Abstract
Clinical manifestations of pulmonary tuberculosis (TB) may depend on a complex interaction between the host and the pathogen. Clinical outcomes of pulmonary tuberculosis are variable, ranging from asymptomatic lifelong infection to parenchymal lung destruction, resulting in cavitary lesions. To investigate the hypothesis that local cellular immune response may affect presentation and outcome in tuberculosis, we performed bronchoalveolar lavage (BAL) in lung segments affected by cavitary and non-cavitary tuberculosis. We then correlated the type of cellular response at the level of the involved lung segments with clinical evolution in terms of cavity formation. We found alveolar lymphocytosis in patients with both cavitary and non-cavitary pulmonary tuberculosis, with increased CD4+ lymphocytes in patients with non-cavitary pulmonary tuberculosis. A predominant Th1 immune response has been observed in non-cavitary patients, while cavitary involved segments exhibit the presence of Th2 lymphocyte subsets. These data, while confirming the importance of Th1-type CD4+ cells and IFN-γ in effective cellular immunity in active pulmonary tuberculosis, also suggest that the presence of Th2 lymphocytes may contribute to tissue necrosis phenomena associated with cavitary evolution of pulmonary tuberculosis. Our observations indicate the importance of the type of local immune response at the site of disease in the development of different clinical characteristics and outcome in pulmonary tuberculosis.
Keywords: BAL, cavitary, non-cavitary, pulmonary tuberculosis, Th1, Th2
INTRODUCTION
Tuberculosis (TB) is a granulomatous disorder caused by Mycobacterium tuberculosis, in which the precise clinical manifestations result probably from a complex interaction between the host and the pathogen [1].
M. tuberculosis infections can lead to a broad range of clinical outcomes, from asymptomatic lifelong infection to progressive primary tuberculosis, to disease reactivation with pulmonary infiltrates and often parenchymal lung destruction resulting in cavitary lesions. Although interstrain virulence differences among M. tuberculosis isolates may drive the outcome after initial infection, the type of active disease that develops is dictated generally by the state of the host immune system.
M. tuberculosis, in immunocompetent subjects, induces an early and late immune response that ultimately destroys most tubercle bacilli and usually prevents development of clinical disease. Whereas the early response is characterized by an influx of phagocytic cells, mainly macrophages, that provide a first line of defence against mycobacteria in co-operation with other immune cells, the late response is dependent on the acquisition of CD4+ T cell-mediated immunity and characterized by granuloma formation consisting of epithelioid and multi-nucleated giant cells [2,3].
CD4+ T lymphocytes are recognized to be the major effector cells in cell-mediated immunity in TB [4], although CD4+ lymphocytic alveolitis has been detected in many, but not all, patients with active pulmonary TB [5]. T lymphocytes can be recruited to the macrophage and stimulate it to inhibit growth of, or kill mycobacteria [6] and furthermore cytotoxic T lymphocytes can ingest macrophages that have engulfed mycobacteria [7].
In recent years, a paradigm for thinking about the functions of CD4+ T cells and their relationship to the manifestations of disease has developed initially from observations in the murine model and now supported in a variety of human disease, holding that CD4+ helper T cells can be separated into at least two phenotypic classes: Th1 and Th2. These cells derive from so-called Th0, or null cells, and their differentiation from these precursor cells may be under the control of several cytokines. Th1 cells are characterized mainly by their ability to produce the cytokines interferon (IFN)-γ and interleukin (IL)-2, whereas Th2 cells produce cytokines such as IL-4, IL-5, IL-10 and IL-13 [8–10]. Experimental investigations in animals have shown that IFN-γ plays a pivotal role in protective immunity to M. tuberculosis[11]. More recent investigations have associated clinically less advanced active pulmonary TB, in the absence of cavitary lesions on chest radiographs, to alveolitis with lymphocytes producing Th1-associated cytokines such as IFN-γ, but not Th2 cytokines [5].
However, there is also evidence of Th2 cytokine production in peripheral blood mononuclear cells in vitro stimulated with mycobacterial antigens [12].
In this study we have explored the hypothesis that differences in tuberculosis outcome, in terms of the development of cavitary lesions, are associated with different patterns of cellular immune response at the level of the diseased lung. We have collected bronchoalveolar lavage (BAL) samples from two well-characterized homogeneous patient groups with active tuberculosis (exhibiting cavitary and non-cavitary lung lesions, respectively) to investigate whether specific T lymphocyte profiles determine cavitary evolution in pulmonary TB.
MATERIALS AND METHODS
patient recruitment criteria
Patients admitted to the Monaldi Hospital with suspected pulmonary tuberculosis were approached about the study; suspected pulmonary tuberculosis was defined as any patient regardless of HIV status with a chest radiograph suggestive of tuberculosis with or without symptoms of cough, fever and weight loss. Patients were considered eligible for this study according to the following criteria: (1) positive tuberculous skin testing; (2) positive sputum cultures for tuberculosis; (3) presence by high-resolution computed tomography (HRCT) of cavitary or non-cavitary lesions; and (4) no evidence of other concurrent diseases. Elegible patients underwent fibre-bronchoscopy and BAL; both fibre-bronchoscopy and biological studies were performed in the Department of ‘Scienze CardioToraciche e Respiratorie – SUN’ located in Monaldi Hospital.
Study population
Twenty-five subjects, newly diagnosed active pulmonary TB patients, were approached regarding the study, of whom 18 agreed to participate. Four patients were excluded later based on negative sputum cultures for M. tuberculosis. All 14 patients enrolled had positive tuberculous skin testing, positive sputum cultures for M. tuberculosis and no evidence of other concurrent diseases. Patient characteristics are reported in Table 1. Seven normal healthy volunteers were enrolled as a control population. All patients gave informed consent and the study was approved by the Institutional Board of the local Ethical Committee.
Table 1.
Demographics of enrolled patients
| Total recruited | 18 |
| Total eligible | 14 |
| Sex | |
| Male | 14 |
| Female | 0 |
| Age, years | |
| Median | 34 |
| Range | 25–67 |
| Mycobacterium sputum culture positive (no.) | 14 |
| Radiological features (HRTC) | |
| Cavitary lesions | 7 |
| Non-cavitary lesions | 7 |
Hrct
All patients underwent chest HRCT analysis in the supine position. HRCT studies were performed on Toshiba Xpress GX (Toshiba, Japan) and were evaluated for the presence, distribution and extent of the following signs: miliary nodules; nodules; panlobular and polylobular consolidation; and cavity. HRCT were reviewed prior to bronchoscopy/BAL by two independent chest radiologists to define the areas of major involvement.
BAL
For each subject, BAL was performed with a flexible bronchoscope with local xylocaine anaesthesia. Three 50-ml aliquots of sterile saline solution were instilled and subsequently recovered by gentle suction from radiographically involved segments [13]. In normal volunteers, the right median lobe was lavaged and the BAL fluid was coded by number in a blinded fashion. The BAL fluid was filtered through two layers of sterile cotton gauze to remove mucus and centrifuged at 1000 r.p.m. for 10 min. Supernatants were aliquoted and stored frozen.
The alveolar cell population was counted by optic microscope using a Burker chamber. Cell viability was determined by trypan blue exclusion. All recovered cells showed>90% viability. Cell pellets and supernatants were separated by centrifugation at 1000 r.p.m. for 5 min and two slides prepared by smears were stained using May–Grunwald–Giemsa in order to obtain cytograms and differential cell counts. We used smears for bronchoalveolar differential cell counts because of the highest accuracy of this technique compared to cytospin [14,15].
The BAL cells were washed twice in RPMI-1640 (Boehringer Mannheim, Mannheim, Germany), resuspended at 106 cells per ml in serum-free RPMI and than assessed by FACScan flow cytometer, as described in the following section.
Three-colour flow cytometry analysis
BAL cell analysis was performed using a three-colour flow cytofluorimeter (Becton Dickinson immunocytometry system, FACScan, San Jose, CA, USA) according to the method reported previously [16,17].
In brief, 100 µl of cellular suspension were incubated in the dark for 30 min at 4°C with 20 µl of the following monoclonal antibodies (MoAb):
CD45PERCP, CD3FITC, CD4PE; CD45PERCP, CD3FITC, CD8PE; CD45PERCP, CD3FITC, CD19PE; CD45PERCP, CD3FITC, CD16PE; CD45PERCP, CD3FITC, HLA-DRPE; CD45PERCP, CTRFITC, CTRPE (BD Biosciences, San Jose, CA, USA).
Cells were washed three times with PBS 1% FCS (Boehringer Mannheim, Germany) and then resuspended in 2 ml of PBS. Using an acquisition gate on lymphocytes in the side scatter (SSC) versus fluorescence (Fl)3 (CD45PERCP) dot-plot, we collected 5000 events for each sample. All values were expressed as mean ± standard deviation (s.d.) of percentages of cell phenotypes.
Flow cytometry for intracellular cytokines
Analysis of Th1- and Th2-like populations in BAL was performed utilizing a three-colour flow cytofluorimeter (Becton Dickinson immunocytometry system, FACScan) according to the method described elsewhere [18,19], with minor modification. In brief, two aliquots of 200 µl of BAL lymphoid cells from the two groups of lung TB patients (cavitary TB, non-cavitary TB) were used to perform the experiments. BAL fluid was filtered in sterile gauze and then centrifuged; cellular pellets were washed thrice in PBS 1% FCS and resuspended at the concentration of 1 × 106 cells/ml in RPMI-1640 + 10% FCS + Pen/Strep (550 IU/ml) (500 µg/ml) (Boehringer Mannheim, Germany). In the first aliquot, cells were stimulated in vitro by phorbol myristate acetate (PMA) (50 ng/ml) + ionomycin (1 µg/ml) (Sigma-Aldrich, Milan, Italy). To detect intracellular storage of specific Th1/Th2-associated cytokines, cell secretion was blocked by adding brefeldin A (5 µg/ml) (Sigma-Aldrich, Italy). Cells were cultured for 6 h at 37°C 5% CO2.
Subsequently cells were incubated with the monoclonal antibody CD4PERCP, fixed and permeabilized by paraformaldehyde 5%/saponin 0·1% (Sigma-Aldrich, Italy).
Each sample was separated into two aliquots. One was incubated with MoAb anti-IFN-γFITC and IL4-PE, the other with control mouse isotopic MoAbs (BD Biosciences, San Jose, CA, USA).
To exclude any dead and contaminating non-CD4+ lymphocytes from the analyses, acquisition of data by flow cytometry was performed selecting an immunological gate on CD4-positive lymphocytes on the dot-plot SSC versus Fl3(CD4PERCP), acquiring 5000 events for each experiment.
Analysis of the data was carried out by three independent observers (F.P., A.B., D.D.A) to avoid bias. Percentage results of Th1-like (CD4+ IFN-γFITC+) and Th2-like (CD4+ IL4PE+) lymphocyte subsets were obtained subtracting values acquired on control samples.
Statistical analysis
Wilcoxon's signed-rank test for paired data was applied to the results. Probability values of <0·05 were considered significant.
RESULTS
Differential and absolute cell counts in bronchoalveolar lavage fluid
Patients with tuberculosis exhibited a significant increase in the number of recovered cells (cavitary: mean 15·08 ± 0·9 × 106; non-cavitary: mean 15·9 ± 0·55 × 106) compared to normal control subjects (mean 4·3 ± 0·6 × 106); no differences were found between cavitary and non-cavitary patients. A significant increase in number of lymphocytes as well as neutrophils was found in patients with TB (both cavitary and non-cavitary) over normal healthy subjects (P < 0·05), in parallel with a significant decrease in alveolar macrophage number. Increase of neutrophils has also been observed in cavitary compared to non-cavitary patients (P < 0·05); conversely, lymphocytes are enhanced in non-cavitary compared to cavitary patients (P < 0·05). Table 2 summarizes data referring to absolute and differential cell counts in BAL fluid.
Table 2.
BAL differential cell count in the involved lobe
| Cavitary TB | Non-cavitary TB | Normal healthy volunteers | |
|---|---|---|---|
| BAL recovered (ml) | 58 ± 7·2 | 59·7 ± 6·7 | 55·1 ± 6·0 |
| Total cells collected | 15·08 ± 0·9 × 106† | 15·9 ± 0·55 × 106‡ | 4·3 ± 0·6 × 106 |
| Cells/ml | 2·4 ± 0·1 × 105† | 2·6 ± 0·2 × 105‡ | 0·5 ± 0·18 × 105 |
| Macrophages percentage | 68·8 ± 3·13*† | 61·4 ± 3·4‡ | 87·5 ± 1·8 |
| Neutrophils percentage | 5·85 ± 1·06*† | 4 ± 0·8‡ | 1·6 ± 0·5 |
| Eosinophils percentage | 0·8 ± 0·8 | 0·5 ± 0·5 | 0·8 ± 0·4 |
| Lymphocytes percentage | 24 ± 2·1*† | 30·2 ± 1·5 | 10·5 ± 2·07 |
Mean value ± s.d. of cellular components of bronchoalveolar lavage of patients with pulmonary cavitary tuberculosis, pulmonary non-cavitary tuberculosis and normal healthy voluntaries.
P < 0·05 for cavitary versus non-cavitary;
P < 0·05 for cavitary versus controls;
P < 0·05 for non-cavitary versus controls.
Flow cytofluorimeter analysis of BAL cell pellets
Table 3 shows FACScan analysis of lymphocyte subpopulations retrieved in BAL of subjects with cavitary and non-cavitary TB, as well as normal healthy subjects as the control population. BAL of cavitary subjects showed significant enhancement of a percentage of CD8-positive cells (P < 0·05) over non-cavitary subjects, associated with a significant decrease in CD4-positive cells, as well as the CD4/CD8 ratio (P < 0·05). Patients with cavitary tuberculosis showed an increased percentage of CD8+ and CD3+ HLA-DR+ (P < 0·05) over normal subjects.
Table 3.
BAL T lymphocyte subsets in the involved lobe
| Lymphocyte subsets | Cavitary TB | Non-cavitary TB | Normal healthy volunteers |
|---|---|---|---|
| CD3% | 92·5 ± 1·9 | 92·2 ± 2·14 | 91·5 ± 1·37 |
| CD4% | 48 ± 2·1* | 62·4 ± 2·9 | 56·1 ± 1·9 |
| CD8% | 41·5 ± 1·7*† | 24 ± 2·3‡ | 35·7 ± 3·01 |
| CD4/CD8 | 1·15 ± 0·06* | 2·6 ± 0·3 | 1·3 ± 0·15 |
| CD3+ HLA-DR+% | 10 ± 2·6† | 9·1 ± 2·1 | 5·1 ± 0·6 |
| CD 19% | 0·2 ± 0·2 | 0·16 ± 0·17 | 0·1 ± 0·1 |
| CD 16% | 3·4 ± 1·7 | 4·81 ± 1·06 | 3·1 ± 0·7 |
Mean value ± s.d. of lymphocyte subsets from bronchoalveolar lavage of patients with pulmonary cavitary tuberculosis, pulmonary non-cavitary tuberculosis and normal healthy voluntaries.
P < 0·05 for cavitary versus non-cavitary;
P < 0·05 for cavitary versus controls;
P < 0·05 for non-cavitary versus controls.
BAL cell pellets from non-cavitary patients exhibited a significant increase in the percentage of CD4+, CD3+ HLA-DR+, but a decrease in CD8+ (P < 0·05) compared to normal subjects. No significant differences for CD3-, CD16- and CD19-positive cells were found between the three groups of subjects studied.
Intracellular analysis of Th1/Th2 subsets by flow cytofluorimeter in non-cavitary and cavitary TB
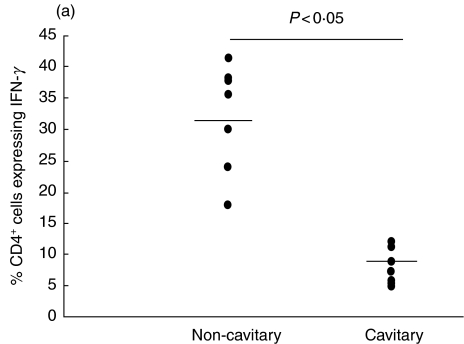
CD4 T lymphocytes from BAL of lung segments affected by non-cavitary pulmonary TB exhibited a predominant type-1 (IFN-γ) response (Fig. 1a).
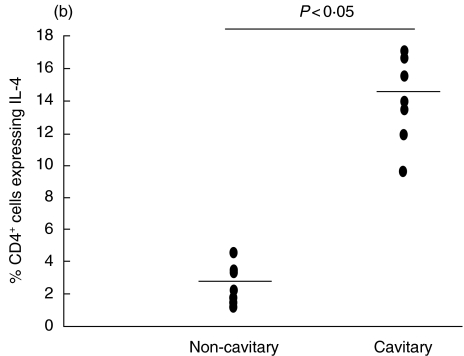
Fig. 1.
Expression of interleukin (IL)-4 and interferon (IFN)-γ in pulmonary tuberculosis patients with and without cavity. The percentages of BAL CD4+ cells expressing IFN-γ(a) and IL-4 (b) are reported. Each dot represents one individual. Horizontal bar is the mean value.
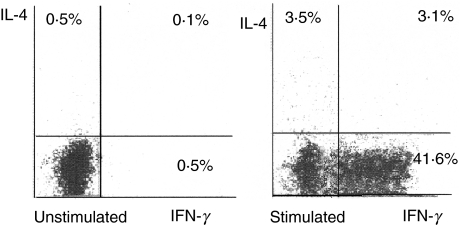
Figure 2 shows the simultaneous detection of IFN-γ and IL-4 in BAL CD4+ cells from a representative patient with non-cavitary TB. BAL from this patient shows 41·6% of IFN-γ-producing cells and 3·5% of IL-4-producing cells as well as 3·1% of cells producing both IFN-γ and IL-4.
Fig. 2.
Expression of interleukin (IL)-4 and interferon (IFN)-γ in BAL CD4+ cells from a patient with non-cavitary pulmonary tuberculosis. Flow cytometric assessment of cell-specific cytokine response in BAL cells was performed by use of immunofluorescent antibodies against CD4, IFN-γ and IL-4. Depicted is the percentage of CD4+ cells expressing IFN-γ and/or IL-4.
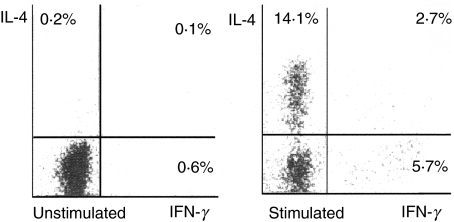
BAL cells collected from radiographically involved cavitary lesions showed a significant presence of Th2-like lymphocytes with concurrent detection of a Th1 subset, as well as T lymphocytes CD4+ exhibiting both Th1 and Th2 intracellular cytokines (Fig. 1b). Figure 3 shows the simultaneous detection of IFN-γ and IL-4 in BAL CD4+ cells from a representative patient with cavitary TB. BAL from this patient shows 5·7% of IFN-γ-producing cells and 14·1% of IL-4-producing cells, as well as 2·7% of cells producing both IFN-γ and IL-4.
Fig. 3.
Expression of interleukin (IL)-4 and interferon (IFN)-γ in BAL CD4+ cells from a patient with cavitary pulmonary tuberculosis. Flow cytometric assessment of cell-specific cytokine response in BAL cells was performed by use of immunofluorescent antibodies against CD4, IFN-γ and IL-4. Depicted is the percentage of CD4+ cells expressing IFN-γ and/or IL-4.
DISCUSSION
In this study alveolar lymphocytosis in patients with both cavitary and non-cavitary pulmonary TB has been found. An increased percentage of CD4+ lymphocytes was present in patients with non-cavitary pulmonary tuberculosis, but not with cavitary lesions. A significant increase of neutrophils was demonstrated in lung segments affected by cavitary disease compared to non-cavitary lesions. Phenotypic analysis of cells retrieved from pulmonary segments exhibiting cavitary lesions showed a significant enhancement in the percentage of CD8-positive lymphocytes, with a parallel reduction in the CD4/CD8 ratio (P < 0·05) compared to non-cavitary lesions.
We have also explored the percentage of CD3-, CD16- and CD 19-positive cells. This enabled both the exclusion of false-positive and -negative cells from our measurements and the identification of the presence of non-lymphocyte related events contaminating the gated region. No differences were found for CD3-, CD16- and CD19-positive cells between the two groups of subjects studied.
Using cytofluorimeter flow analysis, the expression of type-1 (IFN-γ) and type-2 (IL-4) cytokines in bronchoalveolar lymphocytes was also assessed. We have shown a predominant Th1 immune response in patients affected by non-cavitary pulmonary TB. These results are consistent with experimental investigations supporting the importance of Th1-type CD4+ cells, IL-12 and IFN-γ in effective local cellular immunity in active pulmonary TB [11,20–23]. To highlight further the role of Th1 lymphocytes in TB, other investigations indicate that patients who did not exhibit an IFN-γ-associated lymphocytic alveolitis initially did so later in their course, and elevated levels of this cytokine were observed as patients improved clinically [5].
Interestingly, in our experiments we have found the presence of Th2 lymphocyte subsets at the level of pulmonary segments radiographically affected by cavitary TB. To our knowledge, this is the first observation correlating cavitary pulmonary TB with a type 2 response at the site of the disease. However, we failed to find detectable levels of IL-4 in supernatant of the bronchoalveolar fluid after 20-fold concentration by means of AMICON concentrators, measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) (data not shown).
In agreement with our results, other investigations indicate that IL-4 may not be detectable in culture supernatants [24,25] because production of IL-4 splice variants, or binding of secreted IL-4 to soluble or cellular receptors, interferes with this assay [26]. To override this problem and to allow for cell-specific analysis of Th status in tuberculosis we used flow cytometric detection of intracellular cytokine expression, which is a reliable method as it is unaffected by the natural release of cytokine inhibitors or receptors [19].
Other studies have already documented the presence of Th-2 lymphocytes in peripheral blood of patients with TB. Sourcel and co-workers [12], stimulating in vitro peripheral blood mononuclear cells with mycobacterial antigens, have indeed found that patients with tuberculosis have an increased proliferation of cells secreting IL-4 but not IFN-γ when compared with cells from healthy subjects. Other investigations have indicated that patients with tuberculosis have a Th2 response in peripheral blood, whereas tuberculin-positive subjects have a Th1-type response [27]. More recently, Van Crevel and co-workers have demonstrated a marked increase of IL-4 production in circulating T lymphocytes from cavitary pulmonary TB [28]. These results, although not performed at the site of disease, are in agreement with our observation that pulmonary cavitary TB exhibits a predominant Th2 response.
Consistent with other infectious disease such as leprosy and leishmaniasis, where Th-2 lymphocytes have been associated with poor outcome [28], release of type-2 cytokines IL-4 and IL-5 has also been found at the site of disease depending on the severity of tuberculosis [29,30].
According to several observations, the presence of Th2 lymphocytes could be involved in tissue necrosis phenomena that characterize cavitary pulmonary TB. Indeed, studies report the involvement of IL-4 in tissue damage in mycobacterial infection in mice [31]. Separate investigations indicate that, while TNF-α exhibits beneficial effects in term of enhancing mycobacterium tuberculosis control in the presence of a predominant Th1 response, a combination of Th2 cytokines and TNF-α causes a significant increase in tissue damage [32–34].
Our findings support these observations further and indicate the importance of the type of local immune response in presentation and clinical outcome of TB. Although additional studies are needed, our investigations suggest a possible implication for a Th2-type response in the tissue necrosis phenomenon related to cavitary evolution of TB. A better understanding of the role of cytokines in influencing the balance between protective immunity and immunopathology may provide further insight into both critical mechanisms underlying the variability of pulmonary TB pathology and new therapeutic approaches in TB treatment.
Acknowledgments
Supported by Istituto Superiore di Sanità (ISS) Programma Nazionale Tubercolosi, Italy, grant 1997–98 to G.M., Istituto Superiore di Sanità (ISS) Programma Nazionale Tubercolosi, Italy, grant 1997–98 to A.S. and UNESCO short fellowships 2001 to A.S.
References
- 1.Wallis RS, Ellner JJ. Cytokines and tuberculosis. J Leukoc Biol. 1994;55:676–81. doi: 10.1002/jlb.55.5.676. [DOI] [PubMed] [Google Scholar]
- 2.Dunn P, North RJ. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect Immun. 1995;63:3428–37. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauffman SHE. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 4.Boom WH. The role of T cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:73–81. [PubMed] [Google Scholar]
- 5.Condos R, Rom WN, Liu YM, Schluger NW. Local immune response correlates with presentation and outcome in tuberculosis. Am J Respir Crit Care Med. 1998;157:729–35. doi: 10.1164/ajrccm.157.3.9705044. [DOI] [PubMed] [Google Scholar]
- 6.Rom WN, Schluger N, Law K, et al. Human host response to Mycobacterium tuberculosis. Schweiz Med Wochenschr. 1995;125:2178–85. [PubMed] [Google Scholar]
- 7.Stenger S, Mazzaccaro RJ, Uyemura K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 8.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S. Understanding the role of Th1/Th2 cells in infection. Trends Microbiol. 1996;4:470–3. doi: 10.1016/s0966-842x(97)82906-x. [DOI] [PubMed] [Google Scholar]
- 10.Mazzarella G, Bianco A, De Palma R, Abbate GF, Catena E. Th1/Th2 lymphocytes polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russel DG, Orme IM. Disseminated tuberculosis in interferon-gamma-gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sourcel HM, Trye-Blomberg M, Paulie S, Anderson G, Moreno C, Pasvol Ivanyi G. Th1/Th2 profiles in tuberculosis based on proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis. 1987;35:250–63. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- 14.Mordelet-Dambrine M, Arnoux A, et al. Processing of lung lavage fluid causes variability in bronchoalveolar cell count. Am Rev Respir Dis. 1984;130:305–6. doi: 10.1164/arrd.1984.130.2.305. [DOI] [PubMed] [Google Scholar]
- 15.Laviolette M, Carreau M, Coulombe R. Bronchoalveolar lavage cell differential on mycroscope glass cover. A simple and accurate technique. Am Rev Respir Dis. 1988;138:451–7. doi: 10.1164/ajrccm/138.2.451. [DOI] [PubMed] [Google Scholar]
- 16.Ponticiello A, Perna F, Sturkenboom MCJM, Marchetiello I, Bocchino M, Sanduzzi A. Demographic risk factors and lymphocyte populations in patients with tuberculosis and their healthy contacts. Int J Tuberc Lung Dis. 2001;5:1148–55. [PubMed] [Google Scholar]
- 17.Catena E, Mazzarella G, Peluso G, Micheli P, Cammarata A, Marsico SA. Phenotypic features of alveolar macrophages in atopic asthmatic patients. Monaldi Arch Chest Dis. 1993;1:6–15. [PubMed] [Google Scholar]
- 18.Elson LH, Nutman TB, Metcalfe DD, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+CD27− lymphocyte subpopulation. J Immunol. 1995;154:4294–301. [PubMed] [Google Scholar]
- 19.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Meth. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 20.Schulger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 21.Cesarini M, Ameglio F, Alemanno L, et al. Cytokine levels correlate with a radiologic score in active pulmonary tuberculosis. Am J Respir Crit Care Med. 1999;159:143–8. doi: 10.1164/ajrccm.159.1.9803066. [DOI] [PubMed] [Google Scholar]
- 22.Taha RA, Kotsimbos TC, Song YL, Menzies D, Hamid Q. IFN-gamma and IL-12 are increased in active tuberculosis. Am J Respir Crit Care Med. 1997;155:1135–9. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Gately MK, Wang E, et al. Interleukin-12 at the site of disease in tuberculosis. J Clin Invest. 1994;93:1733–9. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Lin Y, Iyer DV, Gong J, Abrams JS, Barnes PF. T cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–4. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Zhang M, Iyer DV, Hofman FM, Gong J, Barnes PF. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–6. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atamas SP, Choi J, Yurowsy VV, White B. An alternative splice variant of human IL-4, IL-4 delta 2, inhibits IL-4 stimulated T cell proliferation. J Immunol. 1996;156:435–41. [PubMed] [Google Scholar]
- 27.Sanchez FO, Rodriguez JI, Agudelo G, Garcia LF. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–8. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Crevel R, Karyadi E, Preyers F, et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J Infect Dis. 2000;181:1194–7. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 29.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–62. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somoskowy A, Zissel G, Zipfel PF, et al. Different cytokine patterns correlate with the extension of disease in pulmonary tuberculosis. Eur Cytokine Netw. 1999;10:135–42. [PubMed] [Google Scholar]
- 31.Hernandez Pando R, Orozcoe H, Sampieri A, et al. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimenal pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 32.Al Attiyah R, Moreno C, Rook GAW. TNF-alfa-mediated tissue damage in mouse footpads primed with mycobacterial preparation. Res Immun. 1992;143:601–10. doi: 10.1016/0923-2494(92)80041-i. [DOI] [PubMed] [Google Scholar]
- 33.Rook GA, Hernandez-Pando R. T cell helper types and endocrines in the regulation of tissue-damaging mechanisms in tuberculosis. Immunobiology. 1994;191:478–92. doi: 10.1016/S0171-2985(11)80454-7. [DOI] [PubMed] [Google Scholar]
- 34.Rook GA, Hernandez-Pando R. Cellular immune responses in tuberculosis-protection and immunopathology. Med Mal Infect. 1996;26:904–10. [Google Scholar]