Abstract
The high number of γ/δ-expressing T cells found in the epithelial lining layer suggests that they form a first line of defence against invading pathogens. To evaluate the role of γ/δ T cell-receptor (TCR)-expressing cells in cutaneous infection caused by Staphylococcus aureus, mice lacking γ/δ-expressing T cells (TCRδ−/−) were inoculated intradermally with S. aureus, and compared with S. aureus-infected congeneic TCRδ+/− control mice. The number of bacteria recovered from the skin of TCRδ−/− mice was significantly higher (P = 0·0071) at early time-points after inoculation compared to the number of bacteria isolated from infected TCRδ+/− congeneic controls. Nevertheless, inflammatory responses measured as serum IL-6 levels, were significantly lower in TCRδ−/− mice than in the control group. A possible explanation for this discrepancy was the observation of significantly decreased overall numbers of infiltrating cutaneous T lymphocytes, which are important producers of IL-6. These results support the notion that the γ/δ-expressing T cells that reside at the epithelial lining layer of the skin is of importance for early containment of the bacteria, thereby limiting their replication and spread.
Keywords: dermatitis, gamma/ delta TCR, mice, Staphylococcus aureus
INTRODUCTION
Recent experiments have demonstrated that gamma/delta (γ/δ) T cell-receptor (TCR)-expressing cells are involved in the regulation and resolution of inflammatory processes that are associated with infectious diseases and autoimmunity. Nevertheless, the physiological role of γ/δ T cells is not fully understood. γ/δ T cells have been shown to accumulate at sites of inflammation that are associated with certain viral and parasitic infections, such as malaria and Toxoplasma gondii[1]. γ/δ T lymphocytes also accumulate in certain human infectious diseases that are associated with granulomatous responses, such as the reactive lesions of leprosy and cutaneous leishmaniasis [2]. Experimental infection with intracellular bacteria, such as Listeria monocytogenes, has indicated that γ/δ T lymphocytes may be involved in the regulation of the infection by suppressing the formation of liver lesions [3]. In addition, experimental infection with Nocardia asteroides showed an essential role for intraepithelial γ/δ lymphocytes in the survival of the host [4].
The majority of T cells bear the Tcell-receptor (TCR) alpha/beta complex (α/β) that recognizes antigen peptides only in the context of self major histocompatibility complex (MHC) molecules [5]. In man, γ/δ TCR lymphocytes constitute on average 5% of all the T cells in normal peripheral blood [6], in organized lymphoid organs and in the skin- and gut-associated lymphoid tissues [2]. Most human γ/δ T cells display the Vγ9Vδ2 rearrangement and lack the expression of both CD4 and CD8 accessory molecules. These T cells constitute a small subset both in the neonatal and adult thymus, but the population expands with age in the peripheral blood, which suggests that positive selection occurs in the periphery following sustained antigenic stimulation [7,8].
In the mouse, γ/δ T cell populations with distinct clonal compositions have been found in the epidermis, intestine, lung, female reproductive tract, tongue and lactating mammary gland. In the murine epidermis, the vast majority of T lymphocytes express TCR γ/δ rather than TCR α/β. In the mouse epidermis, the γ/δ T cells which are also known as Thy-1+ dendritic epidermal T cells (DETCs) [9,10], expresses essentially a single type of γ/δ heterodimer [11], which is known as the junctionally monomorphic Vγ5/Vδ1+ TCR [12]. The γ/δ T cells vary in density between different skin sites and different strains [9], with values as high as 580 cells/mm2 in ear epidermis, measured in whole mounts of epidermis (horizontal sections). Precursors of DETC appear primarily in the fetal liver and traverse the fetal thymus as a single wave of cells on their way to the epidermis. At later time-points in thymic ontogeny, cells that express other subsets of γ/δ receptors appear in similar waves but migrate preferentially to other tissues [13–15]. The DETCs that appear in the skin cannot be replaced in the adult animal by new precursors that express the same subset of γ/δ TCR receptors, because the generation of DETC precursors is restricted to specific stages of fetal development. In animals that congenitally lack these γ/δ DETC, the epidermis is populated by bone marrow-derived TCR α/β, CD8+ DETCs [16].
To investigate the role of γ/δ TCR-expressing lymphocytes in cutaneous staphylococcal infection, we compared bacterial clearance in γ/δ TCR knock-out (TCRδ−/−) mice and heterozygous littermate control mice (TCRδ+/−) using a recently described model of cutaneous S. aureus infection [17,18]. Our results indicate that resident cutaneous γ/δ T cells act as an early antibacterial defence.
MATERIALS AND METHODS
Mice
Male mice of mixed (129 × C57BL/6) background, which lacked γ/δ T cells as a result of targeted germline mutation in the TCRD gene [19], were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mutated TCRD locus was back-crossed onto the DBA/1 (originally from the Jackson Laboratory) background for six generations. By subsequent intercrossing of mice that were either heterozygous or homozygous for the mutated TCRD, we obtained both homozygous (γ/δ T cell-deficient) or heterozygous (normal T cell phenotype) offspring littermates. The different T cell phenotypes were determined by flow cytometry analysis of blood cells, using a monoclonal antibody (GL3) that was specific for TCRγδ.
Female, 12–26-week-old DBA/1 mice, were housed in the animal facility of the Department of Rheumatology and Inflammation Research, University of Göteborg, under standard conditions of light and temperature and fed standard laboratory chow and water ad libitum.
Bacterial strain
The DBA/1 mice were inoculated intracutaneously with S. aureus LS-1 strain, which is harboured naturally on the skin of many mouse strains [20]. LS-1 strain has been shown to produce large amounts of toxic shock syndrome toxin 1 (TSST-1) and to be coagulase and catalase positive. The bacteria were kept frozen at − 20°C in 5% bovine serum albumin and 10% dimethylsulphoxide (C2H6OS) in phosphate buffered saline (PBS) (pH 7·4) until use. Just prior to use, the bacteria were thawed and washed in PBS. Viable counts were used to check the number of live bacteria in each bacterial solution.
Experimental protocol
Two experiments were performed using DBA/1 mice. The mice were inoculated with bacteria intracutaneously on the shaved back during neurolept analgesia (Dormicum®, Hoffmann-La Roche Ltd., Basel, Switzerland; Hypnorm®, Janssen Pharmaceuticals, Beerse, Belgium). In experiment I, 31 γ/δ T cell receptor knock-out mice (TCRδ−/−) and 31 congeneic control mice (TCRδ+/−) were inoculated at two sites with 0·1 ml of saline that contained either 2 × 108 or 2 × 107 colony-forming units (CFU) of S. aureus (a total of 4 × 108 or 4 × 107 CFU per mouse). In experiment II, 19 γ/δ T cell receptor knock-out mice (TCRδ−/−) and 19 control mice (TCRδ+/−) were inoculated at a single site with 1 × 108 CFU of S. aureus (a total of 1 × 108 CFU per mouse). The mice were monitored individually and sacrificed by cervical dislocation 2 days or 7 days after bacterial inoculation. The mice were evaluated clinically for local inflammatory reactions and weight development. In experiment I, skin samples corresponding to single injection sites on each mouse were dissected for histopathological and immunohistochemical evaluation at the time intervals indicated. Skin samples of the second injection site were resected for bacterial analysis. Blood samples were taken for bacterial counts, granulocyte counts, total white blood cell counts, interleukin-6 (IL-6) levels, immunoglobulins (IgG1, IgG2a, IgG3 and IgM), and for specific antibodies to staphylococcal cell walls and TSST-1 (see below).
In experiment II, skin samples from the injection site as well as blood samples were obtained for bacterial analysis at the time intervals indicated. Blood samples were also analysed for granulocyte counts and total white blood cell counts.
Histopathological examination
Skin samples from 62 mice (experiment I) were examined histopathologically after routine fixation and staining with haematoxylin–eosin. Blinded microscopical evaluation was carried out to characterize the size and density of the inflammatory infiltrates.
Immunohistochemical examination
Skin samples from the mice in experiment I were also analysed regarding the occurrence of CD11b+ cells (i.e. macrophages) and CD3+-expressing T cells. Briefly, skin samples were frozen in isopentane prechilled with liquid nitrogen, and kept at −70°C until cryosectioned. All the sections were fixed in cold acetone for 5 min and washed in PBS. The sections were incubated overnight in a humid atmosphere at + 4°C with unlabelled rat anti-CD11b (Mac-1; M1/70) [21] or hamster anti-CD3 (clone 145–2C11, PharMingen, San Diego, CA, USA) monoclonal antibodies [22], which were diluted in PBS containing 1% bovine serum albumin (BSA). After several washes, endogenous peroxidase was depleted by treatment with 0·3% H2O2 for 5 min. Biotin-labelled rabbit antirat Ig (Vector Laboratories, Burlingame, CA, USA) and mouse antihamster IgG (were a cocktail of clones G70-204 and G94-56; PharMingen) diluted in PBS–BSA were used as secondary antibodies. The binding of biotin-labelled secondary antibodies was detected by stepwise incubation with streptavidin–biotin-complex/HRP (Dako, Denmark) and 3-amino-9-ethyl-carbazole containing H2O2. All sections were counterstained with Meyer's haematoxylin.
Bacterial culture
Skin and blood samples were obtained for bacterial analysis. After surface disinfection with 70% alcohol, skin samples that corresponded to the injection site were deposited in sterile plastic bags, homogenized and suspended in 10 ml PBS. Skin suspensions were plated in appropriate dilutions on agar plates containing 5% horse blood and incubated at 37°C for 24 h. The number of CFUs per skin sample and per 100 µl of blood were counted and the bacterial colonies were tested for coagulase and catalase activity.
Serological analyses
IL-6 assay
The cell line B13·29, which is dependent on interleukin-6 (IL-6) for growth, has been described previously [23–25]. For IL-6 determinations, the more sensitive subclone B9 was used. B9 cells were harvested from tissue culture flasks, seeded into microtiter plates (Nunc, Roskilde, Denmark) at a concentration of 5000 cells per well, and cultured in Iscove's medium supplemented with 5 × 10−5M 2-mercaptoethanol, 10% FCS (Seralab, Sussex, UK), gentamycin (50 µg/ml) and L-glutamine. The serum samples were added for 68 h and [3H]thymidine was then added for 4 h prior to harvesting. Each sample was tested for IL-6 in a series of twofold dilutions and compared with a recombinant IL-6 standard. B9 cells have been shown previously not to react with recombinant cytokines, such as IL-1α, IL-1β, IL-2, IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, tumour necrosis factor-α and gamma interferon and had only weak reactivity with IL-4 [25].
Immunoglobulins
Total serum levels of immunoglobulin (Ig) G1, IgG2a, IgG3, and IgM were measured by the radial immunodiffusion technique [26]. Antisera and immunogobulin standards specific for IgG1, IgG2a, IgG3 and IgM were purchased from Sigma (Sigma Chemical Co., St Louis, MO, USA).
Anti-TSST-1 antibodies
Serum levels of IgM and IgG antibodies to TSST-1 were estimated by ELISA using 0·5 µg/ml of highly purified TSST-1 (Toxin Technology, Sarasota, FL, USA) as a solid phase coating. All sera were diluted serially in 0·5% PBS–BSA and incubated in the wells. To measure the level and class specificity of anti-cell-wall antibodies that were bound to the solid phase, affinity-purified and biotinylated F(ab′)2 fragments of goat antimouse IgG and IgM (Jackson Laboratories), which were diluted 1 : 3000 in PBS-Tween 20, were added to wells. This was followed stepwise by the addition of 0·5 µg/ml extravidin–horseradish peroxidase (Sigma) and 2·5 mg/ml of the enzyme substrate 2,2-azino-bis-(3-ethylbenzothiazoline sulphonic acid) (Sigma) in citrate buffer (pH 4·2) containing 0·0075% H2O2.The absorbance at 414 nm (A414) was measured in a SpectraMax Plus spectrophotometer (Molecular Devices). All optical density values were converted to antigen-specific arbitrary units using calibration curves that were based on the optical density values obtained from serial dilutions of a pool of reference sera. The calibration curves were constructed with a computer program that employed weighted logit-log models [27,28].
Antibodies to cell walls of S. aureus
Serum levels of IgM antibodies that were specific for S. aureus cell-wall constituents were estimated by an ELISA in which poly L-lysine (25 µg/ml) was used to precoat the wells, and 100 µl of whole, formalin-treated (4% for 20 min) S. aureus LS-1 cells were used (108/ml) to coat the wells.The subsequent steps in the assay were similar to those described above.
Haematological analysis
Total white blood cell counts (WBC) were determined using a haemacytometer (Toa Medical Electronics, Kobe, Japan). Blood smears were prepared and stained by the May–Grunewald–Giemsa method for differential counts.
Statistical evaluation
The differences between mean values were tested for significance using the non-parametric Mann–Whitney U-test. P-values ≤ 0·05 were considered to be statistically significant.
RESULTS
Bacterial cultures
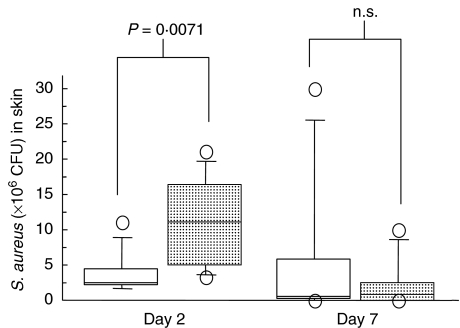
Skin samples from γ/δ T cell-deficient mice that received 2 × 108 CFU of bacteria had significantly higher numbers of bacteria than littermate controls 2 days after inoculation (P = 0·0071) (Fig. 1). There were no significant differences in bacterial numbers between mice that received 2 × 108 CFU of S. aureus 7 days after bacterial inoculation, and no significant differences between groups of mice that were inoculated with 2 × 107 CFU of S. aureus (data not shown). Bacterial cultures from blood samples 2 days after bacterial inoculation were positive in six of eight mice (75%) in the γ/δ T cell-deficient group that received a total of 4 × 108 CFU, and in eight of nine mice (89%) in the control group (n.s.) that received the same dose of bacteria. Seven days after staphylococcal inoculation, 2/7 mice (29%) in both groups showed bacteraemia. Bacteraemia was absent in mice that received a total of 4 × 107 CFU of S. aureus.
Fig. 1.
Numbers of S. aureus (± s.d.) in skin samples (n = 7–9 for each time period and group) recovered at defined time intervals after initial intracutaneous inoculation with 2 × 108 CFU of S. aureus. □, γ/δ TCR+/-;  , γ/δ TCR-/-.
, γ/δ TCR-/-.
Microscopic evaluation
Microscopic evaluation of infected skin specimens revealed a dense infiltrate of granulocytes and macrophages at days 2 and 7 in mice that received 2 × 108 CFU of S. aureus. The bacteria were clearly visible in some skin specimens. In contrast, mice that received 2 × 107 CFU of S. aureus showed only mild inflammatory infiltrates and the bacteria were not easily detectable. This result is in accordance with previously reported findings [17]. There were no clear-cut differences between the KO and control mice with respect to inflammatory infiltrate size.
Immunohistochemical analysis
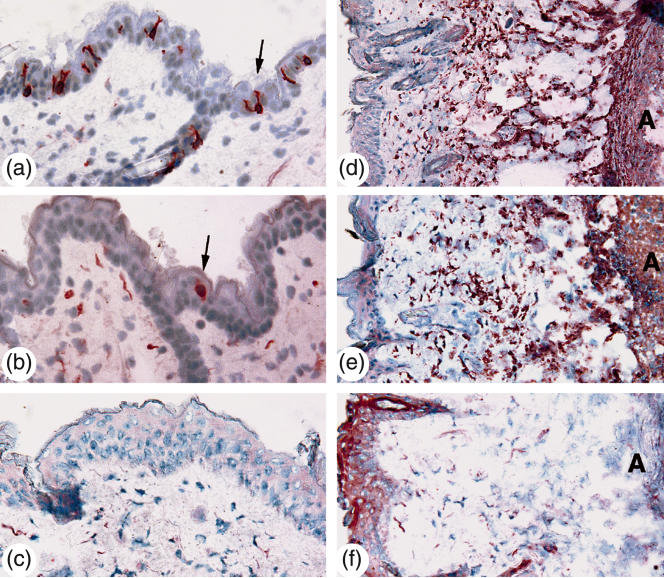
Immunohistochemical analysis was performed to determine the occurrence of CD11b+ cells (phagocytic cells) and CD3+ cells (T lymphocytes). The number of CD3+ T cells in the epidermis was significantly higher in the control mice compared to the γ/δ T cell-deficient animals (Fig. 2), both in mice that received 2 × 108 CFU of S. aureus and in mice that were inoculated with 2 × 107 CFU of S. aureus at days 2 and 7 (Table 1). No significant differences were noted between the groups regarding the number of CD3+ cells in the dermis, where a number of approximately 10 CD3+ T cells/mm2 were noted.The number of CD11b+ (Mac-1) cells was high (>50/mm2) in all the mouse groups, with no significant differences noted at days 2 or 7 (Fig. 2).
Fig. 2.
Left row (a, b and c): CD3+ epidermal T lymphocytes 2 days after bacterial inoculation with 2 × 108 CFU of S. aureus. Arrows indicate single CD3+ T lymphocytes in (a) control group, and (b) γ/δ T cell-deficient mice. (c) Tissue section stained with appropriate hamster IgG control antibodies. Right row (d, e and f): CD11b+ cells 2 days after bacterial inoculation with 2 × 108 CFU of S. aureus in (d) control group, and (e) γ/δ T cell-deficient mice. (f) Tissue section stained with appropriate rat IgG control antibodies. The letter A indicates abscess formation.
Table 1.
Number of epidermal CD3+ T cells (mean ± s.e.m./skin section) is significantly decreased in TCR δ-/- mice. Two to five sections were counted per mouse
| No. of S. aureus inoculated | TCR status (n = 5–8) | Day 2 | Day 7 |
|---|---|---|---|
| 108 | TCR δ+/− | 48 ± 11 | 40 ± 10 |
| P | <0·002 | <0·004 | |
| 108 | TCR δ−/− | 3 ± 1 | 3 ± 1 |
| 107 | TCR δ+/− | 40 ± 7 | 49 ± 9 |
| P | <0·004 | <0·001 | |
| 107 | TCR δ−/− | 4 ± 1 | 4 ± 1 |
Serological analyses
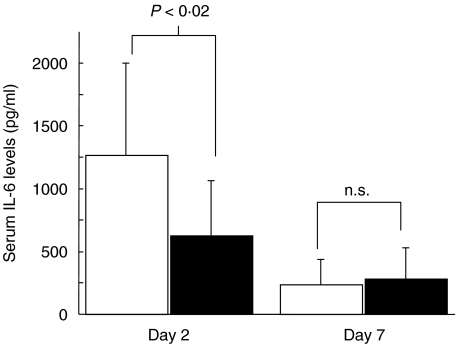
IL-6.The serum levels of IL-6 in mice that received a total of 4 × 108 CFU S. aureus were significantly lower in γ/δ knock-out mice than in control mice (P < 0·02), with peak values around 600 pg/ml in γ/δ T cell-deficient mice compared to 1200 pg/ml in the controls (Fig. 3). No significant differences were noted at day 7 in groups of mice that received a total of 4 × 108 CFU or 4 × 107 CFU of S. aureus.
Fig. 3.
Serum IL-6 levels (pg/ml) ± s.d. at specified time intervals following intracutaneous inoculation with 2 × 108 CFU of S. aureus (n = 7–9). □, γ/δ TCR+/-; ▪, γ/δ TCR-/-.
B lymphocyte responses
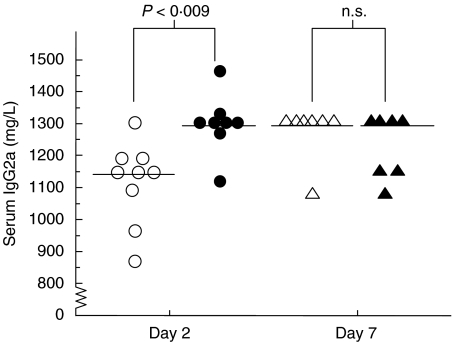
Because IL-6 is an efficient B lymphocyte-stimulating factor, we decided to analyse the serum levels of immunoglobulins and specific antibodies to staphylococcal components. Serum levels of IgG1 and IgG3 were not increased significantly 7 days after bacterial inoculation in any mouse group, and there were no significant differences between the γ/δ T cell-deficient and control mice. In contrast, the serum levels of IgG2a were significantly higher at day 2 in γ/δ T cell-deficient mice compared to controls (P < 0·009) (Fig. 4). Serum IgM levels were increased at day 7 in mice that received a total of 4 × 108 CFU of S. aureus, but no significant differences were noted between the groups. The serum levels of S. aureus cell-wall-specific antibodies of IgM class increased significantly (P = 0·01) between day 2 and day 7 in control mice (Table 2), with a twofold increase also seen in γ/δ T cell-deficient mice (n.s) that were inoculated with a total of 4 × 108 CFU of S. aureus. However, there were no significant differences between the experimental and control groups of mice that received 4 × 108 or 4 × 107 CFU of bacteria. The serum levels of TSST-1-specific antibodies of IgM class were also significantly increased (P = 0·006) between day 2 and day 7 in control mice that received 4 × 108 CFU of bacteria, while the serum levels of TSST-1 specific IgM antibodies in γ/δ T cell-deficient mice showed only a slight increase (n.s.). No increase in serum levels of TSST-1 specific antibodies of IgG class were detected (data not shown).
Fig. 4.
Serum IgG2a levels (mg/l). Horizontal bars represent the median values at specified time intervals following intracutaneous inoculation with 2 × 108 CFU of S. aureus (n = 7–9). ○, γ/δ TCR+/-; •, γ/δ TCR-/-; ▵, γ/δ TCR +/-; ▴, γ/δ TVR-/-.
Table 2.
In vivo antibody responses to S. aureus components following intradermal inoculation with TSST-1 secreting S. aureus in congeneic mice with respect to γ/δ TCR expression
| Staphylococcal cell wall antibody* | TSST-1 antibody* | ||||
|---|---|---|---|---|---|
| Size of inoculum (CFU) | Mice | Day 2 | Day 7 | Day 2 | Day 7 |
| 4 × 108 | γ/δ-/- | 30 300 ± 7513 | 59 090 ± 29 102 | 805 ± 473 | 1226 ± 634 |
| n.s. | n.s | n.s. | n.s. | ||
| γ/δ+/− | 33 458 ± 11 137 | 74 812 ± 17 013 | 634 ± 64 | 1331 ± 331 | |
| 4 × 107 | γ/δ-/- | 25 437 ± 4905 | 36 600 ± 12 567 | 446 ± 38 | 1107 ± 270 |
| n.s. | n.s. | n.s. | P = 0·05 | ||
| γ/δ+/− | 33 500 ± 14 285 | 34 650 ± 16 570 | 529 ± 124 | 642 ± 221 | |
IgM, arbitrary units ± s.d.
Clinical and haematological analysis
No significant changes in weight development were noted after inoculation with S. aureus between γ/δ T cell-deficient mice and their congeneic controls. Analysis of blood smears showed an increase in the percentage and number of granulocytes after bacterial inoculation, but no significant differences were noted between the groups (Table 3).
Table 3.
Number of leucocytes in peripheral blood 7 days after intracutaneous inoculation with S. aureus LS-1. The values shown represent mean ± s.d., n = number of mice
| Mice | (n) | Size of total inoculum (CFU) | WBC (106/ml) | Total granulocytes (106/ml) | Granulocytes (%) |
|---|---|---|---|---|---|
| γ/δ−/− | 9 | 4 × 107 | 4·8 ± 3·3 | 0·9 ± 0·7 | 18·8 ± 6·0 |
| γ/δ+/− | 8 | 4 × 107 | 4·1 ± 2·2 | 0·8 ± 0·5 | 18·8 ± 4·5 |
| γ/δ−/− | 10 | 1 × 108 | 7·6 ± 2·1 | 1·7 ± 0·5 | 22·9 ± 3·3 |
| γ/δ+/− | 10 | 1 × 108 | 5·8 ± 2·0 | 1·5 ± 0·6 | 24·8 ± 3·8 |
| γ/δ−/− | 7 | 4 × 108 | 9·0 ± 2·7 | 2·8 ± 0·9 | 28·1 ± 3·1 |
| γ/dgr;+/− | 7 | 4 × 108 | 9·8 ± 2·9 | 2·7 ± 1·2 | 29·0 ± 6·1 |
DISCUSSION
The presence of large numbers of γ/δ TCR-expressing lymphocytes at epithelial surfaces suggests that they have a role in the first line of defence against invading pathogens. Nevertheless, the timing of influx and regulatory responses of γ/δ-expressing T cells is debated and controversial. Some investigators have shown that γ/δ T cells are more prominent during subacute infections, or in the recovery phase following certain viral infections. The predominance of early or late γ/δ T cell responses following infection may be related to the type and dose of bacteria as well as to the site of infection and sampling time-points [1]. Indeed, our study suggests that the cutaneous deposition of 2 × 108 CFU of S. aureus but not of lower bacterial doses leads to differential immune responses in mice that have intact T cell repertoires and those being knocked out for the γ/δ T cell receptor. The reasons for this threshold effect are presently unknown. In the present study, significantly higher numbers of S. aureus were recovered from skin samples 2 days after intracutaneous inoculation of bacteria in TCRδ−/− mice compared to heterozygous TCRδ+/− littermate control mice. Similar findings have been reported in experimental Listeria monocytogenes infection, in which mice that were depleted of γ/δ T cells succumbed to infection induced by high doses of this intracellular bacterium [1]. In that report, the authors proposed that one function of γ/δ+ T cells was to down-regulate the responses of infection-activated macrophages by inducing their apoptosis, thereby restoring macrophage homeostasis and preventing the development of chronic inflammation. The mechanisms by which γ/δ T cells kill activated macrophages have not been determined, but examples of cytotoxicity induced by γ/δ+ T cells have been reported, which include the perforin pathway and Fas ligation on target cells [29,30].
Although it has been demonstrated clearly that γ/δ TCRs do not recognize processed peptide Ags that are complexed to self-MHC molecules [31], there is evidence that they can recognize highly conserved non-protein Ags. In this respect, peptidoglycans and/or teichoic acid, both of which are constituents of the staphylococcal cell wall may provide activating signals to γ/δ T cells that, directly or indirectly, enhance bacterial elimination. In addition, it has been recently shown that γ/δ+ T cells may be activated directly by staphylococcal TSST-1 [32], a superantigen that is produced by the staphylococcal strain used in the present study. In the present study, skin infection with TSST-1-producing staphylococci induced predominantly IgG2a production in mice that lacked γ/δ TCR-expressing cells. Furthermore, decreased levels of IL-6, which is a Th2 cytokine, were found in the same mice. This finding indicate either decreased ability to produce IL-6 or decreased inflammatory stimulus (i.e. number of bacteria). Since the γ/δ knockout mice displayed higher rather than lower bacterial load, the first hypothesis is more valid. Interestingly, it has been suggested previously that IL-6 stimulates antibacterial macrophage activities [33], thereby decreasing the risk of infection.
Our observations of early and transient deficiencies in bacterial elimination in γ/δ T cell-deficient mice need further clarification. One possibility, put forward recently by Ottones et al. [34], is that activated γ/δ T cells act directly as antibacterial effector cells in the early stages of infection. A paucity of γ/δ T cells would thus hamper the efficient early elimination of staphylococci, whereas in the later stages of infection compensatory mechanisms would have emerged. Altogether, our results strongly support the notion that cutaneous γ/δ-expressing T cells act as an early barrier to prevent staphylococcal infections of the skin.
Acknowledgments
We thank Lena Svensson and Ing-Marie Jonsson for excellent technical assistance and Vincent Collins for careful reading of the manuscript. This study was supported by grants from the Göteborg Medical Society, Swedish Medical Research Council, the A.M.E. Wolff Foundation, the Welander Foundation and the University of Göteborg (LUA).
References
- 1.Egan PJ, Carding SR. Downmodulation of the inflammatory response to bacterial infection by gammadelta T cells cytotoxic for activated macrophages. J Exp Med. 2000;191:2145–58. doi: 10.1084/jem.191.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modlin RL, Pirmez C, Hofman FM, et al. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–8. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts P, Arnoldi J, Russ F, et al. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 4.King DP, Hyde DM, Jackson KA, et al. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–6. [PubMed] [Google Scholar]
- 5.Marrack P, Kappler J. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. Adv Immunol. 1986;38:1–30. doi: 10.1016/s0065-2776(08)60005-x. [DOI] [PubMed] [Google Scholar]
- 6.Bos JD, Teunissen MB, Cairo I, et al. T-cell receptor gamma delta bearing cells in normal human skin. J Invest Dermatol. 1990;94:37–42. doi: 10.1111/1523-1747.ep12873333. [DOI] [PubMed] [Google Scholar]
- 7.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poccia F, Malkovsky M, Gougeon ML, et al. Gammadelta T cell activation or anergy during infections; the role of nonpeptidic TCR ligands and HLA class I molecules. J Leukoc Biol. 1997;62:287–91. doi: 10.1002/jlb.62.3.287. [DOI] [PubMed] [Google Scholar]
- 9.Bergstresser PR, Tigelaar RE, Dees JH, et al. Thy-1 antigen-bearing dendritic cells populate murine epidermis. J Invest Dermatol. 1983;81:286–8. doi: 10.1111/1523-1747.ep12518332. [DOI] [PubMed] [Google Scholar]
- 10.Stingl G, Gunter KC, Tschachler E, et al. Thy-1+ dendritic epidermal cells belong to the T-cell lineage. Proc Natl Acad Sci USA. 1987;84:2430–4. doi: 10.1073/pnas.84.8.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asarnow DM, Kuziel WA, Bonyhadi M, et al. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–47. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 12.Tigelaar RE, Lewis JM, Bergstresser PR. TCR gamma/delta+ dendritic epidermal T cells as constituents of skin-associated lymphoid tissue. J Invest Dermatol. 1990;94:58S–63S. doi: 10.1111/1523-1747.ep12875138. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Bonneville M, Takagaki Y, et al. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci USA. 1989;86:631–5. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 15.Born WK, O'Brien RL, Modlin RL. Antigen specificity of gamma delta T lymphocytes. Faseb J. 1991;5:2699–705. doi: 10.1096/fasebj.5.12.1717333. [DOI] [PubMed] [Google Scholar]
- 16.Shiohara T, Moriya N. Epidermal T cells: their functional role and disease relevance for dermatologists. J Invest Dermatol. 1997;109:271–5. doi: 10.1111/1523-1747.ep12335465. [DOI] [PubMed] [Google Scholar]
- 17.Molne L, Tarkowski A. An experimental model of cutaneous infection induced by superantigen-producing Staphylococcus aureus. J Invest Dermatol. 2000;114:1120–5. doi: 10.1046/j.1523-1747.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- 18.Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68:6162–7. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itohara S, Mombaerts P, Lafaille J, et al. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–48. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 20.Bremell T, Lange S, Svensson L, et al. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–44. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- 21.Springer T, Galfre G, Secher D, et al. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–6. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 22.Leo O, Foo M, Sachs DH, et al. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374–8. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansdorp PM, Aarden LA, Calafat J, et al. A growth-factor dependent B-cell hybridoma. Curr Top Microbiol Immunol. 1986;132:105–13. doi: 10.1007/978-3-642-71562-4_14. [DOI] [PubMed] [Google Scholar]
- 24.Aarden LA, De Groot ER, Schaap OL, et al. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–6. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 25.Helle M, Boeije L, Aarden LA. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–40. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- 26.Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235–54. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 27.Lue C, Tarkowski A, Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988;140:3793–800. [PubMed] [Google Scholar]
- 28.Russell MW, Brown TA, Radl J, et al. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Meth. 1986;87:87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- 29.Lin T, Brunner T, Tietz B, et al. Fas ligand-mediated killing by intestinal intraepithelial lymphocytes. Participation in intestinal graft-versus-host disease. J Clin Invest. 1998;101:570–7. doi: 10.1172/JCI896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeine R, Pon R, Ladiwala U, et al. Mechanism of gammadelta T cell-induced human oligodendrocyte cytotoxicity: relevance to multiple sclerosis. J Neuroimmunol. 1998;87:49–61. doi: 10.1016/s0165-5728(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 31.Schild H, Mavaddat N, Litzenberger C, et al. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 32.Fikri Y, Denis O, Pastoret P, et al. Purified bovine WC1+ gamma delta T lymphocytes are activated by staphylococcal enterotoxins and toxic shock syndrome toxin-1 superantigens: proliferation response, TCR V gamma profile and cytokines expression. Immunol Lett. 2001;77:87–95. doi: 10.1016/s0165-2478(01)00182-1. [DOI] [PubMed] [Google Scholar]
- 33.Flesch IE, Kaufmann SH. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (interleukin-6) Infect Immun. 1990;58:269–71. doi: 10.1128/iai.58.1.269-271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottones F, Dornand J, Naroeni A, et al. V gamma 9V delta 2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. J Immunol. 2000;165:7133–9. doi: 10.4049/jimmunol.165.12.7133. [DOI] [PubMed] [Google Scholar]