Abstract
The tryptic FAD-peptide carrying the flavin in 8α-(N3)histidyl linkage as natural hapten was isolated by HPLC from the bacterial enzyme 6-hydroxy-d-nicotine oxidase. The same flavin protein linkage is found in the mitochondrial succinate dehydrogenase flavoprotein subunit, the predominant flavoprotein with covalently bound FAD in mitochondria of cardiomyocytes. Peripheral blood mononuclear cells (PBMC) were isolated from four patients with acute myocarditis, seven patients with dilated cardiomyopathy (DCM) and from four healthy control individuals. The response of PBMC to the FAD-peptide was evaluated by measuring proliferation ([3H]-dThd incorporation) and cytokine secretion [interferon (IFN)-γ]. PBMC from all patients with acute myocarditis showed positive responses to the FAD-peptide, in contrast to PBMC from patients with DCM or control individuals. Following the recovery of the patients from the acute inflammation of the heart, PBMC no longer exhibited a proliferation response to the FAD-peptide. A chemically synthesized FAD-free peptide with identical amino acid sequence induced no response of PBMC. The results are consistent with a recall response by activated T cells, specific for the normally cryptic mitochondrial flavin-hapten, which may be liberated following cardiomyocyte destruction during the inflammation of the heart.
Keywords: FAD-peptide, flavin-hapten, flavoproteins, hapten-specific T cells, myocarditis
INTRODUCTION
Haptens, low molecular weight organic molecules not immunogenic by themselves, when bound to carrier proteins induce a strong immune response in immunized animals. Di- and trinitrophenyl (TNP) derivatives, attached covalently to lysine residues of albumin as carrier protein, are presented in the peptide-binding groove of MHC-I and MHC-II complexes [1]. The MHC-restricted contacts between haptens such as TNP and the corresponding T cell receptors (TCR) reflect closely the contacts between TCR and nominal peptide antigens [2–4].
Drugs such as penicillins and sulphonamides, that are often responsible for allergic reactions, may be immunogenic due to their hapten feature. Thus, the β-lactam ring of penicillin opens spontaneously under physiological conditions and the formed penicilloyl can bind covalently to lysine residues of proteins. This may lead to the presentation of β-lactam-modified peptides to T cells [5]. Drug-specific T cell clones from allergic individuals are MHC-restricted CD4+ or CD8+ cells [6].
All organisms contain naturally occurring haptens in the form of enzyme cofactors bound covalently to their apoproteins. Well-known examples are biotin in carboxylases, lipoic acid in mitochondrial 2-oxo-acid dehydrogenase complexes and flavin adenine dinucleotide (FAD) in the mitochondrial succinate dehydrogenase complex of the citric acid cycle (also known as respiratory chain complex II). Serum antibodies directed against endogenous haptens have been reported in humans and an increased incidence of these autoantibodies has been correlated to specific pathological states. Thus, serum antibodies against the lipoamide moiety of the E3 subunit of oxo-acid dehydrogenases are diagnostic in primary biliary cirrhosis [7,8]. An increased incidence of antimitochondrial antibodies (AMA) directed against the covalently attached flavin moiety of flavoenzymes has been described in patients with myocarditis and dilated cardiomyopathy (anti-M7 sera) [9,10]. These antiflavoprotein (αFp) antibodies have been shown to be flavin-specific as well as flavin-peptide-specific [11], characteristic for a hapten induced immune response. αFp antibodies have also been found in other muscular diseases, e.g. in occular myasthenia gravis, and were shown to be directed against the flavoprotein(Fp)-subunit of the succinate dehydrogenase (SucDH) complex [12]. Therefore the presence of these antibodies in the serum of patients may be a more general indication of a degenerative process affecting muscle cells.
The most common type of covalent attachment of FAD to apoproteins is by a riboflavin-8α-(N3)histidyl linkage [13]. This type of linkage is characteristic for the SucDH-Fp subunit of mitochondria, as well as for the bacterial enzyme 6-hydroxy-d-nicotine oxidase (6HDNO), used as model covalent flavoprotein in previous studies [11,14]. The most common cause of a myocarditis is a viral infection, frequently with viruses of the Coxsackie group. If α-Fp antibodies were induced following myocyte destruction associated with the inflammation of the myocard and liberation of mitochondrial antigens, one would expect to detect them in the serum of mice infected with Coxsackie virus B3. Indeed, mice infected with this virus present α-Fp antibodies in their sera [15].
In the context of the viral infection of the heart, mitochondrial protein complexes may be liberated from cardiomyocytes, taken up by APC and presented on MHC-complexes to T cells. Among the presented peptides may be peptides carrying as natural hapten the covalently bound flavin, which may induce flavin-specific T cell responses. We tested the hypothesis of the presence of FAD-peptide reactive T lymphocytes under the conditions of a recall response in vitro, using peripheral blood mononuclear cells (PBMC) from patients with myocarditis, dilated cardiomyopathy and healthy controls. Here we show for the first time, to our knowledge, that PBMC isolated during the acute phase of myocarditis will respond to this natural hapten with proliferation and the secretion of IFN-γ, a typical T cell cytokine.
MATERIALS AND METHODS
Patients
Four patients with acute myocarditis, m1 to m4 (males aged 48, 52, 58 and 60 years), seven patients with idiopathic dilated cardiomyopathy (DCM) (two females and five males, aged 50–80 years) and four healthy controls (two females and two males, aged 35–48 years) volunteered as blood donors. Blood was obtained by consent of the patients and according to the guidelines of the ethic commission of the University of Freiburg. The diagnosis of acute myocarditis was based on the presence of at least four diagnostic criteria: (1) ubiquitous transient ST-segment changes; (2) transient elevation of cardiac troponin T; (3) elevation of C-reactive protein; and (4) demonstration of a normal coronary angiogram. All patients met all of these criteria. Based on strict clinical criteria, our patients had an unequivocal presentation of acute myocarditis. We therefore did not consider endomyocardial biopsy to corroborate our clinical diagnosis. The diagnosis of idiopathic dilated cardiomyopathy was based on demonstration of a global reduction in left ventricular function (left ventricular ejection fraction below 40% on echocardiography or left ventricular angiography), absence of laboratory signs of a systemic inflammatory response, absence of significant coronary artery disease on coronary angiography and absence of a history or suspected history of secondary causes for reduced left ventricular function (e.g. previous myocarditis, alcohol abuse, hypertension).
The sera of the patients with acute myocarditis and two of the seven sera from patients with idiopathic DCM gave a characteristic immunoreaction with mitochondrial antigens when tested in immunoblots [14].
Isolation of PBMC
PBMC were isolated from freshly drawn blood by centrifugation on Ficoll-Hypaque as recommended by the supplier (Sigma, Germany). The cells (viability> 95%) were resuspended in RPMI-1640 tissue culture medium supplemented with 5% human serum and placed in 96-well flat-bottomed plates at a density of 1 × 105 cells/100 µl.
Protease digestion of 6HDNO and isolation of the FAD-peptide by HPLC
One mg of affinity purified 6HDNO was digested in 40 mm HEPES buffer, pH 7·4, 0·5 m NaCl, at 37°C for 4 h in the dark with sequencing grade modified trypsin (Promega, Germany). Trypsin digestion generates a 21-amino acid FAD-peptide from 6HDNO, corresponding to the amino acid residues 68–88 of the flavoprotein. The trypsin digested samples (0·2 mg protein) were applied to an analytical C18 HPLC Column (300 A°, 5 µm, 250 × 4·4 mm; Pharmacia, Germany). The samples were eluted with a flow rate of 1 ml/min and fractionated using a linear gradient from 100% eluent A (0·01% TFA)/0% eluent B (70% acetonitril, 0·01 TFA) to 0% eluent A/100% eluent B for 60 min. The absorption at 440 nm was registered and the peak corresponding to the FAD-peptide collected, lyophilized and the pellet dissolved in lipopolysaccharide (LPS)-free water (Acila, Walldorf, Germany). The FAD-peptide solution was tested for LPS contamination with the aid of the limulus amebocyte lysate assay (Pyroquant Diagnostik, Walldorf). The peptide preparation was kept at −20°C until use.
In vitro stimulation of PBMC
PBMC were incubated at 37°C, 5% CO2 either alone or in the presence of the following additions: FAD-peptide (100 ng/ml), PHA (1 µg/ml), tetanus toxoid (TT, 10 µg/ml) or staphylococcal enterotoxin B (SEB, 100 ng/ml) (all from BD Biosciences, Heidelberg, Germany). Each group consisted of six individual cultures.
Assays for proliferation and cytokine secretion
Proliferation was measured by [3H]-dThd incorporation. Seven days after the initiation of the in vitro stimulation 0·5 µCi [3H]-dThd (Amersham Life Science, UK) was added to each well. Plates were harvested 18 h later (Wallac 1295–001 Cell Harvester) and the radioactivity incorporated into the DNA was assessed on a Betaplate counter (Wallac, Turku, Finland). Data are presented as stimulation index (SI = cpm of PBMC incubated with additive/cpm of PBMC incubated without additive).
SN were harvested after 7 days. IFN-γ secreted during this time was quantified by ELISA as described by the manufacturer (Pharmingen, Heidelberg, Germany).
RESULTS
Isolation of the FAD-peptide of 6HDNO
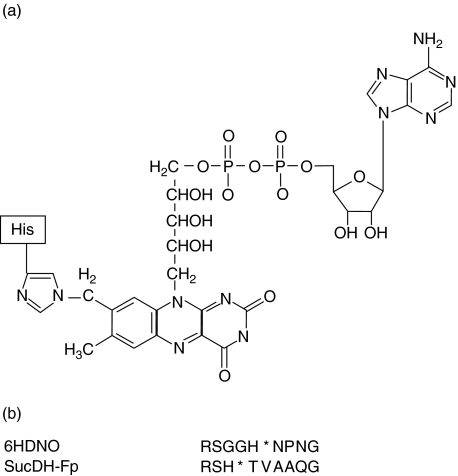
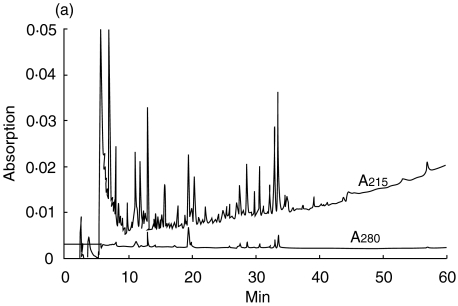
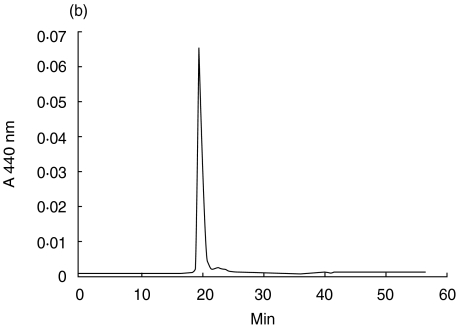
The 21 amino acid long tryptic FAD-peptide of 6HDNO with the sequence SGGHNPNGYATNDGGIVLDLR was isolated by HPLC as described in Material and methods. FAD is attached to His71 (bold, underlined). The structure of the FAD-hapten bound via an 8(α)-(N3)histidyl to the peptide is presented in Fig. 1a. This structure is the same in 6HDNO and the mitochondrial mammalian flavoenzymes. Figure 1b shows the alignment of the amino acid residues surrounding the FAD-binding histidine in 6HDNO and in SucDH-Fp. Besides the His-FAD, the residues ArgSer and Gly are conserved in all SucDH-FP subunits as well as in 6HDNO. The conserved ArgSer residues are separated from the His-FAD by two Gly residues in 6HDNO. A typical HPLC elution profile of the tryptic peptides of 6HDNO, monitored at 215 nm, the absorption maximum of the peptide bond, and at 280 nm, the absorption maximum of aromatic amino acids, is shown in Fig. 2a. The peptide eluting at 20 min was identified by its absorption at 440 nm as the FAD-peptide, collected and rechromatographed (Fig. 2b). The resulting preparation was essentially pure FAD-peptide. N-terminal sequencing of the isolated FAD-peptide confirmed its identity with the predicted tryptic FAD-peptide of 6HDNO. The 6HDNO preparation and the FAD-peptide preparation when tested by the limulus test for the presence of LPS turned out to be LPS-free (not shown).
Fig. 1.
Structure of the naturally occurring FAD-hapten. (a) Covalent attachment of FAD via the 8α-group of the izoaloxazine ring of riboflavin to the N3 nitrogen of a histidine residue of the polypeptide chain. (b) Alignment of the amino acid residues surrounding the FAD-binding His (indicated by a star) of the bacterial flavoenzyme 6HDNO and of the mitochondrial SucDH Fp subunit.
Fig. 2.
HPLC purification of the FAD-peptide of 6HDNO. The tryptic digest of 6HDNO and the isolation of the FAD-peptide was performed as described in Material and methods. (a) Tryptic peptide pattern of 6HDNO monitored at 215 nm and 280 nm; (b) the yellow-coloured peptide fraction eluting at 20 min in (a) was rechromatographed and showed a typical flavin absorption at 440 nm.
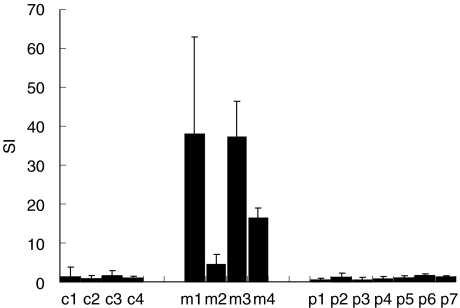
Only PBMC from patients with myocarditis obtained during the acute phase are stimulated to proliferate by the FAD-peptide
PBMC were isolated from patients with acute myocarditis, with DCM or from healthy control people. PHA, TT and SEB were employed as positive proliferation controls, and resulted in positive stimulation indices in all cases. The FAD-peptide stimulated PBMC from all four patients with acute myocarditis, resulting in stimulation indices (SI) between 4·5 and 37·3 (Fig. 3, m1–m4). Neither PBMC of seven patients with DCM nor PBMC of four controls showed any proliferative response to the FAD-peptide (Fig. 3, p1–p7 and c1–c4, respectively). PBMC of patient m1 were retested for proliferation 2, 4 and 6 months and of patient m2 6 months after the acute phase of myocarditis. At this time the FAD-peptide no longer induced any proliferative response (Fig. 4). As the control responses of patients m1 and m2 to PHA, SEB and TT were comparable at all time-points (not shown), we conclude that the stimulation by the FAD-peptide is correlated directly to the acute phase.
Fig. 3.
Proliferation of PBMC stimulated by the FAD-peptide. PBMC isolated from the blood of controls (c1–c4), of patients with acute myocarditis (m1–m4) or of patients with DCM (p1–p7) were incubated with FAD-peptide for 7 days. Proliferation was determined by [3H]-dThd incorporation and the SI calculated. Given are means and standard deviations.
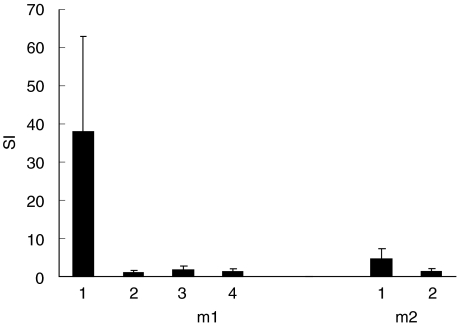
Fig. 4.
Time-dependent proliferative responses of PBMC from two patients with myocarditis to the FAD-peptide. PBMC were isolated repeatedly from the myocarditis patients m1 and m2 and stimulated with the FAD-peptide. The proliferation response was measured by [3H]-dThd incorporation and the SI calculated. m1, 1: SI of PBMC isolated during the acute phase of the myocarditis; m1, 2, 3 and 4: SI of PBMC isolated 2, 4 and 6 months, respectively, after the recovery of the patient from the acute myocarditis; m2, 1: SI of PBMC isolated during the acute phase of the myocarditis; m2, 2: SI of PBMC isolated 6 months after the recovery of the patient from the acute phase of the myocarditis.
The stimulation induced by the FAD-peptide requires the FAD-hapten
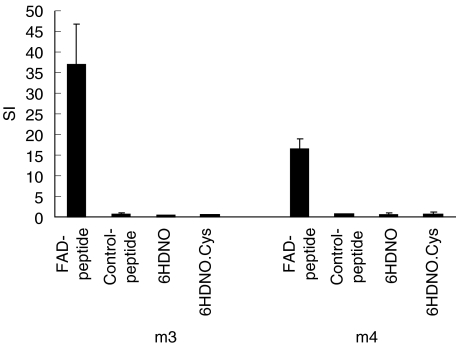
A flavin-free peptide of identical amino acid sequence to the tryptic 6HDNO FAD-peptide was synthesized chemically. 6HDNO and a 6HDNO mutant protein (6HDNO.Cys) with the FAD-binding His71 replaced by Cys, which no longer binds FAD covalently, were produced recombinantly and purified as 6-His tagged proteins. PBMC isolated from m3 and m4 were tested for proliferation in the presence of the FAD-peptide (compare Fig. 3), the FAD-free synthetic peptide, 6HDNO and the FAD-free 6HDNO.Cys mutant. In both experiments (patient m3 and m4) only the FAD-peptide induced a proliferative response of PBMC (Fig. 5).
Fig. 5.
Flavin-hapten-dependent proliferation response of PBMC from two patients with acute myocarditis. PBMC from patients m3 and m4 were stimulated with the FAD-peptide, the FAD-free synthetic peptide with the same amino acid sequence, 6HDNO and the FAD-free 6HDNO.Cys mutant protein. Data are expressed as SI (means and standard deviation).
The FAD-peptide induces PBMC to secrete IFN-γ, as does tetanus toxoid, a well-established recall antigen
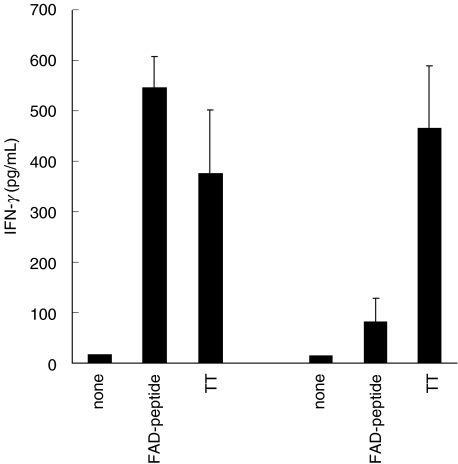
Supernatants (SN) of the experiments with patients m3 and m4 were taken on day 7 of the culture period and assayed for IFN-γ. The results correlated very well with the data from the proliferation assay, as the stimulation with the FAD-peptide and the positive controls PHA, SEB and TT induced the secretion of IFN-γ, whereas medium alone and the synthetic control peptide, 6HDNO and 6HDNO.Cys did not (Fig. 6). As IFN-γ is secreted most often by T lymphocytes upon antigen-specific stimulation, we interpret this result as an indication for the activation of FAD-hapten-specific T lymphocytes present in PBMC from patients with acute myocarditis.
Fig. 6.
FAD-peptide-induced secretion of IFN-γ. PBMC of patients m3 and m4 were stimulated as described in the legend to Fig. 5 (same experiments). SN were taken on day 7 and the amount of IFN-γ quantified. Negative groups had values below the limit of detection (15·6 pg/ml: medium only, addition of the synthetic peptide, 6HDNO or 6HDNO.Cys), positive controls included PHA and SEB (both> 700 pg/ml). Means and standard deviation are given.
DISCUSSION
The observed FAD-peptide specific in vitro activation of PBMC from patients with acute myocarditis suggests the existence of autoreactive T lymphocytes with specificity for autologous intracellular haptens. We hypothesize that haptens such as the FAD-hapten might enter the pathway leading to antigen processing and presentation under the special conditions of a disease. An acute inflammation of the heart may represent such a condition and lead to the presentation of previously cryptic epitopes, e.g. from the flavoprotein subunit of the SucDH complex, either by heart muscle cells themselves or by professional APC. These APC present the hapten, e.g. FAD, anchored via a peptide, and induce hapten-specific T lymphocytes. Such T lymphocytes enter the circulation and respond in vitro to the FAD-hapten even if bound to another yet related peptide.
Our data suggest that T lymphocytes proliferate in vitro and synthesize IFN-γ, but still are without formal proof. The effects are specific for the FAD-hapten, and are observed only in patients with acute myocarditis during a restricted time window, indicating that the presence of a specific cell is required. Both parts of the FAD-peptide do not induce any reactions, as long as they are added to the system independently of each other. The synthetic peptide alone is not sufficient, nor is the presence of the FAD-hapten, e.g. on the complete bacterial enzyme 6HDNO. The most probable interpretation is that APC directly binding the peptide allow recognition of the FAD-hapten by specific T lymphocytes, whereas the processing of 6HDNO does not result in presentation of the FAD-hapten. It therefore appears highly probable that the proliferation induced by the presence of the FAD-peptide represents a recall response by previously activated T cells.
The structure of the flavin-hapten–histidine complex is the same in the FAD-peptide isolated from 6HDNO and the Fp subunit of SucDH, although the amino acid sequences flanking the hapten are similar but not identical. The way in which the flavin-peptide is presented by APC during the induction of the T cells is not known and remains to be established. The pyrophosphate group of FAD is labile to acidic conditions and may be cleaved by pyrophosphatases ubiquitously present in cells. Therefore we assume that the adenine part of FAD will be removed during processing of the epitope. In this context it may be worth mentioning that the αFp-antibody in human sera were shown to be specific for the riboflavin part of FAD and not for the AMP moiety of the molecule [11].
The myocarditis patients presented αFp-antibodies. When two of these patients were retested 6 months after they fell ill, αFp-antibodies were still present in their sera. Unfortunately it was not possible (because of the scarcity of myocarditis patients) to follow systematically over time the persistence of the αFp-antibodies in these patients. The presence of αFp-antibodies in some patients with idiopathic DCM may indicate that under this condition an immune response including B and T lymphocytes also occurs. In patients with IDM the appearance of specific T cells in the periphery was restricted to a narrow time window. Specific T cells responsible for the αFp-antibody response in DCM patients were probably not detected because our assays missed the right time and compartment. At this stage our investigation is limited by the lack of histopathological data diagnostic of myocardial inflammation. Clearly, these results have to be extended and supported in future studies by the histopathological analysis of myocardial biopsies taken from patients with myocarditis and DCM in order to define the time-course of the T cell and the αFp-antibody response.
In conclusion, we demonstrate for the first time the presence of a cellular immune response, most probably performed by T lymphocytes, specific for the natural flavin-hapten of mitochondrial proteins in the conditions of a disease.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to RB and IM.
References
- 1.Bour H, Peyron E, Gaucherand M, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–10. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 2.Von Bonin A, Plaga S, Ruh H, et al. Analysis of major histocompatibility complex class I-restricted hapten recognition by mutation of the V–J joining of T cell receptor alpha chains. Eur J Immunol. 1996;26:179–86. doi: 10.1002/eji.1830260128. [DOI] [PubMed] [Google Scholar]
- 3.Luescher IF, Anjuere F, Peitsch MC, Jongeneel CV, Cerottini JC, Romero P. Structural analysis of TCR–ligand interactions studied on H-2Kd-restricted cloned CTL specific for photoreactive peptide derivative. Immunity. 1995;3:51–63. doi: 10.1016/1074-7613(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 4.Kohler J, Martin S, Pflugfelder U, Ruh H, Vollmer J, Weltzien HU. Cross-reactive trinitrophenylated peptides as antigens for class II major histocompatibility complex-restricted T cells and inducers of contact sensitivity in mice. Limited T cell receptor repertoire. Eur J Immunol. 1995;25:92–101. doi: 10.1002/eji.1830250118. [DOI] [PubMed] [Google Scholar]
- 5.Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziger T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J Immunol. 1995;155:462–72. [PubMed] [Google Scholar]
- 6.Brander C, Mauri-Hellweg D, Bettens F, Rolli H, Goldman M, Pichler WJ. Heterogeneous T cell responses to beta-lactam-modified self-structures are observed in penicillin-allergic individuals. J Immunol. 1995;155:2670–8. [PubMed] [Google Scholar]
- 7.Maeda T, Loveland BE, Rowley MJ, Mackay IR. Autoantibody against dihydrolipoamid dehydrogenase, the E3 subunit of the 2-oxoacid dehydrogenase complexes: significance for primary biliary cirrhosis. Hepatology. 1991;14:994–9. [PubMed] [Google Scholar]
- 8.Berg PA, Klein R. Antimitochondrial antibodies in primary biliary cirrhosis and other disorders: definition and clinical relevance. Dig Dis. 1992;10:85–101. doi: 10.1159/000171347. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Maisch B, Kochisek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1984;58:283–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Klein R, Berg PA. Anti-mitochondrial antibodies (anti-M7) in heart diseases recognize epitopes on bacterial and mammalian sarcosine dehydrogenase. Clin Exp Immunol. 1990;82:289–93. doi: 10.1111/j.1365-2249.1990.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stähle I, Brizio C, Barile M, Brandsch R. Anti-mitochondrial flavoprotein autoantibodies of patients with myocarditis and dilated cardiomyopathy (anti–M7): interaction with flavin-carrying proteins, effect of vitamin B2 and epitope mapping. Clin Exp Immunol. 1999;115:404–8. doi: 10.1046/j.1365-2249.1999.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunji K, Skolnik C, Bednarczuk T, et al. Eye muscle antibodies in patients with ocular myasthenia gravis: possible mechanism for eye muscle inflammation in acetylcholine-receptor antibody-negative patients. Clin Immunol Immunopahtol. 1998;87:276–81. doi: 10.1006/clin.1998.4536. [DOI] [PubMed] [Google Scholar]
- 13.Decker K, Brandsch R. Flavoproteins with a covalent histidyl (N3)-8α-riboflavin linkage. Biofactors. 1991;3:69–81. [PubMed] [Google Scholar]
- 14.Otto A, Stähle I, Klein R, Berg PA, Pankuweit S, Brandsch R. Anti-mitochondrial antibodies in patients with dilated cardiomyopathy (anti-M7) are directed against flavoenzymes with covalently bound FAD. Clin Exp Immunol. 1998;111:541–7. doi: 10.1046/j.1365-2249.1998.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicek G, Vuorinen T, Stähle P, Stepanek P, Freudenberg N, Brandsch R. Coxsachievirus B3 infection induces anti-flavoprotein antibodies in mice. Clin Exp Immunol. 2000;122:404–9. doi: 10.1046/j.1365-2249.2000.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]