Abstract
Accumulating data show that fibroblasts are important regulators in the development and maintenance of allergic airway inflammation. However, most studies so far have used individual recombinant cytokines in high concentrations, unlikely to be found in vivo. We aimed to investigate how cytokines produced by peripheral blood mononuclear cells (PBMC) affect fibroblast functions. Primary airway fibroblasts where incubated with allergen-stimulated or non-stimulated PBMC supernatants from allergic patients. The levels of cytokines in PBMC supernatants were measured and the expression of CD54, CD40 and CD106 as well as the production of eotaxin, interleukin (IL)-6 and IL-8 were assessed in fibroblasts. Although the levels of single cytokines measured in PBMC supernatants were low, a significant up-regulation of the surface molecules as well as of IL-6 and IL-8 production was found in fibroblasts cultured with allergen-stimulated PBMC supernatants as compared to non-stimulated, while the increase in eotaxin production was not significant. The evaluation of correlations between cytokines produced by PBMC and effects seen on fibroblasts did not indicate a crucial role for any single cytokine. Furthermore, the addition of comparably low concentrations of recombinant interferon (rIFN)-γ or recombinant tumour necrosis factor (rTNF)-α did not induce the same effects as PBMC supernatants, the only exception being TNF-α as a direct inducer of CD54 expression. Our results show that synergistic mechanisms has a more important role than single mediators, highlighting important differences between in vitro experiments, where effects of individual mediators are studied, versus the actual situation in vivo.
Keywords: adhesion molecules, airway inflammation, allerge, cytokine network, fibroblast
INTRODUCTION
The allergic reaction in human airways is characterized by an ongoing inflammatory response. Protagonists in the typical allergic inflammation are mast cells, eosinophils, neutrophils and lymphocytes and the general recruitment of inflammatory cells into the injured site involves a series of events such as adhesion, transendothelial migration and chemotactic movement, in which adhesion molecules play an important role [1]. Furthermore, when activated, these cells produce proinflammatory cytokines that amplify the inflammatory reaction and may induce remodelling of the airway [2].
Activation of fibroblasts by inflammatory cells is associated with cell proliferation and increased deposition of collagen and fibronectin, causing the thickening of the basal membrane and the appearance of a subepithelial fibrosis [3]. Previously, fibroblasts have been considered mainly a physical barrier, but recent studies have shown that they may be important modulators of local inflammation due to their capacity to release a variety of proinflammatory mediators such as eotaxin, interleukin (IL)-6 and IL-8 [4,5]. In particular, eotaxin is a potent chemoattractant for eosinophils and Th2 cells, which are hallmarks of the inflammation associated with allergic disease [6].
The findings that IL-4, IL-13, tumour necrosis factor (TNF)-α and interferon (IFN)-γ can act directly on fibroblast cell lines [7–10] also suggests that they may be important effector cells in the pathogenesis of airway inflammation and remodelling and consequently targets for new treatment strategies. In fact, some commonly used anti-allergic and anti-inflammatory drugs have been shown to down-regulate adhesion molecule expression and proliferation in fibroblasts [11,12]. Considering that approximately 30% of the lung and airway tissues consist of fibroblasts [12] it is very probable that their functions as well as the modulation of these functions will have a profound impact on the inflammatory response.
We used fibroblasts isolated from nasal polyps, which represent a good model of chronic airway inflammation, because many histopathological features are shared [13]. The recently proposed concept of united airway disease (UAD) provides evidence for a strict connection between upper and lower respiratory tracts [14]. The same kinetics of inflammation, the same pattern of recruited cells, and the up-regulation of the same adhesion molecules occur after specific challenge in the mucosa of the nose, conjunctiva and bronchi of allergic patients [15–17].
Previous studies on fibroblast regulation by cytokines have been performed using one or a combination of recombinant cytokines in high concentration ranges [7–9,18,19]. However, in vivo, many known and unknown mediators, present in minute concentrations, are likely to contribute to the immune regulation in a synergistic network.
In this study we aimed to investigate how the mediators produced by peripheral blood mononuclear cells (PBMC), in the concentrations actually produced by these cells, can regulate proinflammatory functions of fibroblasts.
MATERIALS AND METHODS
Patients
PBMC were isolated from 14 patients with documented allergy to the house dust mite Dermatophagoides pteronyssinus (Dp). Fibroblasts were obtained from nasal polyps of six patients undergoing nasal surgery. None of the patients received anti-inflammatory drug treatment during a minimum of 2 weeks before cells were isolated.
Informed consent was obtained from all subjects participating in the study and appropriate ethical approval was obtained from Comitato Etico, Dipartemento di Medicina Interna e Specialità Mediche, Genoa University, Genoa, Italy.
Preparation and stimulation of PBMC
PBMC were prepared from heparinized whole blood samples using Ficoll gradient centrifugation (Lympholyte-H, Cedarlane Laboratory Ltd, Canada). The cells were recovered in RPMI-1640 (Gibco, Milan, Italy) supplemented with 10% FCS (Gibco), 50 µg/ml penicillin (Sigma, St Louis, MO, USA), 50 µg/ml streptomycin (Sigma) and 2 mm glutamine (Sigma) and seeded at a concentration of 1 × 106 cells/ml in 96-well microtitre plates for proliferation and in six-well plates for collection of supernatants. For specific allergen stimulation 10 µg/ml Dp extract (Lofarma, Milan, Italy) was used. This concentration was shown to give optimal cell activation in our preliminary studies. This is also a concentration of in vivo relevance as the risk level of sensitization is 2 µg/ml of the major allergen Der p1 [20].
As a negative control of the specific proliferation, PBMC from five non-allergic subjects were stimulated in the same way. The proliferation was measured on day 7 after allergen stimulation. During the last 18 h of incubation 1·0 µCi/well of [3H]thymidine was added and the thymidine incorporation was determined by scintillation counting in a beta-counter. All samples were set up in triplicate and the mean value was calculated. Stimulation index (SI) was calculated as counts per minute (cpm) values after allergen stimulation divided by cpm value without stimulation.
Cell free supernatants were collected after 5 days of allergen stimulation and stored at − 20°C until use.
Preparation and stimulation of primary fibroblasts
Nasal tissue was cut into small fragments and incubated in RPMI-1640 medium containing a mixture of 10 UI/ml DNAse, 500 UI/ml collagenase type IV and 30 UI/ml hyaluronidase (all enzymes purchased from Sigma) on a magnetic stirrer for 2 h at 37°C. The cells were then cultured in RPMI supplemented with 10% FCS, glutamine and antibiotics, as described above. The fibroblast cultures were characterized in flow cytometry using the specific mouse IgG1 monoclonal antibody ASO2 (Dianova, Hamburg, Germany), that according to the manufacturer reacts specifically with a membrane-bound protein of human fibroblasts, and anticytokeratin antibody (Immunotech, Marseille, France) by intracytoplasmic immunofluorescence staining. In addition, using immunoperoxidase staining the presence of vimentin (Biogenex, CA, USA), fibronectin (kindly provided by Prof L. Zardi, IST, Genoa, Italy), α-smooth muscle actin (α-SMA) (Dako, CA, USA), smoothelin and myosin (both kindly provided by Prof G. Gabbiani, Department of Pathology, University of Geneva, Switzerland) was assessed. The characterization is presented in Table 1.
Table 1.
Characterization of fibroblast cell cultures by immunohistochemistry†or flowcytometry‡
| Antigen | Immunoreactivity* |
|---|---|
| Cytokeratin‡ | − |
| Thy-1 (CD90)‡ | + + |
| Vimentin† | + + |
| Fibronectin† | + + |
| Smoothelin† | − |
| α-Smooth muscle actin† | + − |
| Myosin† | + |
Indicates no positive staining; ± indicates <5% positive staining; + indicates <50% positive staining; ++ indicates>50% positive staining. In flow cytometry – indicates no positive cells, and ++ indicates 100% positive cells.
Cells were seeded in 24-well culture plates and grown close to confluence, before incubation with PBMC supernatants (stimulated as described above). Cell-free supernatants were collected after 24 h of incubation at 37°C and stored at − 20°C, and the cells were recovered for flow cytometry analysis. Preliminary studies showed that by increasing the incubation time up to 48 h no additional effects on adhesion molecule expression or cytokine production were detected. Individual effects of TNF-α and IFN-γ were studied by incubating the fibroblasts with recombinant (r) TNF-α or rIFN-γ (Euroclone, Milan, Italy) at concentration ranges (250–15000 pg/ml) comparable to those found in the PBMC supernatants plus a higher concentration, similar to what has been used in previous studies [7–9,18,19].
Inhibition experiments
The inhibition of TNF-α was studied in PBMC from four subjects by preincubation of supernatants with 72 ng/ml of anti-TNF-α (Peprotech EC Ltd, UK) for 1 h at 37°C before addition to fibroblasts. This concentration was calculated to achieve 50% blocking efficiency of the highest concentration of TNF-α found in our supernatants.
Flowcytometry analysis of stimulated fibroblasts
Fibroblast surface expression of CD54 (mouse IgG1, Cymbus Biotechnology, Southampton, UK), CD106 (mouse IgG1, Cymbus Biotechnology) and CD40 (mouse IgG1, Ancell Corporation, MN, USA) was assessed by indirect immunoflourescence staining and analysed on a Coulter EPICS XL flow cytometer as described before [18,19]. As a secondary antibody, fluorescein isothiocyanate (FITC)- labelled goat antimouse IgG (Southern Biotechnology Association, Birmingham, AL, USA) was used.
Cytokine quantification in PBMC and fibroblast supernatants
Concentrations of IL-4, IL-6, IL-8, IL-13, TNF-α, IFN-γ and eotaxin were measured in PBMC supernatants and IL-6, IL-8 and eotaxin were assessed in fibroblast supernatants. Commercially available ELISA kits for IL-4, IFN-γ, IL-6, TNF-α, IL-8 (Euroclone, detection limits 0·5 pg/ml; 5 pg/ml; 2 pg/ml; 10 pg/ml; 25 pg/ml, respectively), IL-13, and eotaxin (R&D Systems UK, detection limits 32 pg/ml; 5 pg/ml, respectively) were used according to the manufacturers’ instructions. The final concentrations of cytokines produced by fibroblasts were obtained by subtracting the concentration of that particular cytokine measured in the PBMC supernatant used as stimulus.
Statistical analysis
To evaluate the significance of surface molecule expression and cytokine production, paired Student's t-test was used. For evaluating correlation between cytokine concentrations in PBMC supernatants and effects on fibroblast, Spearman's rank correlation was assessed. In order to increase the power of the correlation analysis, data were included from both allergen and non-stimulated PBMC. These results reflect the direct correlation between the absolute concentration of a cytokine in the PBMC supernatant used as stimulus and the effect on the fibroblasts. A P-value < 0·05 was considered significant. Outliers are excluded in the figures in order to clarify the presentation of the data, but they are included in all statistical calculations.
RESULTS
Proliferation and cytokine production in PBMC cultures
In allergic subjects the Dp-induced specific proliferation was significantly increased compared to non-stimulated cell cultures, calculated as SI (P = 0002) (SI range: 1·0–5·4, mean: 2·5). In the five non-allergic control subjects no significant specific proliferation was found (P = 0·3) (SI range: 0·3–3·1, mean: 1·2), excluding nonspecific stimulation by contamination in the allergen extract.
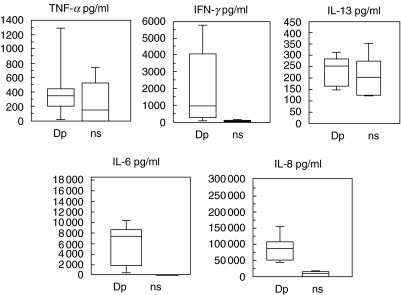
The production of cytokines was measured in the PBMC from six allergic patients with and without Dp stimulation. These supernatants were also used to stimulate fibroblasts. In Fig. 1 the production of cytokines, with and without Dp specific stimulation, is shown. TNF-α, IFN-γ, IL-6 and IL-8 were augmented following Dp stimulation; however, only IL-6 and IL-8 levels reached a statistically significant increase (P = 0·01 and P = 0·009, respectively). IL-13 expression was largely unchanged. IL-4 and eotaxin production were under the detection limit in all samples, and therefore not included in the figure.
Fig. 1.
Cytokine production in PBMC supernatants (n = 6) with or without Dp stimulation for 5 days. Dp = Dp stimulation; ns = no stimulation. The following concentration ranges and mean values in pg/ml were measured: TNF-α: Dp (0–1387; 455), ns (0–762; 267); IFN-γ: Dp (0–6000; 2036), ns (0–138; 59); IL-13: Dp (146–316; 236), ns (122–275; 219); IL-6: Dp (309–10 516; 6018), ns (0–75; 13); IL-8: Dp (43 954–160 185; 88 962), ns (0–16 337; 7701).
Cytokine production in fibroblast cultures
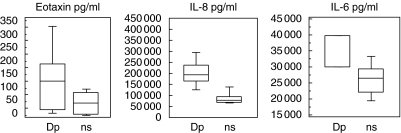
The production of cytokines in fibroblast cultures is shown in Fig. 2. The production of IL-6 and IL-8 was significantly higher in fibroblasts cultured with Dp-stimulated PBMC supernatants compared to non-stimulated supernatants (P = 0·02 and P = 0·002, respectively). The production of eotaxin found after incubation with Dp-stimulated supernatants did not reach statistical significance but a calculated P-value of 0·07 suggests an increase. This is also indicated by the fact that in all patients an increase in eotaxin production was observed (individual data not shown).
Fig. 2.
Cytokine production in fibroblast cultures, after incubation with PBMC supernatants (n = 6). Dp = Dp stimulated PBMC; ns = not stimulated PBMC. The final concentrations were corrected by subtracting the amount of the respective cytokine present in the PBMC supernatants used as stimulus of the fibroblasts. The following concentration ranges and mean values in pg/ml were measured: eotaxin: Dp (8–343; 136), ns (0–92; 37); IL-6: Dp (30 000–39 800; 33 282), ns (18 900–34 200; 26 330); IL-8: Dp (122 100–300 000; 194 522), ns (72 300–147 500; 82 470).
Table 2 shows statistical evaluation of the correlation's between cytokines in the PBMC supernatants and mediators produced by the stimulated fibroblasts. We found a positive correlation between the concentration of eotaxin produced by fibroblasts and the amount of TNF-α and IFN-γ in PBMC supernatants. The production of IL-6 and IL-8 in fibroblasts correlated with the levels of IL-6, IL-8 and IL-13 as well as IFN-γ.
Table 2.
Statistical correlations between cytokines concentrations in the PBMC supernatants used as stimulus (IL-6, IL-8, IL-13, TNF-α and IFN-γ) and the consequent expression of eotaxin, IL-6, IL-8, CD54, CD106, and CD40 by fibroblasts
| Fibroblast expression | ||||||
|---|---|---|---|---|---|---|
| PBMC release | Eotaxin | IL-6 | IL-8 | CD54 | CD40 | CD106 |
| IL-6 | 0·1 | 0·05* | 0·004** | 0·02* | 0·3 | 0·2 |
| IL-8 | 0·07 | 0·06 | 0·02* | 0·004** | 0·5 | 0·2 |
| IL-13 | 0·1 | 0·004** | 0·08 | 0·6 | 0·2 | 0·1 |
| TNF-α | 0·007** | 0·6 | 0·9 | 0·08 | 0·09 | 0·4 |
| IFN-γ | 0·03* | 0·03* | 0·008** | 0·005** | 0·03* | 0·02* |
Values of cytokine concentrations from both Dp stimulated and non-stimulated PBMC supernatants are included in the calculations. A Spearman's rank correlation test was used
a P-value = 0·05 was considered as significant.
P-value = 0·01 as highly significant
Up-regulation of surface markers
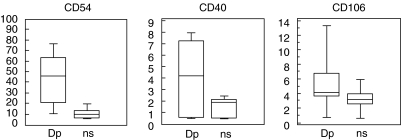
In fibroblasts cultured with allergen-stimulated PBMC a significant up-regulation of all three surface molecules was found, compared to non-stimulated cultures (CD54, P = 0·0001; CD40, P = 0·0005; CD106, P = 0·005). The results are presented in Fig. 3. The amounts of IFN-γ in the PBMC supernatants correlated significantly with the degree of up-regulation of all three surface molecules while IL-6 and IL-8 correlated mainly with CD54 expression (Table 2).
Fig. 3.
Expression of surface molecules on fibroblasts after incubation with PBMC supernatants (n = 14). Dp = Dp stimulated PBMC; ns = not stimulated PBMC. CD54: Dp (9·6–78; 43·8), ns (5·2–20·5; 10·7); CD40: Dp (0·5–8; 4·2), ns (0·4–2·5; 1·5); CD106: Dp (0·5–14; 5·6), ns (0·5–6·1; 3·3).
Effects of rTNF-αand rIFN-γ
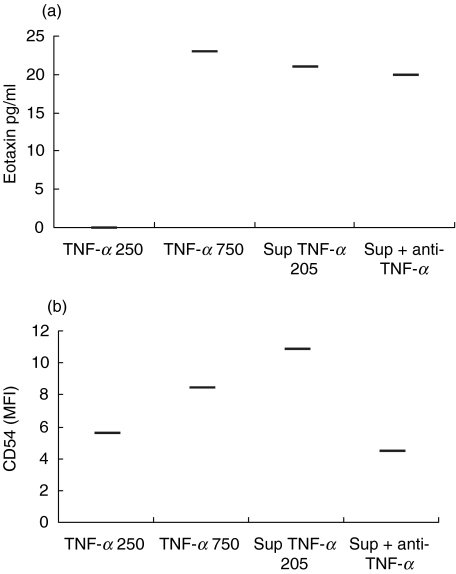
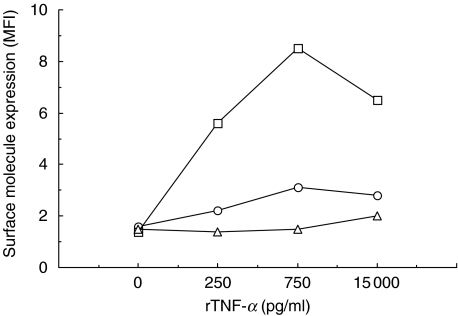
In order to evaluate their single effects, rTNF-α and rIFN-γ were added to fibroblast cultures at concentration ranges similar to those found in PBMC supernatants plus one higher concentration (range 250–15 000 pg/ml). Using a concentration of 250 pg/ml rTNF-α, no eotaxin was produced by the fibroblasts, while a production was induced by PBMC supernatant containing 205 pg/ml of TNF-α (Fig. 4a). An almost fourfold increase in rTNF-α (750 pg/ml) was required to obtain a comparable production to this PBMC supernatant. Blocking TNF-α in the supernatant provided only marginal down-regulation in eotaxin production (Fig. 4a). CD54 production was induced by rTNF-α already at the lowest concentration and showed a dose-dependent response while CD40 and CD106 were unaffected (Fig. 5). Furthermore, CD54 expression was specifically inhibited by the addition of anti-TNF-α antibody (Fig. 4b). It should be noted that the highest expression of CD54 was induced by PBMC supernatant. rIFN-γ did not have any effect on either eotaxin production or surface molecule expression by fibroblasts (data not shown).
Fig. 4.
Eotaxin production (a) and CD54 expression (b) by fibroblasts after stimulation with two concentrations of rTNF-α (250 and 750 pg/ml), a PBMC supernatant (TNF-α: 205 pg/ml) and the same PBMC supernatant preincubated with anti-TNF-α blocking antibodies. This figure presents one representative experiment of four.
Fig. 5.
Effect of four different concentrations of rTNF-α on the expression of surface molecules by primary fibroblast cultures. MFI = mean fluorescence intensity. □, CD54; ○, CD106; Δ, CD40.
rIFN-γ and rTNF-α were not able to induce IL-6 and IL-8 release from fibroblasts in a comparable way to the PBMC supernatants (Table 3).
Table 3.
Induction of IL-6 and IL-8 production in fibroblasts by recombinant TNF-α and IFN-γ, respectively, or PBMC supernatants with a comparable amount of these cytokines. One representative experiment of four is shown
| Fibroblast expression | ||
|---|---|---|
| IL-6 | IL-8 | |
| Supernatant (708 pg/ml TNF-α) | 21 146 | 79 098 |
| 750 pg/ml rTNF-α | 6 336 | 16 873 |
| Supernatant (621 pg/ml IFN-γ) | 29 852 | 190 643 |
| 750 pg/ml rIFN-γ | 328 | 363 |
DISCUSSION
It is now becoming evident that fibroblasts are not merely barrier cells, but also important modulators of the inflammatory response by their capacity to secrete mediators and express surface molecules [7–9,21,22]. Previous in vitro studies have shown important effects of recombinant individual cytokines on fibroblasts isolated from different target organs as well as on cell lines. In this study we have investigated the effects of allergen-stimulated PBMC supernatants on primary nasal fibroblasts, thereby trying to mimic a more in vivo-like environment regarding the presence of both known and unknown mediators which may act synergistically, even in very low concentrations. An important observation is that the cytokine concentrations we found in our PBMC supernatants were lower than those used in most studies applying pure cytokines in fibroblast cultures. These differences were remarkable in some cases. For example, different studies have shown induction of eotaxin production in dermal or nasal fibroblasts by TNF-α, IL-4 and IL-13 using concentration ranges from 20 to 104 times more than we found in our PBMC supernatants [4,7–10]. Although the concentrations of individual cytokines in our supernatants were low, they had profound effects on fibroblasts by inducing chemokine/cytokine production and up-regulation of surface molecules. This indicates that, in vivo, the cells may be regulated by very subtle changes in the cytokine network rather than highly polarized effects exerted by single mediators. We studied the effects of TNF-α in more depth because this cytokine has been reported to have important effects on fibroblast functions and because we found some significant correlations between TNF-α concentrations in PBMC supernatants and effects on fibroblasts. However, when stimulating fibroblasts with the recombinant cytokine, the effects on fibroblasts were different to those found using PBMC supernatants, although the concentration of the individual cytokine was comparable. For example, to obtain the same level of eotaxin production, a fourfold higher concentration of rTNF-α was required. Moreover, when blocking TNF-α in the PBMC supernatants no significant decrease was found in the chemokine production. This supports further the view that synergistic effects orchestrate the cytokine network and that small changes may be crucial to the final response rather than large alterations in a single cytokine. Regarding CD54 expression, the role of TNF-α seems to be clearer, as the expression of this adhesion molecule was induced directly by recombinant TNF-α at the same concentrations as found in our PBMC supernatants and its expression was significantly down-regulated by blocking TNF-α. Interestingly, no significant correlation was found between TNF-α in PBMC supernatants and CD54 expression, indicating that an inhibitory synergism may also be present in the cytokine network. Furthermore, the reverse situation was found with IFN-γ that correlated significantly with CD54 expression, but when adding rIFN-γ to fibroblasts no effects were found. However, when adding rIFN-γ together with rTNF-α an up-regulation, even higher than with rTNF-α alone, was observed (unpublished observations), again supporting the important role of synergism. Also in other studies the important role of synergistic effects have been highlighted. Concerning eotaxin production in fibroblasts, several combinations of cytokines have been shown to be important; TNF-α and IL-4, as well as TNF-α and IL-13, enhance eotaxin production when added together, while IFN-γ, on the other hand, inhibits TNF-α-induced eotaxin production [7,9,10].
Because various cytokines have different kinetic patterns and any chosen time-point cannot represent the optimal production of all individual cytokines, we performed preliminary experiments to evaluate the influence of different incubation time points. We stimulated PBMC for 3, 5 and 7 days and measured the effects on surface molecules up-regulation on fibroblasts after stimulation with these supernatants. The 5-day stimulation was shown to give the best specific response according to the ratio obtained between stimulated and non-stimulated fibroblasts. The fact that we chose one time-point may be the reason why IL-4 production was under the detection limit in our measurements. This is in accordance with previous studies showing difficulties in detecting IL-4 [23], and that IL-4 is produced during a short time-frame with a rapid decline [24]. We cannot, therefore, draw any conclusions about the role of this cytokine in our supernatants. However, of the fibroblast effects that we are studying, Azzarone and colleagues have shown that IL-4 alone only influence CD106 expression [4]. Furthermore, as IL-4 and IL-13 have largely been shown to have overlapping effects on fibroblasts [4,9] it is plausible that in our experiments IL-13 has the most important effect, due to the higher concentrations found.
The specific mediators released by fibroblasts that we measured in this study all have possible important impacts on airway inflammation; IL-6 induces T cell activation and proliferation [25], and the recently characterized CC chemokine eotaxin selectively recruits eosinophils in the airways [26]. IL-8, a CXC chemokine mainly associated with neutrophil function, has been linked to a variety of inflammatory diseases in the lung, including adult respiratory distress syndrome and chronic bronchitis, and in allergic asthma IL-8 levels correlate with the severity of the disease [27,28].
An important signalling between lymphocytes and resident cells in the tissue can also be delivered through direct cell contact mediated by surface molecules. The up-regulation of CD54 and CD40 on fibroblasts following incubation with allergen-stimulated PBMC supernatants is in accordance with the hypothesis of an enhancing cross-talk between fibroblasts and leucocytes [29]. The CD40–CD40L interaction has, for example, been shown to directly induce IL-6 and IL-8 production by fibroblasts [30].
This cross-talk including resident fibroblasts expressing increased amounts of CD54 and CD40 may lead to an enhancement of the inflammatory process. More studies using co-culture systems are needed to increase the knowledge of these mechanisms.
Taken together, these and previous results show clearly that fibroblasts are able to act as specific immune modulators in the allergic inflammation and should therefore be considered as important therapeutic targets. Furthermore, our study indicates that the regulating cytokines function in a delicate synergistic network that may not be mimicked properly with individual mediators. Consequently, results from in vitro studies must be interpreted carefully when cytokines in high concentrations are applied individually.
Acknowledgments
We would like to thank Prof. G Passalacqua and Prof. A De Maria for valuable help with statistical evaluation. Financial support for this study was received from the ARMIA Foundation, the Italian Medical Research Council (CNR), The Swedish Medical Research Council (MFR), the Hesselman Foundation, the Swedish Medical Society, the Tore Nilsson Research Foundation, the UCB Institute of Allergy, the Swedish Society for Medical Research and the Pharmacia Allergy Research Foundation.
References
- 1.Canonica GW, Ciprandi G, Buscaglia S, Pesce G, Bagnasco M. Adhesion molecules of allergic inflammation: recent insights into their functional roles. Allergy. 1994;49:135–41. doi: 10.1111/j.1398-9995.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 2.Passalacqua G, Venturi S, Zoccali P, et al. Cytokine and airways: recent insights and therapeutic implications. Pulmonary Pharmacol Ther. 1998;II:375–9. doi: 10.1006/pupt.1999.0165. [DOI] [PubMed] [Google Scholar]
- 3.Brewester CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–11. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 4.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol. 1998;10:1421–33. doi: 10.1093/intimm/10.10.1421. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi R, Yamashita N, Matsui S, Maruyama M, Sugiyama S, Kobayashi M. Bradykinin stimulates interleukin-8 production by human lung fibroblasts. Immunology. 1998;95:507–11. doi: 10.1046/j.1365-2567.1998.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provide a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–56. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 7.Teran LM, Mochizuki M, Bartels J, et al. Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am J Respir Cell Mol Biol. 1999;20:777–86. doi: 10.1165/ajrcmb.20.4.3508. [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki M, Schroder JM, Christophers E, Yamamoto S. IL-4 induces eotaxin in human dermal fibroblasts. Int Arch Allergy Immunol. 1999;120:19–23. doi: 10.1159/000053587. [DOI] [PubMed] [Google Scholar]
- 9.Terada N, Hamano N, Nomura T, et al. Interleukin-13 and tumor necrosis factor-α synergistically induce eotaxin production in human nasal fibroblasts. Clin Exp Allergy. 2000;30:348–55. doi: 10.1046/j.1365-2222.2000.00750.x. [DOI] [PubMed] [Google Scholar]
- 10.Miyamasu M, Yamaguchi M, Nakajima T, et al. Th1-derived cytokine IFN-gamma is a potent inhibitor of eotaxin synthesis in vitro. Int Immunol. 1999;11:1001–4. doi: 10.1093/intimm/11.6.1001. [DOI] [PubMed] [Google Scholar]
- 11.Cagnoni F, Oddera S, Semino C, Mincarini M, Melioli G, Canonica GW. Cetirizine-induced downregulation of airway fibroblast proliferation and function: a rationale for a different approach to allergy treatment? Immunol Lett. 2000;72:31–4. doi: 10.1016/s0165-2478(00)00151-6. [DOI] [PubMed] [Google Scholar]
- 12.Meyer J, Berner I, Teran LM, et al. RANTES production by cytokine-stimulated nasal fibroblasts: its inhibition by glucocorticoids. Int Arch Allergy Immunol. 1998;117:60–7. doi: 10.1159/000023991. [DOI] [PubMed] [Google Scholar]
- 13.Jahnsen FL, Haraldsen G, Aanesen JP, Haye R, Brandtzaeg P. Eosinophil infiltration is related to increased expression of vascular cell adhesion molecule-1 in nasal polyps. Am J Respir Cell Mol Biol. 1995;12:624–32. doi: 10.1165/ajrcmb.12.6.7539273. [DOI] [PubMed] [Google Scholar]
- 14.Passalacqua G, Ciprandi G, Canonica GW. The nose–lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001;1:7–13. doi: 10.1097/01.all.0000010978.62527.4e. [DOI] [PubMed] [Google Scholar]
- 15.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–76. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 16.Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma after bronchial provocation. Am Rev Respir Dis. 1989;139:806–17. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- 17.Bousquet J, Vignola AM, Campbell AM, Michel FB. Pathophysiology of allergic rhinitis. Int Arch Allergy Immunol. 1996;110:207–18. doi: 10.1159/000237289. [DOI] [PubMed] [Google Scholar]
- 18.Postiglione L, Montagnani S, Riccio A, et al. Expression of GM-CSF receptor and ‘in vitro’ effects of GM-CSF on human fibroblasts. Life Sci. 1998;63:327–36. doi: 10.1016/s0024-3205(98)00281-1. [DOI] [PubMed] [Google Scholar]
- 19.Sato E, Nelson DK, Koyama S, Hoyt JC, Robbins RA. Inflammatory cytokines modulate eotaxin release by human fibroblast cell line. Exp Lung Res. 2001;27:173–83. doi: 10.1080/019021401750069401. [DOI] [PubMed] [Google Scholar]
- 20.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der pI) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;23:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 21.Minshall EM. Fibroblasts: a cell type central to eosinophil recruitment? Clin Exp Allergy. 2000;30:301–3. doi: 10.1046/j.1365-2222.2000.00753.x. [DOI] [PubMed] [Google Scholar]
- 22.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 23.Nurse B, Puterman AS, Haus M, Berman D, Weinberg EG, Potter PC. PBMCs from both atopic asthmatic and nonatopic children show a TH2 cytokine response to house dust mite allergen. J Allergy Clin Immunol. 2000;106:84–91. doi: 10.1067/mai.2000.107397. [DOI] [PubMed] [Google Scholar]
- 24.van Bever HP, Vereecke IF, Bridts CH, De Clerck LS, Stevens WJ. Comparison between the in vitro cytokine production of mononuclear cells of young asthmatics with and without immunotherapy (IT) Clin Exp Allergy. 1998;28:943–9. doi: 10.1046/j.1365-2222.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–32. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsner J, Hochstetter R, Kimmig D, Kapp A. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur J Immunol. 1996;26:1919–25. doi: 10.1002/eji.1830260837. [DOI] [PubMed] [Google Scholar]
- 27.Kunkel SL, Standiford T, Kasahara K, Strieter RM. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991;17:17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- 28.Shute JK, Vrugt B, Lindley IJ, et al. Free and complexed interleukin-8 in blood and bronchial mucosa in asthma. Am J Respir Crit Care Med. 1997;155:1877–83. doi: 10.1164/ajrccm.155.6.9196089. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Cao HJ, Graf B, Meekins H, Smith TJ, Phipps RP. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol. 1998;160:1053–7. [PubMed] [Google Scholar]
- 30.Kaufman J, Graf BA, Leung EC, et al. Fibroblasts as sentinel cells: role of the CD40–CD40 ligand system in fibroblast activation and lung inflammation and fibrosis. Chest. 2001;120:53S–55S. doi: 10.1378/chest.120.1_suppl.s53. [DOI] [PubMed] [Google Scholar]