Abstract
In mice, the roles of cytokines in the initiation of epidermal Langerhans’ cell (LC) migration are well documented; however, the mechanism of this response in humans is less well defined. The purpose of the present investigation was to examine the contribution of interleukin (IL)-1β to human epidermal LC migration and to define further the mechanisms of this response. We demonstrate here that homologous recombinant IL-1β administered intradermally to healthy human volunteers provides a stimulus for LC migration, with significant (P < 0·01) reductions in LC densities being observed at both 2 h and 4 h following treatment. At the later time-point of 4 h, injection of IL-1β was also accompanied by activation of those LC remaining in the epidermis. Analysis of fluid aspirated from suction blisters formed at injection sites revealed significant (P < 0·01) tumour necrosis factor (TNF)-α production (2·99 ± 1·18 pg TNF-α/mg protein; mean ± s.d. of n = 10) in response to IL-1β treatment compared with saline control injections (0·90 ± 1·05 pg TNF-α/mg protein). Prior topical application of human recombinant lactoferrin (LF), an iron-binding protein found in exocrine secretions and skin, inhibited IL-1β-mediated LC migration and also compromised the production of TNF-α protein as measured in suction blister fluids derived from each of the treatment sites. Taken together, these data demonstrate that IL-1β is associated with both the stimulation of human epidermal LC migration and local TNF-α production. Topical treatment with LF compromises both these responses. These data suggest that topical LF may potentially represent a novel therapeutic in the treatment of skin inflammation where TNF-α is an important mediator.
Keywords: cytokines, dendritic cells, epidermis, inflammation
INTRODUCTION
Investigations in mice have revealed that the initiation and regulation of Langerhans’ cell (LC) mobilization and migration are orchestrated by cutaneous cytokines and chemokines [1,2]. Collectively, the available data reveal that under normal circumstances the stimulation by chemical allergens of LC migration away from the epidermis is dependent upon the local availability of interleukin (IL)-1β, IL-18 and tumour necrosis factor (TNF)-α[3–6] and that this process may be inhibited by IL-10 [7]. Moreover, the directed movement of emigrating cells towards, and their localization within, lymph nodes draining the skin are governed by chemokines, and by induced changes in chemokine receptor expression [8–12]. The process of LC migration and dendritic cell (DC) accumulation in regional lymph nodes is potentially susceptible to regulation by a variety of exogenous factors. Studies in mice have demonstrated that topical application of homologous recombinant lactoferrin (LF; an iron binding protein found in exocrine secretions and in skin) is associated with a significant inhibition of LC migration [5].
Experience to date suggests that LC migration is regulated similarly in humans. Thus, exposure of human volunteers either to a contact allergen (diphenylcyclopropenone; DPC) by topical administration, or to TNF-α by intradermal injection, in both instances results in a significant reduction in the frequency of epidermal LC [13–15]. Furthermore, DPC-induced LC migration was found to be inhibited markedly by prior treatment of the same skin site with human recombinant LF [14].
The purpose of the investigations described here was to elucidate further the regulation of LC migration in humans, and within this context there were two primary objectives.
The first of these was to examine the influence of IL-1β on LC mobilization and morphology. It is clear from studies in mice that local administration of IL-1β alone is able to stimulate LC migration [16], and that this cytokine is in fact required for both chemical allergen-induced LC migration and optimal contact sensitization [4,6,17]. Our purpose here was to examine whether in humans local exposure to IL-1β is also associated with the initiation of LC migration.
The second objective was to determine the mechanism through which LF causes an inhibition of allergen-induced LC mobilization. Previously circumstantial evidence, in both man and mouse, has suggested that the inhibitory activity of LF in this respect is secondary to a down-regulation of TNF-α[14,18,19]. However, direct confirmatory data have been lacking. The strategy we have adopted here has been to examine whether IL-1β induces local production of TNF-α protein as measured in suction blister fluids derived from treated skin sites and whether prior exposure to LF is associated with a decrease in IL-1β-induced TNF-α release.
MATERIALS AND METHODS
Volunteers
Twenty-five healthy human volunteers (14 male, 11 female; mean age 34·3 years, range 19–51) were recruited for these studies. All subjects gave written, informed consent and the investigations were approved by the Salford and Trafford Local Research Ethics Committee.
Treatment with IL-1β and biopsy
Volunteers received 50 µl intradermal injections of homologous recombinant IL-1β (specific activity>1 × 107 U/mg, endotoxin content <1 pg/100 U; Insight Biotechnology Ltd, Middlesex, UK) diluted in sterile normal saline to the appropriate concentration, or normal saline alone, to sites identified on non-sun-exposed hip or buttock. Punch biopsies (6 mm) were taken from the injection sites 2 or 4 h later under local anaesthesia (1% lidocaine). Biopsies were processed immediately for either analysis of epidermal LC frequency or for histological examination.
Preparation and analysis of epidermal sheets
Biopsies were placed immediately in 0·02 m ethylenediamine tetraacetic acid (Sigma, St Louis, MO, USA) dissolved in phosphate buffered saline (PBS) and incubated for 2 h at 37°C. The epidermis was separated from the dermis using forceps, washed in PBS and fixed in acetone for 20 min at −20°C. After washing in PBS, epidermal sheets were incubated at room temperature for 30 min with monoclonal antibodies to either CD1a [clone NA1/34 (mouse IgG2a); Dako Ltd, Cambridge, U.K.] or HLA-DR [clone DK22 (mouse IgG2a); Dako], each diluted to 10 µg/ml in PBS containing 0·1% bovine serum albumin (BSA). Sheets were washed prior to incubation for a further 30 min with fluorescein isothiocyanate-conjugated goat F(ab′)2 antimouse immunoglobulins (Dako) diluted 1 : 100 in 0·1% BSA/PBS. Finally, sheets were washed in PBS and mounted on microscope slides in Citifluor (Citifluor Ltd, London, UK) and sealed with nail varnish. Samples were examined by fluorescence microscopy and the frequency of stained cells assessed in a blinded fashion using an eyepiece with a calibrated grid (0·32 × 0·213 mm at × 40 magnification). For each sample, 50 random fields were examined and the results expressed as the mean ± s.d. number of cells/mm2. No specific staining was observed in control experiments using mouse monoclonal IgG2a (clone DAK-G05; Dako) in place of the primary antibody. Images were acquired digitally using an Olympus BX50 fluorescence microscope coupled with an RS Photometrics Coolsnap colour CCD camera (Princeton Instruments, Marlow, Bucks, UK) and were processed equally for image sharpness and brightness using MetaMorph Imaging System (Version 4·01, Princeton Instruments). Images were printed using a Sony digital colour printer (UP-D1510CNE).
Clinical assessment
Treated sites were assessed hourly for signs of erythema (graded from 0, none to 3, severe) and induration (graded from 0, none to 3, severe) in addition to a subjective assessment of pain. Immediately prior to injection, and at half-hourly intervals thereafter, blood pressure, pulse and temperature were recorded.
Recombinant human lactoferrin
Recombinant human LF was produced in Aspergillus niger var. awamori as described previously [20]. Lyophilized LF was resuspended in sterile deionized water to a stock concentration of 0·1% w/v and stored at −20°C. Stock LF was diluted to 0·04% v/v in aqueous cream BP (Pinewood Healthcare, Tipperary, Ireland) immediately prior to use as described previously [14]. LF in aqueous cream (50 µl) was applied topically to two skin sites (each of 2 cm2 area) identified on non-sun-exposed buttock or hip. A further two sites on the contralateral buttock or hip received 50 µl of aqueous cream alone. Sites were treated with LF or cream alone 2 h prior to further treatment.
Suction blister formation
In one series of experiments, suction blister cups (12 mm, Ventipress Oy, Finland) were applied to treated sites and a vacuum of 250 mmHg applied [21]. After approximately 90–120 min, when suction blisters had formed, the vacuum was released and the fluid aspirated from the blister using a 23-gauge needle. Fluids were centrifuged for 5 min in a microcentrifuge (MSE Micro Centaur; Fisher Scientific, Loughborough, UK) and frozen immediately at −70°C prior to assay for TNF-α content by enzyme-linked immunosorbent assay (ELISA) and for total protein content using a modified Lowry assay [22].
TNF-α ELISA and protein determination
The TNF-α content of blister fluid samples was measured using a specific solid phase sandwich ELISA kit from Biosource International Inc. (human TNF-α US Ultrasensitive; Camarillo, CA, USA). The assay was conducted according to the manufacturer's instructions. Recombinant human TNF-α (32–0·5 pg/ml) was diluted in sample diluent buffer and added to the plate in duplicate to form the standard curve and blister fluid samples were diluted to various extents (1 in 2–1 in 20) with sample diluent buffer and added to duplicate wells. Optical density was measured at 450 nm using an automated plate reader (Multiskan, Flow Laboratories, Irvine, Ayrshire, UK) and the levels of TNF-α were calculated using associated computer software for microplate-based assays (Genesis, Life Sciences International Ltd, Basingstoke, UK). The limit of detection for TNF-α was 1–2 pg/ml, and ranges were typically less than 10%.
Protein content was determined using a modification of the Lowry assay [22], the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA), a colourimetric assay for determination of protein concentration. A standard reference curve was prepared using BSA (10–0·1 mg/ml) in PBS, and the protein concentration in each blister fluid sample was determined using readings obtained for samples diluted between 1 : 10 and 1 : 50 in PBS. The TNF-α content of individual blister fluids was expressed as pg TNF-α/mg protein for each sample.
Statistical analyses
Differences between treatment groups were considered by analysis of variance. The individual was treated as a random effect and pretreatment, treatment and the pretreatment by treatment interaction as fixed effects. Selected comparisons between treatment groups were compared using a two-sided Student's t-test, based on the error mean square in the analysis of variance.
RESULTS
Influence of IL-1β on epidermal LC density and morphology
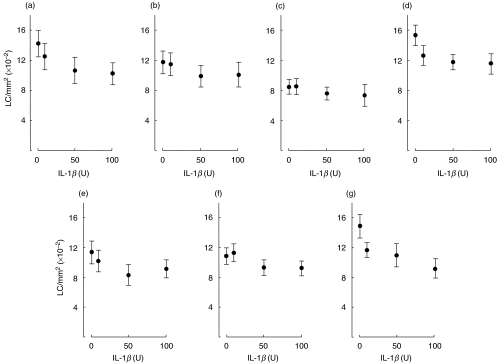
Volunteers (a–g) were exposed by intradermal injection to various concentrations (10, 50 and 100 U) of homologous recombinant IL-1β suspended in sterile normal saline, or to saline alone. Punch biopsies (6 mm) were taken from injected sites 2 h (volunteers a–d) and 4 h (volunteers e–g) later and the frequency of CD1a+ LC in epidermal sheets determined (Fig. 1). As reported previously [13,14], baseline epidermal LC densities (saline-treated sites) varied between volunteers, with mean (± s.d.) LC frequencies for the individuals displayed in Fig. 1 ranging from 847·3 ± 96·2 CD1a+ cells/mm2 for volunteer c to 1536·8 ± 137·7 CD1a+ cells/mm2 for volunteer d. The mean (±s.e.m.; n = 7) LC frequency for volunteers a–g was 1242·0 ± 101·9 cells/mm2, a value similar to that reported previously for control LC numbers [13,14]. Intradermal administration of IL-1β provoked reductions in LC frequencies at both 2 and 4 h. However, responsiveness varied substantially between subjects with 10 U IL-1β stimulating decreases in LC frequencies, ranging from zero to 17·7% (P < 0·05) at 2 h and zero to 21·5% (not significant) at 4 h. Injection of 50 U IL-1β caused reductions in LC densities of 10·1–25·0% (P < 0·01) at 2 h and 14·6–26·9% (P < 0·05) at 4 h and 100 U IL-1β resulted in losses ranging from 13·0% to 27·9% (P < 0·01) at 2 h, and 15·4%– 38·5% (P < 0·01) at 4 h. In a further two volunteers, the influence of IL-1β on LC numbers was examined as a function of HLA-DR expression. In these volunteers, 100 U IL-1β stimulated decreases in LC densities of 16·8% and 12·5% measured 4 h following injection. The wide variation in responsiveness to IL-1β was found not to be associated with either the age or sex of the volunteers. Injection of IL-1β was tolerated in all subjects with minimal systemic side-effects. Pulse rate, blood pressure and body temperature did not change significantly over the 2–4-h period of examination. Local side-effects included erythema graded no more than 1 at 2 h and 1–3 at 4 h.
Fig. 1.
Modulation of human epidermal CD1a+ LC frequency by homologous recombinant IL-1β. Each volunteer received intradermal injections of IL-1β (10, 50 and 100 U) and a single control injection of normal saline alone (0 U). Biopsies for each volunteer were taken 2 h (a–d) or 4 h (e–g) later and epidermal sheets prepared. The frequency of CD1a+ LC was assessed following indirect immunofluorescence staining of epidermal sheets. Results are expressed as the mean ± s.d. number of cells/mm2 derived from examination of 50 fields/sample. The percentage reduction in frequency of CD1a+ LC induced by 10, 50 and 100 U IL-1β, respectively, for each volunteer was: (a) 12·0, 25·0 and 27·9%; (b) 2·0, 15·5 and 14·1%; (c) 0·0, 10·1 and 13·0%; (d) 17·7, 23·4 and 24·7%; (e) 10·0, 26·9 and 19·5%; (f) 0·0, 14·6 and 15·4%; (g) 21·5, 26·2 and 38·5%.
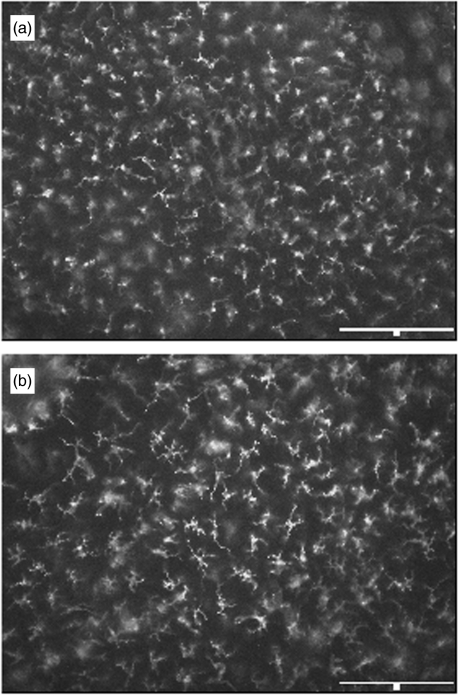
Morphologically, LC remained largely unchanged 2 h following exposure to IL-1β (not shown). However, striking changes were apparent in epidermal sheets prepared 4 h after treatment with IL-1β (Fig. 2). At this time-point, LC displayed characteristics of activation including extended dendritic processes and enhanced cell size. The intensity of staining for CD1a appeared unaltered. Biopsies from one volunteer at 2 h and two individuals at 4 h following treatment with IL-1β were examined histologically (not shown). In these cases, exposure to IL-1β was associated with a cellular infiltration similar to that shown previously for TNF-α[13].
Fig. 2.
Morphological changes induced in human epidermal CD1a+ LC by IL-1β. Epidermal sheets were prepared from volunteer g (Fig. 1) 4 h following intradermal administration of (a) saline, or (b) 50 U IL-1β and stained by indirect immunofluorescence for CD1a expression. Scale bar: 150 µm.
Topical LF inhibits IL-1β-induced LC migration
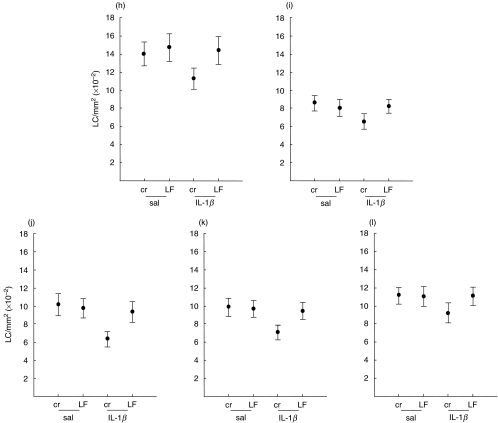
From the dose–response and kinetic analyses shown in Fig. 1, LC migration provoked by 50 U IL-1β was very similar to that achieved for 100 U IL-1β at both 2 and 4 h. Therefore, all subsequent volunteers were exposed to the lower dose of IL-1β (50 U) for the shorter period of time (2 h). Thus, five volunteers (h–l; Fig. 3) were exposed topically to LF (0·04%) suspended in aqueous cream, or to cream alone, 2 h prior to injection into paired sites (one pretreated with LF and the other with cream) of IL-1β (50 U), or saline alone. Biopsies were taken 2 h later and the frequency of CD1a+ LC determined following indirect immunofluorescence staining of epidermal sheets (Fig. 3). The mean (± s.e.m.; n = 5) LC frequency for control sites (cream/saline treated sites) was 1072·7 ± 101·0 cells/mm2, ranging from 856·7 ± 85·4 cells/mm2 (mean ± s.d.) for subject h to 1394·2 ± 122·8 cells/mm2 for subject j. Intradermal administration of IL-1β to sites pretreated with cream stimulated a significant decrease (25·3%; P < 0·01) in LC frequencies from a mean (± s.e.m.) LC value for all five volunteers (h–l) of 1072·7 ± 101·0 cells/mm2−807·7 ± 106·4 cells/mm2. In all cases, prior exposure to LF inhibited significantly (P < 0·01) IL-1β-induced LC migration with the mean (± s.e.m.; n = 5) LC density of 1048·9 ± 120·4 cells/mm2 being not significantly different from control (cream/saline) values. These results confirm and extend our previous preliminary observations [19] and represent here a mean percentage reduction in LC values of only 2·2%. Pretreatment of saline-injected sites with LF caused no significant changes in LC density (1063·9 ± 125·1 cells/mm2; mean ± s.e.m.). Throughout the course of this series of analyses, LC morphology remained unchanged.
Fig. 3.
Inhibition by LF of IL-1β-induced changes in epidermal CD1a+ LC frequency. Volunteers (h–l) were exposed topically at two sites to LF (50 µl) and at a further two sites to an equivalent volume of aqueous cream alone. Two hours later, IL-1β was injected intradermally into paired sites (one pretreated with LF and one with cream) and biopsies taken 2 h later. CD1a+ LC densities were assessed following indirect immunofluorescence staining of epidermal sheets. Results are expressed as the mean ± s.d. number of cells/mm2 derived from examination of 50 fields/sample. The percentage reduction in frequency of CD1a+ LC induced by IL-1β (at sites pretreated with cream) was: (h) 19·0%; (i) 17·6%; (j) 23·9%; (k) 37·6%; (l) 28·2%. In all cases, prior treatment with LF inhibited the IL-1β-induced response.
Topical LF inhibits IL-1β-induced epidermal TNF-α production
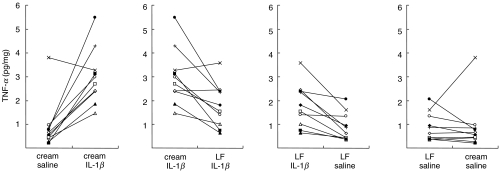
To investigate whether LF may limit LC migration through inhibition of local TNF-α production, a further 10 volunteers were exposed topically to LF (0·04%) or to cream alone, followed 2 h later by injection into treated sites of either IL-1β or saline. Suction blister equipment was applied immediately to all four treated sites and suction blister fluid collected, typically 90–120 min later. Fluids were analysed for TNF-α content by ELISA and for total protein content by modified Lowry. The TNF-α content of individual blister fluids was expressed as pg TNF-α/mg protein for each sample (Fig. 4). The level of total protein measured in blister fluids varied little between volunteers or among treatment groups. Thus, the mean ± s.e.m. (n = 10) total protein measurements for each treatment group were as follows; cream/saline: 21·58 ± 2·07 mg/ml; cream/IL-1β: 23·26 ± 2·32 mg/ml; LF/IL-1β: 23·89 ± 3·14 mg/ml; LF/saline: 23·13 ± 2·38 mg/ml. The TNF-α content of blister fluids taken from control (cream/saline) treated sites was remarkably stable with nine of 10 samples displaying 0·21–0·96 pg/mg protein. Only one volunteer (×; Fig. 4) showed a TNF-α value outside of this range at 3·81 pg/mg protein. Injection of IL-1β into cream-treated sites stimulated a marked increase in the amount of TNF-α detectable in blister fluids taken from nine of 10 subjects. Thus, the mean ± s.e.m. TNF-α measurements for all 10 volunteers increased significantly from 0·90 ± 0·33 pg/mg of protein at cream/saline-treated sites to a mean TNF-α value of 2·99 ± 0·37 pg/mg in response to IL-1β (P < 0·01). Again, the same individual (×; Fig. 4) failed to demonstrate induced TNF-α production following exposure to IL-1β. Pretreatment with LF resulted in an inhibition of IL-1β-induced TNF-α production in eight of 10 volunteers, with two subjects showing a small increase in TNF-α levels (× and e; Fig. 4). Overall, prior exposure to LF inhibited significantly IL-1β-induced TNF-α production by 57·4% (mean for all 10 volunteers; P < 0·01) to a mean TNF-α concentration of 1·79 ± 0·90 pg/mg. Pretreatment with LF was without effect on TNF-α measurements obtained following control (saline) injection (mean TNF-α content: 0·90 ± 0·19 pg/mg).
Fig. 4.
Inhibition by LF of IL-1β-induced epidermal TNF-α production. A further 10 volunteers were exposed topically to LF or cream followed by intradermal injections of IL-1β or saline, as described in the legend to Fig. 3. Each symbol represents the results from one of the 10 volunteers. Immediately following injections, suction blister cups were applied to each of the four treated sites. Suction blister fluids were collected and analysed by ELISA for TNF-α content and total protein expression by modified Lowry [22]. Results are expressed as pg TNF-α/mg protein.
DISCUSSION
The roles of TNF-α and IL-1β in initiating epidermal LC migration in mice are well documented. In human skin the mechanisms of this process are less well defined, although the contribution of TNF-α is known to be very similar, and perhaps identical, to that in mice [13]. The purpose of this investigation was to examine the contribution of IL-1β to epidermal LC migration in human skin and to discern whether, as in mice, this was promoted in part by local release of TNF-α. The results presented in the current series of experiments establish firmly that in humans intradermal administration of IL-1β is able to stimulate migration of LC away from the epidermis and that this process is associated with local release of TNF-α as measured in suction blister fluid. We provide additional evidence that topical administration of the iron-binding protein, LF, abrogates not only IL-1β mediated LC migration but also compromises the local release of TNF-α protein.
We did not determine directly whether numbers of LC within the dermis, or for that matter draining lymph nodes, were increased concomitant with reduction in number of epidermal LC following intradermal administration of IL-1β. However, the observed loss of CD1a+ LC is likely to represent migration out of the epidermis rather than down-regulation of CD1a expression as intradermal administration of IL-1β also produced a reduction in frequency of epidermal HLA-DR+ LC equivalent to that observed for CD1a+ LC. It would be extremely unlikely for IL-1β to have down-regulated cell surface expression of both these markers, bearing in mind their relative reciprocity of expression − mature LC are characterized by a down-regulation of CD1a and an up-regulation of HLA-DR [23,24]. Also, experiments in mice have shown that exogenous IL-1β causes LC migration and the subsequent accumulation of dendritic cells in draining lymph nodes [4,16].
In murine skin there are different kinetics of epidermal LC migration following administration of TNF-α or IL-1β. Intradermal exposure of mice to homologous recombinant TNF-α results in a significant reduction in the number of epidermal LC within 30 min of exposure, whereas a reduction in epidermal LC frequency is first observed 2 h after intradermal injection of recombinant IL-1β. This discrepancy in LC kinetics can be explained by an understanding that, following injection of IL-1β, there is a need for this cytokine to induce the production, by keratinocytes, of TNF-α[1,25] thereby providing the second signal for migration. In the case of migration induced by exogenous TNF-α it is assumed that the rapid migration is due to sufficient constitutive IL-1β being present already within the epidermis to supply the other signal for migration [17,26]. In this series of experiments there was no appreciable difference in reduction of epidermal LC frequency measured at 2 h or 4 h following intradermal administration of 50 or 100 U IL-1β, implying that most migration had occurred by the former time-point. This is in agreement with our previous work with TNF-α[13] where an equivalent (25%) reduction in frequency of human epidermal LC had occurred within 2 h of intradermal injection of homologous TNF-α. We did not look, however, at time-points earlier than 2 h and it is possible that maximal migration had already occurred 30 min post-TNF-α injection: a scenario similar to that observed in mice.
It appears from our experiments that exogenous administration of either TNF-α or IL-1β is able to cause mobilization of only a proportion (20–30%) of human epidermal LC. This is an identical feature of cytokine induced LC migration in mice [16,27]. Our working hypothesis for this observation is that there is functional heterogeneity among human LC [28]. Possible bases for variable responsiveness to triggers for mobilization include differences in adenosine triphosphate (ATPase) activity, or in the expression of the relevant membrane receptors for stimulatory cytokines [29].
One important aspect of the present study is that it provides functional evidence in humans for the ability of exogenous IL-1β to enhance local levels of TNF-α, thus corroborating the hypothesis generated from experiments in mice. Intradermal administration of recombinant IL-1β to control (cream-treated) skin brought about rapid release of TNF-α as measured by ELISA in fluid taken from suction blisters formed within 2 h at the site of injection. Levels of TNF-α, although detectable, were significantly less in suction blister fluid retrieved from control sites treated with cream and then injected with normal saline. This is the first evidence in man that IL-1β induces local production of TNF-α. Whether this is due to release of preformed TNF-α by keratinocytes or is in part dependent on increased gene expression remains to be determined.
The source of suction blister fluid is a topic of debate − it is highly likely that this is primarily derived from epidermis. Work using this technique on ultraviolet light induced skin inflammation has shown discrete differences in epidermal mediators identified in suction blister fluid and dermal mediators as identified by microdialysis [30]. Furthermore, there is a significant difference between cytokine concentrations found in suction blister fluid and plasma [31]. Therefore, it is likely that the suction blister technique allows for ready identification and measurement of cytokines produced primarily by cells within the epidermis, most probably keratinocytes. Although the concentration of TNF-α protein was increased in IL-1β-treated skin we have not excluded the possibility that other cytokines are involved; for instance, elevation of IL-6 or reduction in IL-10. A role for IL-6 is unlikely, as in mice this cytokine is unable to induce LC migration [16]. The converse argument holds true for the ability of LF to inhibit IL-1β-induced migration − i.e. reduction in proinflammatory cytokines other than TNF-α or elevation of IL-10.
LF is an iron-binding protein found in exocrine secretions and skin [18]; its anti-inflammatory effects are thought to be mediated, at least in part, by inhibition of TNF-α synthesis. In mice and humans this theory has been substantiated by circumstantial evidence [14,18,19]. In mice this evidence accrues from the observation that intradermal homologous recombinant LF prevents LC migration provoked either by contact allergen (oxazolone) or intradermal injection of IL-1β but not migration produced by exogenous TNF-α[18]. The conclusion is that LF impairs LC migration secondary to an inhibition of local release of TNF-α by keratinocytes. Thus, the purpose of the current study was to determine whether LF, if applied topically to human skin, would impair IL-1β-induced LC migration and whether this was associated with compromised production of TNF-α protein. The experiments presented herein confirm and extend observations made in mice. Topical administration of homologous LF, prior to intradermal injection of IL-1β, inhibited very significantly (P < 0·01) the migration of CD1a+ LC, to the extent that values were very similar to those in control (saline-treated) sites. Also, LF applied topically prior to local, intradermal injection of IL-1β reduced significantly the level of TNF-α in suction blister fluids. Together, these data suggest that LF may inhibit LC migration via its ability to control local production of TNF-α. Furthermore, that LF exists as a constituent of normal skin [18 and data not shown] raises the possibility that in vivo LF may serve as an endogenous regulator of cutaneous immune function acting to influence and control local cytokine production.
In conclusion, the data reported here confirm that IL-1β supplies a key signal for human epidermal LC migration and that this process may be mediated in part by local release of TNF-α − most probably from keratinocytes. Furthermore, topical LF can inhibit IL-1β-induced migration of LC out of the epidermis and the local release of epidermal TNF-α. LF appears to be a potent inhibitor of LC migration and TNF-α production but these observations need to be translated into clinical practice. For instance, it would be important to ascertain whether topical LF could be used to treat existing skin inflammation (such as psoriasis and sunburn) where TNF-α is known to be an important mediator.
Acknowledgments
We are grateful to June Bowden and Mike Dean for assistance in subject recruitment and suction blister methodology, to Suzanne Camus for collection of biopsy and suction blister material and to Dr Susan Andrew for histological assessment. This work was funded in part by a grant from Agennix Inc., Houston, Texas, USA.
References
- 1.Kimber I, Cumberbatch M, Dearman RJ, et al. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–12. doi: 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 2.Jakob T, Ring CA, Udey MC. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol. 2001;108:688–96. doi: 10.1067/mai.2001.118797. [DOI] [PubMed] [Google Scholar]
- 3.Cumberbatch M, Kimber I. Tumour necrosis factor-α is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Cumberbatch M, Dearman RJ, Kimber I. Interleukin 1β and the stimulation of Langerhans cell migration: comparisons with tumour necrosis factor α. Arch Derm Res. 1997a;289:277–84. doi: 10.1007/s004030050193. [DOI] [PubMed] [Google Scholar]
- 5.Cumberbatch M, Dearman RJ, Antonopoulos C, et al. Interleukin (IL)-18 induces Langerhans cell migration by a tumour necrosis factor-α- and IL-1β-dependent mechanism. Immunology. 2001;102:323–30. doi: 10.1046/j.1365-2567.2001.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonopoulos C, Cumberbatch M, Dearman RJ, et al. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J Immunol. 2001;166:3672–7. doi: 10.4049/jimmunol.166.6.3672. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Zhuang L, Fujisawa FH, et al. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999;162:277–83. [PubMed] [Google Scholar]
- 8.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 9.Gunn MD, Kyuwa S, Tam C, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CL, Suri RM, Rahdon RA, et al. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol. 1998;28:4114–22. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara S, Komura E, Nagafune J, et al. EB11/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161:3096–102. [PubMed] [Google Scholar]
- 13.Cumberbatch M, Griffiths CEM, Tucker SC, et al. Tumour necrosis factor-α induces Langerhans cell migration in humans. Br J Dermatol. 1999;141:192–200. doi: 10.1046/j.1365-2133.1999.02964.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths CEM, Cumberbatch M, Tucker SC, et al. Exogenous topical lactoferrin inhibits allergen-induced Langerhans cell migration and cutaneous inflammation in humans. Br J Dermatol. 2001;144:715–25. doi: 10.1046/j.1365-2133.2001.04125.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhushan M, Cumberbatch M, Dearman RJ, et al. Tumour necrosis factor-α-induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol. 2002;146:32–40. doi: 10.1046/j.1365-2133.2002.04549.x. [DOI] [PubMed] [Google Scholar]
- 16.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin-1β for migration. Immunology. 1997b;92:388–95. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enk AH, Angeloni VL, Udey MC, et al. An essential role for Langerhans cell derived IL-1β in the initiation of primary immune responses in the skin. J Immunol. 1993;150:3698–704. [PubMed] [Google Scholar]
- 18.Cumberbatch M, Dearman RJ, Uribe-Luna S, et al. Regulation of epidermal Langerhans cell migration by lactoferrin. Immunology. 2000;100:21–8. doi: 10.1046/j.1365-2567.2000.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimber I, Cumberbatch M, Dearman RJ, et al. Lactoferrin: influences on Langerhans cells, epidermal cytokines, and cutaneous inflammation. Biochem Cell Biol. 2002;80:103–7. doi: 10.1139/o01-227. [DOI] [PubMed] [Google Scholar]
- 20.Ward PP, Piddington CS, Cunningham GA, et al. A system for the production of commercial quantities of human lactoferrin: a broad spectrum natural antibiotic. Bio/Technology. 1995;13:498–502. doi: 10.1038/nbt0595-498. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes LE, Durham BH, Fraser WD, et al. Dietary fish oil reduces basal and ultraviolet B-generated PGE2 levels in skin and increases the threshold to provocation of polymorphic light eruption. J Invest Dermatol. 1995;105:532–5. doi: 10.1111/1523-1747.ep12323389. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Bio Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.Romani N, Lenz A, Glassel H, et al. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–9. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 24.Aiba S, Katz SI. Phenotypic and function characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791–6. [PubMed] [Google Scholar]
- 25.Enk AH, Katz SI. Early molecular events in the induction phase contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398–402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariizumi K, Kitajima T, Bergstresser OR, et al. Interleukin 1β converting enzyme in murine Langerhans cells and epidermal-derived dendritic cell lines. Eur J Immunol. 1995;25:2137–41. doi: 10.1002/eji.1830250803. [DOI] [PubMed] [Google Scholar]
- 27.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans cell frequency by tumour necrosis factor-α. Immunology. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- 28.Shibaki A, Meunier L, Ra C, et al. Differential responsiveness of Langerhans cell subsets of varying phenotypic states in normal human epidermis. J Invest Dermatol. 1995;104:42–6. doi: 10.1111/1523-1747.ep12613476. [DOI] [PubMed] [Google Scholar]
- 29.Kimber I, Dearman RJ, Cumberbatch M, et al. Langerhans cells and chemical allergy. Curr Opin Immunol. 1998;10:614–9. doi: 10.1016/s0952-7915(98)80078-2. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes LE, Belgi G, Parslew R, et al. Ultraviolet B-induced erythema is mediated by nitric oxide and prostaglandin in combination. J Invest Dermatol. 2001;117:880–5. doi: 10.1046/j.0022-202x.2001.01514.x. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes LE, Hashim JA, McLaughlin PJ, et al. Blister fluid cytokines in cutaneous inflammatory disorders. Acta Derm Venereol. 1999;79:288–90. doi: 10.1080/000155599750010689. [DOI] [PubMed] [Google Scholar]