without lipopolysaccharide (LPS), for 20 h. Cell-free supernatants were analysed for MIP-1α content. COX inhibitors and anti-PGE2 antibody abrogated the inhibitory effect of NNK on MIP-1α release by AMs (no significant inhibition). NNK and exogenous PGE2 significantly (*P < 0·005) inhibited AM MIP-1α release.
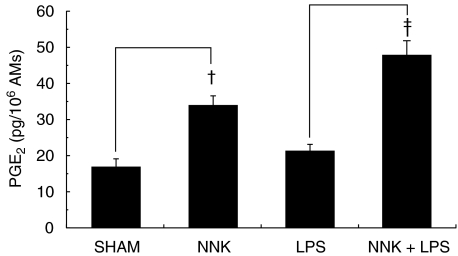
The stimulation, by NNK, of AM PGE2 production was also investigated. AMs were treated with NNK (500 µM) for 2 h, with or without LPS (1 ng/ml), and cell-free supernatants were tested for PGE2 content. NNK significantly increased PGE2 release from unstimulated (twofold) and LPS-stimulated (2·3-fold) AMs (Fig. 7).
Fig. 7.
Modulation of alveolar macrophage (AM) prostaglandin E2 (PGE2) production by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). AMs were treated with NNK (500 µM) for 2 h in the presence or absence of lipopolysaccharide (LPS) (1 ng/ml), and PGE2 levels were measured in cell-free supernatants. NNK significantly increased (†P < 0·005 and ‡P < 0·0005) PGE2 release by unstimulated and LPS-stimulated AMs. Mean values ± standard error of the mean (s.e.m.) of eight experiments are shown.
To evaluate the cumulative effect of NNK on cytokine production, AMs were treated with 1·5 µg/ml (7·2 µM) of NNK for 5 days or with 1·5 µg/ml NNK every day for 5 days, giving a total exposure of 7·5 µg/ml (36 µM). IL-10 levels were measured in cell-free supernatants. Treatment of AMs with NNK every day for 5 days (28·5 ± 8·8 pg/106 AM) significantly (P < 0·04) stimulated IL-10 release compared with the control (10·0 ± 2·1 pg/106 AM), whereas one treatment alone did not significantly increase IL-10 release by AM (20·2 ± 6·9 pg/106 AM). These results suggest a cumulative effect of NNK on AMs.
DISCUSSION
NNK is a procarcinogen present in tobacco and in the particulate phase of tobacco smoke [3]. NNK requires metabolic activation to damage DNA and induce carcinogenesis. Metabolic activation occurs in pulmonary alveolar type II cells and AMs from various species, including rats and humans [22,23]. Carbonyl reduction of NNK to NNAL is the major pathway of NNK biotransformation in many human lung cells, including AMs [23]. NNAL is also a potent carcinogen in rats and mice [4]. Two metabolic pathways, α-hydroxylation and N-oxidation, have also been observed, but the balance between these transformations weighed heavily towards α-hydroxylation [23]. NNK metabolism in the AM cell line, NR8383, resulted in two metabolites: NNAL, as previously observed with human lung cells; and 4-oxo-4-(3-pyridyl)butyric acid (or keto acid), formed by an α-carbon hydroxylation. The latter involves hydroxylation of the methylene carbon adjoining the N-nitroso group of NNK and produces the reactive intermediate, α-hydroxymethylene-NNK, which spontaneously yields methane diazohydroxide and keto aldehyde (Fig. 1) [4]. Further oxidation of this keto aldehyde yields the keto acid (Fig. 1). The absence of activity of the keto acid suggests that the modulation of AM MIP-1α production by NNK may involve NNK directly or the reactive intermediates generated during NNK bioactivation, rather than metabolic end-products such as keto acid.
Levels of NNK in the mainstream smoke of one cigarette may reach up to 1749 ng of NNK, depending on the manufacturer [24,25]. Thus, smokers of one pack/day may theoretically be exposed to 44 µg of NNK. In the present study, the concentration of 250 µM NNK (51·8 µg/ml) required to modulate TNF, MIP-1α, IL-10 and IL-12 is higher than the daily exposure of human lung cells to NNK. However, there may be an additive effect of NNK exposure. Indeed, a low dose of NNK (1·5 µg/ml) given once does not significantly increase AM IL-10 production, but the same concentration given every day for 5 days (a total of 7·5 µg/ml) causes a 2·8-fold increase of IL-10 release, which is higher than the 1·6-fold increase observed at 207 µg/ml (1000 µM; Fig. 5) after 20 h of treatment. Consequently, the concentrations needed to modulate AM mediator production are representative of what may happen in humans.
This is the first study showing that, in addition to its genotoxic effect, NNK can modulate immune responses. NNK inhibits (in a concentration-dependent manner) AM MIP-1α production in the presence or absence of LPS. However, the inhibitory effect of NNK was stronger after a short treatment (6 or 20 h) in unstimulated AMs than in LPS-stimulated AMs, whereas similar inhibition was observed after a longer NNK treatment (48 h). MIP-1α is a member of the chemokine family, a group of proinflammatory chemoattractant cytokines. In addition to its chemotactic properties, MIP-1α enhances neutrophil phagocytosis and AM bacterial killing [26]. A reduced production of MIP-1α by AMs may favour bacterial invasion and increase the risk of pulmonary infections, as observed in smokers. Furthermore, MIP-1α increases the cytotoxic activity of AMs and NK cells [27]. Thus, the inhibition of MIP-1α production by NNK may reduce anti-tumour immunity, leading to an increased risk of lung cancer in tobacco smokers.
Exposure of AMs to tobacco smoke in vitro or in smokers has been show to inhibit TNF production [17]. Our results show that NNK is one of the components of tobacco smoke that inhibits AM TNF production. In contrast, Rioux & Castonguay [20] have reported that NNK induced TNF release from the human monocyte cell line, U937. Although U937 monocytes were differentiated into macrophages using phorbol 12-myristate 13-acetate (PMA), given the heterogeneity of macrophages, this macrophage population may be functionally different from AMs, showing the importance of using AMs in our in vitro study.
NO is involved in many pathophysiological processes and plays a pivotal role in microbicidal and tumoricidal actions of macrophages [28]. Our data show that NNK inhibits AM NO production (by 20%), suggesting that NNK may be responsible, in part, for the reduced level of exhaled NO observed in smokers [29]. Inadequate production of NO caused by NNK in cigarette smoke may contribute to the increased risk of respiratory infection and cancer. Furthermore, given the bronchodilatory effects of NO, a decrease in NO production may contribute to airflow obstruction in cigarette smokers.
IL-12 is produced mainly by macrophages and monocytes [11]. IL-12 has been shown to induce cytokine production, stimulate differentiation of T helper 1 (Th1) cells, enhance cytolytic activity of both NK and T cells, and possess anti-tumour activity, including tumour regression [11]. NNK inhibits IL-12 production by AMs, which may reduce the capacity of AMs to stimulate the cytotoxic activity of NK cells, resulting in an increased risk of developing lung cancer.
IL-10 is an anti-inflammatory cytokine that inhibits various macrophage functions and production of proinflammatory cytokines and chemokines [30]. IL-10 is a potent inhibitor of both AM and NK-cell anti-tumour activities [31,32]. In addition to being produced by tumour cells, the increased AM IL-10 production induced by NNK may favour the development of lung cancer. However, NNK did not modulate the production of TGF-β, which is involved in tumour growth.
COX expression and PGE2 synthesis are induced by NNK in animal and human cells [33]. Two isoforms of COX have been identified: a constitutive form, COX-1; and an inducible form, COX-2. Inflammatory cells and cancer cells mainly express COX-2 [34], which can activate NNK. Both COX inhibitors abrogated the inhibitory effect of NNK on MIP-1α production, suggesting that PGE2 may be involved. Our data with anti-PGE2 support this observation. Furthermore, we have demonstrated that NNK stimulates the release of PGE2, as observed in vivo[33]. PGE2 is known to stimulate proliferation of tumour cells and mediate immune suppression [35], suggesting that the induction of PGE2 synthesis by NNK may contribute to the development of lung cancer.
In summary, NNK reduces the production of proinflammatory mediators and increases the production of anti-inflammatory mediators from AMs, suggesting that NNK contributes to the lung immunosuppression observed in smokers. Furthermore, this immunosuppression caused by NNK provides some basis for the increased risk of pulmonary infections and lung cancer in cigarette smokers. Nevertheless, further investigations are needed to elucidate the mechanism of NNK immunomodulatory effects on AM functions and COX regulation. A better understanding of NNK-mediated immunomodulation may help in the prevention of lung cancer.
Acknowledgments
This study was supported by the Cancer Research Society, Inc., Fondation J.-D. Begin and Canadian Institutes for Health Research (Grant MOP-43972). E.Y.B. is a senior FRSQ scholar.
References
- 1.Williams MD, Sandler AB. The epidemiology of lung cancer. Cancer Treat Res. 2001;105:31–52. doi: 10.1007/978-1-4615-1589-0_2. [DOI] [PubMed] [Google Scholar]
- 2.Wynder EL. Tobacco as a cause of lung cancer: some reflections. Am J Epidemiol. 1997;146:687–94. doi: 10.1093/oxfordjournals.aje.a009342. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 4.Hecht SS. Biochemistry, biology and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:560–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 5.Smith TJ, Stoner GD, Yang CS. Activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human lung microsomes by cytochrome P450, lipoxygenase and hydroperoxides. Cancer Res. 1995;55:5566–73. [PubMed] [Google Scholar]
- 6.Rioux N, Castonguay A. Induction of COXs expression by a tobacco carcinogen: implication of lung cancer chemoprevention. Inflamm Res. 1999;48(Suppl. 2):136–7. doi: 10.1007/s000110050555. [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS, Carmella SG, Foiles PG, Murphy SE, Peterson LA. Tobacco-specific nitrosamines adducts: studies in laboratory animals and humans. Environ Health Perspec. 1993;99:57–63. doi: 10.1289/ehp.939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraal G, Broug E, Thepen T, Van Iwaarden JF, Persoons JHA. The role of alveolar macrophages in pulmonary immune function. In: Lipscomb MF, Russell SW, editors. Lung Macrophages and Dendritic Cells in Health Disease. New York: Marcel Dekker Inc; 1997. pp. 203–20. [Google Scholar]
- 9.Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–53. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S. Nitric oxide-mediated cytotoxic effects of alveolar macrophages on transformed lung epithelial cells are independent of the beta 2 integrin-mediated intercellular adhesion. Immunology. 1998;93:102–8. doi: 10.1046/j.1365-2567.1998.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrzak JA, Brunda MJ. Biology of disease. Interleukin-12, biologic activity, therapeutic utility, and role in disease. Lab Invest. 1995;72:619–37. [PubMed] [Google Scholar]
- 12.De Vita F, Orditura M, Galizia G, et al. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. 2000;117:365–73. doi: 10.1378/chest.117.2.365. [DOI] [PubMed] [Google Scholar]
- 13.Huang M, Wang J, Lee P, et al. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–53. [PubMed] [Google Scholar]
- 14.Harris JO, Swenson EW, Johnson JE. Human alveolar macrophages. Comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970;49:2086–96. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomassen MJ, Wiedemann HP, Barna BP, Farmer M, Ahmad M. Induction of in vitro tumoricidal activity in alveolar macrophages. Cancer Res. 1988;48:3949–53. [PubMed] [Google Scholar]
- 16.Brown GP, Iwamoto GK, Monick MM, Hunninghake GW. Cigarette smoking decreases interleukin 1 release by human alveolar macrophages. Am J Physiol. 1989;256:C260–4. doi: 10.1152/ajpcell.1989.256.2.C260. [DOI] [PubMed] [Google Scholar]
- 17.McCrea KA, Ensor JE, Nall K, Bleecker ER, Hasday JD. Altered cytokine regulation in the lung of cigarette smokers. Am J Respir Crit Care Med. 1994;150:696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 18.Brown R, Pinkerton R, Tuttle M. Respiratory infections in smokers. Am Fam Physician. 1987;36:133–40. [PubMed] [Google Scholar]
- 19.Sirois J, Menard G, Moses AS, Bissonnette EY. Importance of histamine in the cytokine network in the lung through its H2 and H3 receptors. Stimulation of IL-10 production. J Immunol. 2000;164:2964–70. doi: 10.4049/jimmunol.164.6.2964. [DOI] [PubMed] [Google Scholar]
- 20.Rioux N, Castonguay A. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone modulation of cytokine release in U937 human macrophages. Cancer Immunol Immunother. 2001;49:663–70. doi: 10.1007/s002620000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard G, Bissonnette EY. Priming alveolar macrophages by leukotriene D4. Potentiation of inflammation. Am J Respir Cell Mol Biol. 2000;23:572–7. doi: 10.1165/ajrcmb.23.4.4152. [DOI] [PubMed] [Google Scholar]
- 22.Schrader E, Hirsch-Ernst KI, Richter E, Foth H. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in isolated rat lung and liver. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;357:336–43. doi: 10.1007/pl00005176. [DOI] [PubMed] [Google Scholar]
- 23.Smith GBJ, Castonguay A, Donnelly PJ, Reid KR, Petsikas D, Massey E. Biotransformation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in freshly isolated human lung cells. Carcinogenesis. 1999;20:1809–18. doi: 10.1093/carcin/20.9.1809. [DOI] [PubMed] [Google Scholar]
- 24.Fischer S, Castonguay A, Kaiserman K, Spiegelhalder B, Preussmann R. Tobacco-specific nitrosamines in Canadian cigarettes. J Cancer Res Clin Oncol. 1990;116:563–8. doi: 10.1007/BF01637075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer S, Spiegelhalder B, Preussmann R. Tobacco-specific nitrosamines in European and USA cigarettes. Arch Geschwulstforsch. 1990;60:169–77. [PubMed] [Google Scholar]
- 26.Standiford TJ, Kunkel SL, Greenberger MJ, Laichalk LL, Strieter RM. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–8. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukoc Biol. 1996;59:81–9. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- 28.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 29.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–12. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 30.de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537–41. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- 31.Nabioullin R, Sone S, Mizuno K, et al. Interleukin-10 is a potent inhibitor of tumor cytotoxicity by human monocytes and alveolar macrophages. J Leukoc Biol. 1994;55:437–42. doi: 10.1002/jlb.55.4.437. [DOI] [PubMed] [Google Scholar]
- 32.Spagnoli GC, Juretic A, Schultz-Thater E, et al. On the relative roles of interleukin-2 and interleukin-10 in the generation of lymphokine-activated killer cell activity. Cell Immunol. 1993;146:391–405. doi: 10.1006/cimm.1993.1035. [DOI] [PubMed] [Google Scholar]
- 33.Rioux N, Castonguay A. Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998;58:5353–60. [PubMed] [Google Scholar]
- 34.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol. 1997;49:15–9. [PubMed] [Google Scholar]
- 35.Howe LR, Subbaramaiah K, Brown AMC, Dannenberg AJ. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]