Abstract
Mycophenolate mofetil (MMF) is an immunosuppressive drug that acts as a selective inhibitor of inosine monophosphate dehydrogenase (IMPDH). MMF has recently been shown to inhibit the enzymatic activity of inducible NO synthase (iNOS) and subsequent production of the cytotoxic free radical nitric oxide (NO) in endothelial cells. We here investigated the effect of bioactive MMF compound mycophenolic acid (MPA) on iNOS-mediated NO synthesis in fibroblasts, which are important source of NO in rheumatoid arthritis and during rejection of solid organ transplants. MPA exerted dose-dependent inhibition of NO synthesis, measured as nitrite accumulation, in IFN-γ + LPS-stimulated L929 mouse fibroblast cell line and rat primary fibroblasts. The effect of MPA was not mediated through interference with IMPDH-dependent synthesis of iNOS co-factor BH4 and subsequent suppression of iNOS enzymatic activity, as direct BH4 precursor sepiapterin failed to block the action of the drug. MPA suppressed the IFN-γ + LPS-induced expression of fibroblast iNOS protein, as well as mRNA for iNOS and its transcription factor IRF-1, as assessed by cell-based ELISA and semiquantitative RT-PCR, respectively. MPA suppression of fibroblast NO release, iNOS, and IRF-1 activation, was efficiently prevented by exogenous guanosine, indicating that the drug acted through reduction of IMPDH-dependent synthesis of guanosine nucleotides. These results suggest that MPA inhibits NO production in fibroblasts by blocking guanosine nucleotide-dependent expression of iNOS gene, through mechanisms that might involve the interference with the induction of iNOS transcription factor IRF-1.
Keywords: mycophenolic acid, fibroblast, nitric oxide, iNOS IRF-1
INTRODUCTION
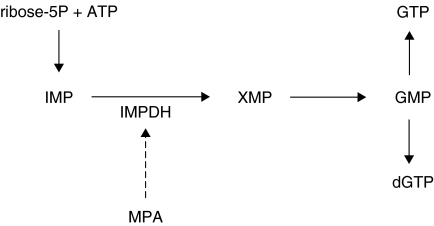
An immunosuppressive xenobiotic drug mycophenolate mofetil (MMF) has been used in preventing transplant rejections [1], and was also shown to be effective in the treatment of adjuvant arthritis, an experimental animal model fairly acceptable for pre-clinical testing of drugs potentially useful in rheumatoid arthritis (RA) [2]. Moreover, MMF has been recently shown to be beneficial in the clinical trials of RA [2,3]. Mycophenolic acid (MPA), the bioactive compound of mycophenolate mofetil (MMF), inhibits the activity of inosine monophosphate dehydrogenase (IMPDH), a rate-limiting enzyme for de novo synthesis of guanosine nucleotides [2] (Fig. 1). By depleting the intracellular concentration of guanosine nucleotides, MPA acts as a powerful proliferation inhibitor in various cell types, especially in lymphocytes [2]. Beneficial effects of MMF in the treatment of allograft rejection in humans, as well as in clinical trials and animal models of various autoimmune diseases, including RA, has been mostly attributed to the influence of MPA on lymphocyte function [1,2]. Although the possible impact of MPA on other cell types involved in the immune response could be of importance for understanding the drug's immunomodulatory properties, such influence has not been thoroughly investigated.
Fig. 1.
Interference of MPA with de novo guanosine nucleotide synthesis. ATP, adenosine monophosphate; IMP, inosine monophosphate; XMP, xanthosine monophosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate; dGTP, deoxyguanosine triphosphate; IMPDH, inosine monophosphate dehydrogenase.
A highly reactive free radical nitric oxide (NO), produced by inducible NO-synthase (iNOS)-mediated oxidation of l-arginine, is an important immune mediator with profound both cytotoxic and regulatory roles [4,5]. It has recently been shown that MPA can block cytokine (IFN-γ, TNF-α)-induced NO production in rodent endothelial cells [6]. Interestingly, although the regulation of iNOS is mainly transcriptional [4], MPA's effect in endothelial cells was exerted through suppression of IMPDH-dependent synthesis of essential iNOS co-factor tetrahydrobiopterin (BH4), and subsequently, iNOS enzymatic activity [6]. Inducible NOS-mediated excessive release of NO has been implicated in joint cartilage destruction in rheumatoid arthritis, and presents a potential target for its treatment [7]. In addition to chondrocytes and infiltrating macrophages, cytokine-activated synovial fibroblasts are a significant source of NO in arthritic joints [8]. Similarly, iNOS-derived NO production is largely responsible for the parenchymal cell death and dysfunction in acutely rejecting allografts [2]. Besides acting as a cytotoxic molecule, NO is a powerful modulator of fibroblast function, showing a mainly positive effect on collagen synthesis in vitro[9,10]. Accordingly, strong up-regulation of iNOS in fibroblasts paralleled the onset and progression of fibrosis in an experimental model of post-transplant obliterative airway disease [11]. Therefore, the interference with fibroblast NO synthesis might be of a therapeutic value in pathological conditions accompanied by excessive fibroblast activation, such as rheumatoid arthritis or the rejection of solid organ transplants.
We here demonstrate that MPA is a potent inhibitor of NO production in rodent fibroblasts. Interestingly, MPA's effect did not stem from the limitation of BH4 availability and resulting suppression of iNOS catalytic activity, as previously observed in endothelial cells, but involved the interference with IFN-γ + LPS-triggered expression of fibroblast iNOS.
MATERIALS AND METHODS
Reagents
Foetal calf serum (FCS), RPMI 1640, and phosphate-buffered saline (PBS) were from ICN (Costa Mesa, CA, USA). Recombinant rat IFN-γ was obtained from Holland Biotechnology (Leiden, the Netherlands). Cycloheximide was purchased from US Biochemical Corporation (Cleveland, OH, USA). Mycophenolic acid (MPA), actinomycin D, guanosine, sepiapterin (3–4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), naphthylenediamine dihydrochloride, sulfanilamide, lipopolysacharide (Esherichia coli 055:B5, LPS) and DMSO were from Sigma (St Louis, MO, USA). Moloney leukaemia virus reverse transcriptase and Taq polymerase were obtained from Eurogentec (Seraing, Belgium). RNA Isolator was purchased from Genosys (Woodlands, TX, USA), and random primers were from Pharmacia (Uppsala, Sweden).
Cells and cell cultures
The murine fibrosarcoma cell line L929 was obtained from the European Collection of Animal Cell Cultures (Salisbury, UK), and grown in HEPES-buffered RPMI 1640 medium supplemented with 10% FCS, l-glutamine and antibiotics (culture medium) at 37°C in a humidified atmosphere with 5% CO2. Plastic-adherent fibroblast-like short-term cell lines were derived from spleen of DA rats (animal facility of Institute for Biological Research, Belgrade, Yugoslavia), as previously described [12]. After the anaesthetized rats were killed by cervical dislocation, spleens were removed, minced into small pieces, and digested for 10 min at 37°C in 2 mg/ml collagenase in PBS. The digest was then washed three times, resuspended in culture medium, and incubated in a cell culture Petri dish for 3 days at 37°C in a humidified atmosphere with 5% CO2. Non-adherent cells and cell debris were removed by replacing culture medium after 24, 48, and 72 h of cultivation. Fibroblasts were cultured to confluence, when they were passaged after 2-min treatment with PBS solution of 0·25% trypsin and 0·02% EDTA. Primary fibroblast preparations obtained by this method do not contain macrophages or other cells of haematopoietic origin [12]. For the experiments, spleen fibroblasts after the second passage, and L929 cells were detached by trypsinization, resuspended in culture medium, seeded in flat-bottom 96-well plates at 2 × 104/well (spleen fibroblasts) or 6 × 104 cells/well (L929 cells) and allowed to grow to confluence. Resident macrophages were obtained from DA rats or CBA mice (animal facility of Institute for Biological Research, Belgrade, Yugoslavia) by peritoneal lavage with cold PBS, followed by adherence to plastic [13]. Confluent fibroblasts, mouse macrophages (1 × 105/well), or rat macrophages (2 × 104/well), were incubated with different agents in 200 µl of culture medium. The cell culture supernatants were collected for nitrite determination after 48 h of cultivation. Total RNA for RT-PCR analysis was isolated from primary fibroblasts after 6 h of incubation. Cell-based ELISA for iNOS in L929 cells was performed after 48 h of cultivation.
Nitrite measurement
Nitrite accumulation, an indicator of NO production, was measured in cell culture supernatants using the Griess reagent [14]. Briefly, 50 µl samples of culture supernatants were mixed with an equal volume of Griess reagent (a mixture at 1 : 1 of 0·1% naphthylenediamine dihydrochloride and 1% sulfanilamide in 5% H3PO4) and incubated at room temperature for 10 min. The absorbance at 570 nm was measured in a microplate reader. The nitrite concentration was calculated from a NaNO2 standard curve.
Cell-based ELISA for iNOS
The expression of iNOS in L929 fibroblasts was determined by slightly modified original protocol for the cell-based ELISA [15]. The cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature (approx, 25°C) and washed three times with PBS containing 0·1% Triton X-100 (PBS/T). Endogenous peroxidase was quenched with 0·6% H2O2 in PBS/T for 20 min, and cells were washed three times in PBS/T. Following blocking with 10% FCS in PBS/T for 1 h, cells were incubated for 1 h with the primary antibody (rabbit anti-mouse iNOS, kindly provided by Dr Carl F. Nathan, Cornell University Medical College) at 1 : 10000 dilution in PBS/T containing 1% BSA at 37°C. After washing the cells four times with PBS/T for 5 min, they were incubated for 1 h with the second antibody (anti-rabbit-HRP; 1 : 500) in PBS/T containing 1% BSA at 37°C. Subsequently, cells were washed and incubated with 200 µl of a solution containing 0·4 mg/ml OPD, 11·8 mg/ml Na2HPO4 × 2H2O, 7·3 mg/ml citric acid and 0·015% H2O2 for 30 min at room temperature in the dark. The reaction was stopped with 50 µl of 3M HCl, and the absorbance at 492 nm was determined in a microplate reader.
RT-PCR determination of iNOS and IRF-1 mRNA
Total RNA from fibroblast cultures was isolated with RNA Isolator, according to manufacturer's instruction. RNA was reverse transcribed using Moloney leukaemia virus reverse transcriptase and random primers. PCR amplification of cDNA with primers specific for iNOS/IRF-1 and GAPDH as a house-keeping gene, was carried out in the same tube in a Thermojet (Eurogentec, Seraing, Belgium) thermal cycler as follows: 30 s of denaturation at 95°C, 30 s of annealing at 50°C for iNOS or 55°C for IRF-1, and 30 s of extension at 72°C. The number of cycles (25 for GAPDH and 30 for iNOS and IRF-1), ensuring non-saturating PCR conditions, was established in preliminary experiments. For iNOS, the sense primer was 5′-AGAGAGATCCGGTTCACA-3′, and the antisense primer was 5′-CACAGAACTGAGGGTACA-3′ corresponding to positions 88–105 and 446–463, respectively, of the published rat iNOS mRNA sequence (GenBank accession number S71597); the PCR product was 376 bp long. For IRF-1, the primers were: sense, 5′-GACCAGAGCAGGAACAAG-3′; antisense, 5′-TAACTTCCCTTCCTCATCC-3′, corresponding to positions 483–500 and 881–899, respectively, of the published rat IRF-1 mRNA sequence (M34253); the PCR product was 417 bp long. The primers for GAPDH were: sense, 5′-GAAGGGTGGGGCCAAAAG-3′; antisense, 5′-GGATG CAGGGATGATGTTCT-3′, corresponding to positions 371–388 and 646–665 of the published rat GAPDH mRNA sequence (AB017801); the PCR product was 295 bp long. PCR products were visualized by electrophoresis through agarose gel stained with ethidium bromide. Gels were photographed and results were analysed by densitometry using Scion Image beta 2 software. Relative expression of iNOS and IRF-1 mRNA was calculated as a ratio between the densities of the iNOS/IRF-1 and GAPDH bands.
Determination of cell viability and total protein content
Cell respiration, as an indicator of cell viability, was assessed by the mitochondrial-dependent reduction of MTT to formazan. At the end of the culture, MTT solution was added to cell cultures in final concentration of 0·5 mg/ml and cells were incubated for an additional 1 h. Thereafter, medium was removed and cells were lysed in DMSO. The conversion of MTT to formazan by metabolically viable cells was monitored by an automated microplate reader at 570 nm. The total protein amount in the cells was measured by Bradford assay and the results were presented as the absorbance at 570 nm.
Statistical analysis
To analyse the significance of the differences between various treatments performed in triplicates, we used analysis of variance (anova), followed by Student-Newman–Keul’s-test. A P-value less than 0·01 was considered significant.
RESULTS
MPA down-regulates IFN-γ + LPS-induced NO production in fibroblasts
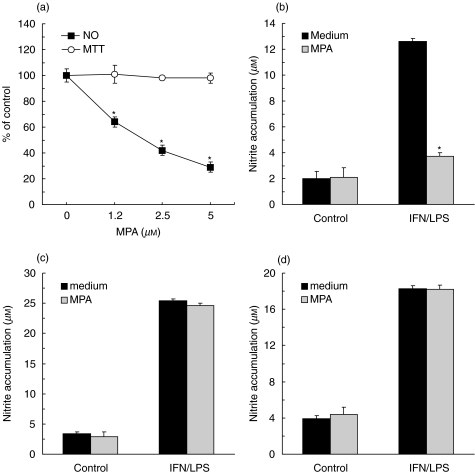
The combination of IFN-γ and LPS has been reportedly used for induction of NO synthesis in various cell types [4]. Accordingly, the stimulation with IFN-γ + LPS effectively up-regulated NO production in mouse L929 fibroblast cell line (Fig. 2a), compared with low basal levels of NO release in unstimulated cultures (<2·0 µm nitrite). A general translation inhibitor – cycloheximide, and a fairly selective iNOS inhibitor – aminoguanidine [16], both prevented the observed NO production (15·6 ± 0·3, 2·4 ± 0·2, 2·1 ± 0·4 µm in control, cycloheximide, and aminoguanidine-treated L929 cultures, respectively; P < 0·01), thus confirming that the NO was synthesized by the inducible NOS isoform. The simultaneous addition of MPA with IFN-γ + LPS markedly down-regulated L929 fibroblast NO synthesis in a dose-dependent manner (Fig. 2a). The inhibitory effect of MPA on IFN-γ + LPS-triggered NO release was confirmed in rat primary fibroblasts (Fig. 2b). The observed MPA action on fibroblast NO synthesis was not a consequence of the drug toxicity, as MTT assay did not show significant changes in cellular respiration upon MPA treatment of L929 cells (Fig. 2a) or primary fibroblasts (data not shown). Interestingly, while both rat and mouse macrophages produced large amounts of NO following IFN-γ + LPS activation, MPA did not have any effect on macrophage NO production (Figs 2c and d).
Fig. 2.
MPA inhibits NO production in fibroblasts, but not macrophages. (a) L929 cells were stimulated for NO production with IFN-γ (250 U/ml) + LPS (5 µg/ml) and treated with various concentrations of MPA. (b–d) Untreated (control) or IFN-γ + LPS-stimulated primary rat fibroblasts (b), mouse (c) or rat (d) macrophages were treated with MPA (5 µm). Results from one of five (a), four (b) or two (c, d) independent experiments with similar results are presented as mean + s.d. (*P < 0·01 refers to cultures without MPA). Control values in (a) were 22·7 µm for nitrite accumulation and 0·373 (A570) for MTT assay.
MPA suppresses iNOS expression, but not its enzymatic activity in fibroblasts
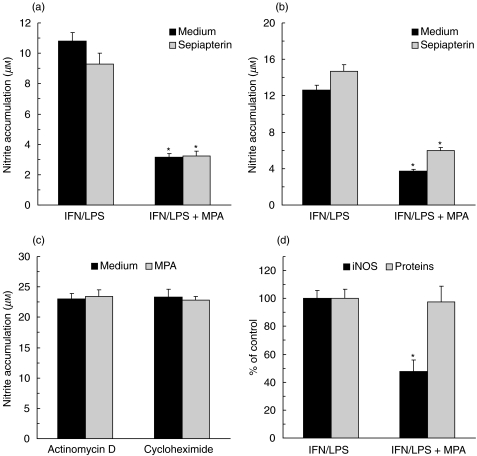
It was recently proposed that MPA might interfere with NO production in endothelial cells by blocking the synthesis of the essential iNOS cofactor BH4 [6]. However, direct BH4 precursor sepiapterin failed to restore NO synthesis in either L929 or primary fibroblasts treated with MPA (Figs 3a and b), indicating that some other mechanism might be responsible for the effect of MPA in these cells. A small increase in nitrite accumulation was observed in sepiapterin-treated primary fibroblasts regardless of the presence of MPA, probably as a consequence of increased availability of BH4. To get further insight into the mechanism of MPA action, the drug was added to L929 fibroblasts in which the iNOS was induced by 24 h pre-treatment with IFN-γ + LPS, and any further iNOS expression was blocked with transcription inhibitor actinomycin D or translation blocker cycloheximide. In these conditions, MPA failed to affect NO release (Fig. 3c), suggesting that IFN-γ + LPS-induced expression of iNOS enzyme, rather than its catalytic activity, was the target for the drug action in fibroblasts. To confirm such an assumption, we examined the influence of MPA on the level of iNOS protein in L929 fibroblasts stimulated with IFN-γ + LPS. In accordance with the data of nitrite measurement, iNOS was almost undetectable in resting cells (data not shown), but it was strongly up-regulated following the activation with IFN-γ + LPS (Fig. 3d). As expected, the addition of MPA during IFN-γ + LPS stimulation markedly reduced the expression of iNOS in L929 fibroblasts. Importantly, MPA did not have a general inhibitory effect on protein synthesis, as it did not significantly alter total protein level in IFN-γ + LPS-treated L929 cells (Fig. 3b).
Fig. 3.
MPA inhibits iNOS expression, but not its catalitic activity in fibroblasts. (a, b) IFN-γ (250 U/ml) + LPS (5 µg/ml)-stimulated L929 cells (a) and primary rat fibroblasts (b) were incubated with MPA (5 µm), in the presence or absence of sepiapterin (100 µm). (c) Primary fibroblasts were stimulated with IFN-γ + LPS for 24 h, thoroughly washed, and cultured in fresh medium containing actinomycin D (5 µg/ml) or cycloheximide (5 µg/ml) for an additional 48 h. (d) IFN-γ + LPS-stimulated L929 cells were incubated for 48 h with MPA (5 µm) and the amount of iNOS and total proteins were determined by cell-based ELISA and Bradford assay, respectively. Representative results from three (a, b) or two (c, d) separate experiments with similar results are presented as mean + s.d. (*P < 0·01 refers to corresponding cultures without MPA).
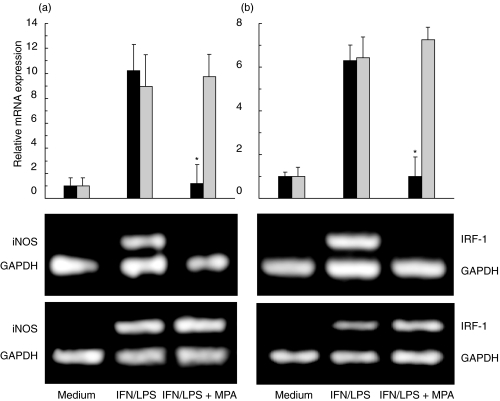
MPA inhibits the induction of iNOS and IRF-1 mRNA in fibroblasts
The inability of MPA to affect fibroblast NO release in the presence of transcription inhibitor actinomycin D (Fig. 3a) indicated that the drug might act by interfering with the transcription of iNOS gene. Therefore, we next investigated the effect of MPA on the expression of mRNA for iNOS and its important transcription factor IRF-1 [17] in primary fibroblasts. Whilst barely detectable in untreated cells, the levels of both iNOS and IRF-1 mRNA were markedly elevated upon the stimulation with IFN-γ + LPS (Fig. 4). In line with the data on nitrite measurement and iNOS expression, MPA almost completely abolished IFN-γ + LPS-triggered accumulation of iNOS mRNA. Furthermore, MPA also decreased the expression of IRF-1 message, thus indicating that the drug might affect iNOS transcription at least partly through inhibition of IRF-1 gene expression. As previously demonstrated at the level of NO production (Fig. 2), MPA did not have any effect on either iNOS or IRF-1 mRNA induction in IFN-γ + LPS-stimulated rat macrophages (Fig. 4).
Fig. 4.
MPA inhibits iNOS and IRF-1 gene expression in fibroblasts. Primary fibroblasts (black bars, and upper photographs) and macrophages (grey bars, and lower photographs) were cultured in medium alone, or stimulated with IFN-γ (250 U/ml) + LPS (5 µg/ml), in the presence or absence of MPA (5 µm). The photographs of the gels from a representative of three separate experiments are presented. The results of RT-PCR analysis, presented as fold increase in iNOS (a) or IRF-1 (b) gene expression relative to the expression in untreated cells, are means ± s.d. of three independent experiments (*P < 0·01 refers to IFN/LPS treatment).
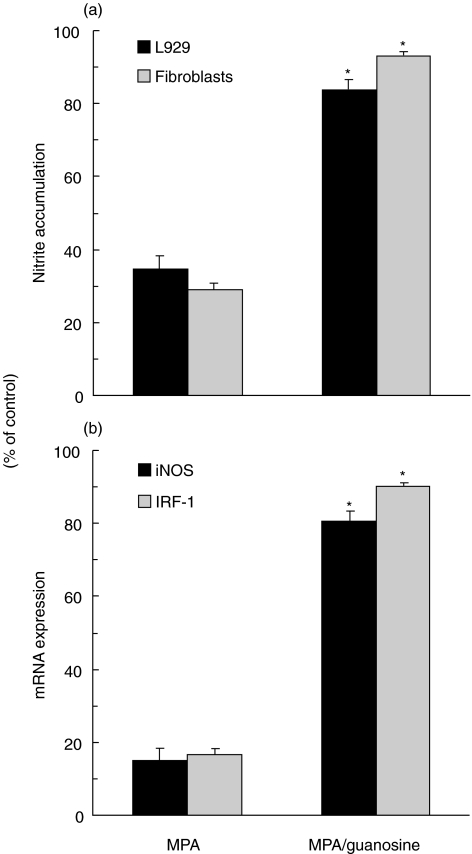
Guanosine blocks MPA's effect on fibroblast iNOS and IRF-1 expression
Finally, we assessed the involvement of IMPDH inhibition in the observed effect of MPA on iNOS-mediated NO synthesis in fibroblasts. As the biological effects of MPA-mediated IMPDH inhibition are the consequence of the reduced pool of guanosine nucleotides, the ability of exogenous guanosine to counteract MPA action in fibroblasts was tested. Indeed, the addition of guanosine almost completely diminished the inhibitory effect of MPA on NO production, as well as the expression of iNOS and IRF-1 mRNA in IFN-γ + LPS-stimulated fibroblasts (Fig. 5). Guanosine itself did not stimulate either iNOS or IRF-1 expression in resting or IFN-γ + LPS-treated cells (data not shown), thus excluding the possibility that the observed effect of guanosine was unrelated to countervailing MPA action. Therefore, it appears that the inhibitory effect of MPA on fibroblast iNOS expression depends mainly on IMPDH inhibition and the subsequent reduction of guanosine nucleotide levels.
Fig. 5.
Guanosine prevents MPA inhibitory action on iNOS and IRF-1 expression in fibroblasts. IFN-γ (250 U/ml) + LPS (5 µg/ml)-stimulated L929 cells (a) and primary rat fibroblasts (a, b) were treated with MPA (5 µm), in the presence or absence of guanosine (100 µm). Results of nitrite measurement (a) and RT-PCR analysis of iNOS and IRF-1 gene expression (b) from one of three independent experiments with similar results are presented as percentage of control, relative to the values obtained in the absence of MPA and guanosine (*P < 0·01 refers to cultures without guanosine).
DISCUSSION
The results of the present study clearly demonstrate that immunosuppresant MPA, at concentrations well below nontoxic levels achievable in vivo[2], inhibits NO production in rodent fibroblasts, but not macrophages. The effect of MPA was probably mediated through reduction of IMPDH activity and subsequent down-regulation of the intracellular level of guanosine nucleotides, resulting in impaired activation of iNOS transcription factor IRF-1 and defective expression of the iNOS gene.
Together with the results by Senda et al., 1995 [6], our data indicate that MPA-mediated inhibition of IMPDH affects NO synthesis in fibroblasts and endothelial cells at different levels. The impairment of iNOS catalytic activity, as a result of BH4 deficiency, appears mainly responsible for the MPA action in endothelial cells [6]. Accordingly, in our hands, direct BH4 precursor sepiapterin was very efficient in reverting the inhibitory effect of MPA on NO production in rat endothelial cells (unpublished observation). However, MPA inhibition of NO release in fibroblasts was largely insensitive to exogenous supplementation of sepiapterin, while it was accompanied by a pronounced decrease of both iNOS mRNA and protein levels. It should be noted that these findings do not exclude the capacity of MPA to affect the activity of fibroblast iNOS through limitation of its essential co-factor BH4, but imply that such an effect would not be readily evident, since preceded by the drug-imposed blockade of iNOS expression. MPA-mediated down-regulation of iNOS mRNA expression was also observed in cytokine-stimulated astrocytes in vitro[18], as well as in renal cortical cells of lupus-prone MRL/lpr mice in vivo[19], thus confirming the drug's ability to interfere with the expression of iNOS gene in different cell types.
A co-ordinated binding of two transcription factors, NF-κB and IRF-1, to their consensus sequences in the iNOS promoter is necessary for optimal iNOS transcription [20]. Unlike NF-κB, IRF-1 is activated mainly at the transcriptional level [21], and a striking down-regulation of IRF-1 mRNA by MPA provides a plausible explanation for the drug effect in the present study. Moreover, it indicates that a similar mechanism might be also responsible for the reduced renal iNOS mRNA expression in MPA-treated lupus mice, as the observed drug action was apparently independent of modulation of NF-κB pathway [19]. While LPS is a prototype NF-κB activator [22], IRF-1 is a principal mediator of IFN-γ intracellular actions [21]. It therefore seems conceivable to assume that MPA might exert its inhibitory action on fibroblast iNOS expression mainly through interference with IFN-γ-derived signals. Accordingly, our unpublished observations show that MPA can inhibit fibroblast NO production triggered by high dose of IFN-γ, in the absence of LPS co-stimulation. The finding that MPA-sensitive expression of iNOS in lupus mice [19] depends mostly on IL-12-induced IFN-γ[23] is also consistent with the putative interference of MPA with IFN-γ signal transduction. However, although the inability of MPA to affect fibroblast NO release when the transcription was blocked, supports transcriptional, possibly IRF-1-dependent iNOS regulation by the drug, the possibility of transcription-dependent drug interference with iNOS mRNA stability could not be completely excluded. The experiments intended to provide support for the putative causal relationship between MPA-mediated inhibition of IRF-1 and iNOS expression in fibroblasts are currently under way in our laboratory.
Guanosine, which acts as a guanosine nucleotide precursor in the salvage pathway [2], completely neutralized MPA-mediated inhibition of fibroblast NO release and restored both IRF-1 and iNOS expression. This strongly indicates that these MPA actions were exerted through inhibition of IMPDH activity and subsequent depletion of the intracellular pool of guanine nucleotides. While the assumption that IMPDH might be required for the optimal induction of IRF-1 and, subsequently, iNOS, is intriguing, there is a question of the intracellular mechanism possibly responsible for that connection. One plausible explanation is that MPA might interfere with guanosine nucleotide-dependent function of G-proteins, which are initiating members of mitogen-activated protein kinase (MAPK) signalling cascade. Indeed, MAPK signalling has recently been implicated in the induction of fibroblast iNOS [24,25], as well as IRF-1 in retinal epithelial cells or hepatocytes [26,27], while IMPDH inhibition was associated with reduced levels of GTP-associated G-protein p21ras and the subsequent impairment of MAPK activation [28]. We are currently investigating the hypothesis that MPA interference with IMPDH activity in fibroblasts might result in deficient MAPK activation, which could then lead to reduced expression of IRF-1 and iNOS.
Interestingly, both rat and mouse macrophages were absolutely resistant to MPA interference with iNOS expression and catalytic activity that was operative in fibroblasts or endothelial cells, respectively. This is unlikely to be due to macrophage insensitivity to MPA-mediated IMPDH inhibition, as MPA causes a rapid down-regulation of guanosine nucleotide content in monocytes [2]. While the possible difference in BH4 availability might account for the distinct MPA effect on the enzymatic activity of macrophage and endothelial iNOS, the absence of MPA influence on the expression of macrophage iNOS indicates that intracellular pathways controlling iNOS activation in fibroblasts and macrophages might differ. Although the data on MAPK involvement in the iNOS induction in macrophages are somewhat conflicting, several studies suggested that the activation macrophage iNOS might be relatively insensitive to MAPK inhibition [25,29–31], thus laying a credible ground for this hypothesis. With the mechanisms still remaining to be established, this cell-specific interference of MPA with iNOS-mediated NO release might have significant therapeutic implications. As macrophage-derived NO could play an important immunoregulatory role in limiting the activation of T cells in lymphoid organs [32,33], its suppression might not be always desirable in T-cell-mediated disorders such as autoimmunity and transplant rejection. Alternatively, selective down-regulation of fibroblast iNOS by MPA might be sufficient to prevent excessive NO release and cell damage in the target tissue, such as inflamed joints in rheumatoid arthritis or parenchymal tissue of the grafted organ. Nevertheless, it is dependent on future studies to confirm whether the interference with iNOS-mediated NO production could contribute to MPA effects in rheumatoid arthritis or the therapy of allograft rejection. Exploring the effect of MPA on NO synthesis in other cell types relevant for the pathology of these inflammatory conditions, such as synoviocytes or chondrocytes, would be valuable for achieving that goal.
References
- 1.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215–45. doi: 10.1016/s0162-3109(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 2.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanism of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 3.Schiff M. Emerging treatments for rheumatoid arthritis. Am J Med. 1997;102:11–5. doi: 10.1016/s0002-9343(97)00411-7. [DOI] [PubMed] [Google Scholar]
- 4.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage fuction. Ann Rev Immunol. 1997;10:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2407–13. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senda M, Delustro B, Eugui E, Natsumeda Y. Mycophenolic acid, an inhibitor of IMP dehydrogenase that is also an immunosuppressive agent, suppresses the cytokine-induced nitric oxide production in mouse and rat vascular endothelial cells. Transplantation. 1995;60:1143–8. doi: 10.1097/00007890-199511270-00015. [DOI] [PubMed] [Google Scholar]
- 7.Amin AR, Attur M, Abramson SB. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol. 1999;11:202–9. doi: 10.1097/00002281-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 8.McInnes IB, Leung BP, Field M, Wei XQ, Huang FP, Sturrock RD, Kinninmonth A, Weidner J, Mumford R, Liew FY. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–24. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffer MR, Efron PA, Thornton FJ, Klingel K, Gross SS, Barbul A. Nitric oxide, an autocrine regulator of wound fibroblast synthetic function. J Immunol. 1997;158:2375–81. [PubMed] [Google Scholar]
- 10.Witte MB, Thornton FJ, Efron DT, Barbul A. Enhancement of fibroblast collagen synthesis by nitric oxide. Nitric Oxide. 2000;4:572–82. doi: 10.1006/niox.2000.0307. [DOI] [PubMed] [Google Scholar]
- 11.Romanska HM, Ikonen TS, Bishop AE, Morris RE, Polak JM. Up-regulation of inducible nitric oxide synthase in fibroblasts parallels the onset and progression of fibrosis in an experimental model of post-transplant obliterative airway disease. J Pathol. 2000;191:71–7. doi: 10.1002/(SICI)1096-9896(200005)191:1<71::AID-PATH560>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Pechold K, Patterson NB, Craighead N, Lee KP, June CH, Harlan DM. Inflammatory cytokines IFN-gamma plus TNF-alpha induce regulated expression of CD80 (B7–1) but not CD86 (B7–2) on murine fibroblasts. J Immunol. 1997;158:4921–9. [PubMed] [Google Scholar]
- 13.Trajkovic V, Stepanovic S, Samardzic T, Jankovic V, Badovinac V, Mostarica Stojkovic M. Cryptococcus neoformans neutralizes macrophage and astrocyte derived nitric oxide without interfering with inducible nitric oxide synthase induction or catalytic activity – possible involvement of nitric oxide consumption. Scand J Immunol. 2000;51:384–91. doi: 10.1046/j.1365-3083.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Versteeg HH, Nijhuis E, Van Den Brink GR, Evertzen M, Pynaert GN, Van Deventer SJ, Coffer PJ, Peppelenbosch MP. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J. 2000;3:717–22. [PMC free article] [PubMed] [Google Scholar]
- 16.Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, McDaniel ML, Williamson JR, Currie MG. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 17.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh SI, Kimura T, Green SJ, Mak TW, Taniguchi T, Vilcek J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–5. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 18.Miljkovic D, Samardzic T, Cvetkovic I, Mostarica Stojkovic M, Trajkovic V. Mycophenolic acid down-regulates inducible nitric oxide synthase induction in astrocytes. Glia. 2002;39:247–55. doi: 10.1002/glia.10089. [DOI] [PubMed] [Google Scholar]
- 19.Yu CC, Yang CW, Wu MS, Ko YC, Huang CT, Hong JJ, Huang CC. Mycophenolate mofetil reduces renal cortical inducible nitric oxide synthase mRNA expression and diminishes glomerulosclerosis in MRL/lpr mice. J Laboratory Clin Med. 2001;138:69–77. doi: 10.1067/mlc.2001.115647. [DOI] [PubMed] [Google Scholar]
- 20.Saura M, Zaragoza C, Bao C, McMillan A, Lowenstein CJ. Interaction of interferon regulatory factor-1 and nuclear factor kappaB during activation of inducible nitric oxide synthase transcription. J Mol Biol. 1999;289:459–71. doi: 10.1006/jmbi.1999.2752. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–55. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 23.Huang FP, Feng GJ, Lindop G, Stott DI, Liew FY. The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. J Exp Med. 1996;83:1447–59. doi: 10.1084/jem.183.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Brecher P. Salicylate inhibition of extracellular signal-regulated kinases and inducible nitric oxide synthase. Hypertension. 1999;34:1259–64. doi: 10.1161/01.hyp.34.6.1259. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic V, Samardzic T, Stosic-Grujicic S, Popadic D, Trajkovic V. Cell-specific inhibition of inducible nitric oxide synthase activation by leflunomide. Cell Immunol. 2000;199:73–80. doi: 10.1006/cimm.1999.1600. [DOI] [PubMed] [Google Scholar]
- 26.Faure V, Hecquet C, Courtois Y, Goureau O. Role of interferon regulatory factor-1 and mitogen-activated protein kinase pathways in the induction of nitric oxide synthase-2 in retinal pigmented epithelial cells. J Biol Chem. 1999;274:4794–800. doi: 10.1074/jbc.274.8.4794. [DOI] [PubMed] [Google Scholar]
- 27.Varley CL, Dickson AJ. Hepatocyte isolation stimulates formation of interferon stimulatory response element DNA-protein complexes. Biochem Biophys Res Commun. 1999;263:627–31. doi: 10.1006/bbrc.1999.1431. [DOI] [PubMed] [Google Scholar]
- 28.Vallee S, Fouchier F, Brauger D, Marvaldi J, Champion S. Ribavirin-induced resistance to heat shock, inhibition of the Ras-Raf-1 pathway and arrest in G(1) Eur J Pharmacol. 2000;404:49–62. doi: 10.1016/s0014-2999(00)00596-3. [DOI] [PubMed] [Google Scholar]
- 29.Caivano M. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 1998;429:249–53. doi: 10.1016/s0014-5793(98)00578-x. [DOI] [PubMed] [Google Scholar]
- 30.Chan ED, Winston BW, Uh ST, Wynes MW, Rose DM, Riches DW. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-gamma and TNF-alpha in mouse macrophages. J Immunol. 1999;162:415–22. [PubMed] [Google Scholar]
- 31.Paul A, Cuenda A, Bryant CE, Murray J, Chilvers ER, Cohen P, Gould GW, Plevin R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell Signal. 1999;11:491–7. doi: 10.1016/s0898-6568(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 32.Bogdan C. The multiplex function of nitric oxide in (auto) immunity. J Exp Med. 1998;187:1361–5. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew FY. Regulation of lymphocyte functions by nitric oxide. Curr Opin Immunol. 1995;7:396–9. doi: 10.1016/0952-7915(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 34.Schoedon G, Schneemann M, Hofer S, Guerrero L, Blau N, Schaffner R. Regulation of the 1-arginine-dependent and tetrahydrobiopterin-dependent biosynthesis of nitric oxide in murine macrophages. Eur J Biochem. 1993;213:833–9. doi: 10.1111/j.1432-1033.1993.tb17826.x. [DOI] [PubMed] [Google Scholar]