Abstract
Several recombinant clones expressing antigens from Echinococcus granulosus were isolated previously from a parasite cDNA library using cystic hydatid disease (CHD) patients’ sera or rabbit hyperimmune antiserum against a lipoproteic fraction from bovine cyst fluid. Six of these antigens were expressed in Escherichia coli and the purified recombinant proteins were tested in enzyme-linked immunosorbent assay (ELISA) for specific IgG with a panel of sera from patients with surgically confirmed (n = 58) or immunologically diagnosed (n = 71) CHD. Sera from clinically normal individuals (n = 203) and sera from individuals with other helminthic infections (n = 65) were assayed for the assessment of specificity. A cut-off value was determined by receiver-operating-characteristic plots for each antigen. A recombinant antigen B subunit (AgB8/2) presented the highest sensitivity (93·1%), considering the group of sera from patients with CHD surgically confirmed, and specificity (99·5%) and is proposed as the basis for an immunodiagnostic test. The other recombinant antigens tested presented sensitivities between 58·6% and 89·7%, and three of them were considered of complementary value. In subclass-specific ELISA, different IgG isotypes showed dominance in the response for each of the recombinant antigens. There was a clear predominance of IgG4 response for all antigens tested, indicating that this would be the subclass of choice to be assessed for these recombinant proteins.
Keywords: Echinococcus granulosus, cystic hydatid disease, ELISA, immunodiagnosis, recombinant antigens
INTRODUCTION
Cystic hydatid disease (CHD) is a zoonosis caused by the infection with the metacestode stage of Echinococus granulosus (Cestoda, Taeniidae). In humans, the metacestodes develop as large cysts, especially in the liver and lungs, causing unspecific symptoms that depend on the size and localization of the lesions [1–3]. Most human CHD cases are initially discovered by clinical examinations using different imaging techniques, such as radiology or ultrasonography [4]. However, this preliminary diagnosis must be complemented by more specific tests, such as immunological assays based on the detection of antiparasite circulating antibodies in patients’ sera [5]. The serological diagnosis in a routine laboratory depends mainly on the detection of immunoglobulin class G (IgG) antibodies directed against different antigens of E. granulosus. Nevertheless, several reports demonstrate the value of analysing specific IgG subclass antibodies for a sensitive and specific serological diagnosis of CHD or to follow-up studies after surgery or after initiation of chemotherapy [5–7]. The immunodiagnosis of CHD might be improved by the detection of subclass-specific antibodies against distinct antigens of E. granulosus[8], and the problem of cross-reaction with sera from patients with other parasitic diseases appears to be reduced significantly with the use of IgG subclass detection [9,10].
Classically, tests such as immunoelectrophoresis, double diffusion in agar or indirect haemagglutination were used as references for human CHD diagnosis [8,11]. Nowadays, these tests are being replaced by more sensitive assays, such as enzyme-linked immunosorbent assay (ELISA), immunoblot and immunofluorescence. Due to its high sensitivity, ELISA is strongly recommended to be used for the detection of specific antibodies in human CHD cases [12–14].
The efficiency (sensitivity and specificity) of any immunodiagnostic test for human CHD depends on the characteristics of the panel of sera used (e.g. from patients with or without surgical confirmation of infection) and the quality of the parasite antigens utilized in the assay. Crude or partially purified antigen preparations are useful, but often present problems associated with low concentration of specific antigens and the presence of cross-reactive ones [13,15]. As an alternative, E. granulosus antigen-encoding genes can be cloned and expressed in heterologous systems, in order to obtain recombinant antigens that can be produced and purified easily, and may be less prone to cross-reactivity [10,16,17].
Our laboratory, individually or in association with other research groups, has cloned several E. granulosus genes, among which are those coding for two different AgB subunits [18,19], a malate dehydrogenase [20,21], an actin filament fragmenting protein [22] and a calcium binding protein [23]. These proteins were expressed in Escherichia coli and the recombinant antigens produced showed, in preliminary tests, potential for use in the immunodiagnosis of human CHD [19,21]. Here, we present an extensive serological survey, testing each of these recombinant antigens in total IgG ELISA with a panel of 129 sera from CHD patients, 58 of them with surgical confirmation of the infection. We have also performed specificity assays with 203 sera from clinically normal individuals, and with 18 sera from toxocariasis and 47 sera from cysticercosis patients, potentially cross-reactive infections prevalent in CHD endemic areas. Finally, we have assessed the specific IgG subclass response to the recombinant antigens in sera from CHD patients. Our results confirmed that the use of these sets of antigens might compose a dependable ELISA or related assay (e.g. immunodot) to complement the clinical diagnosis of human CHD.
MATERIALS AND METHODS
Patients and control sera
Serum samples were obtained from 58 CHD patients confirmed by surgery (group I) recruited in Uruguay by the Cátedra de Inmunología (Universidad de la Republica, Montevideo, Uruguay). All blood samples were drawn before the surgical intervention. All patients had liver disease but four of them also exhibited lung, spleen or peritoneum localization of cysts. Forty-nine patients presented primary CHD and nine showed relapsed CHD. Most of the cysts were hialin and only three patients had partially calcificed cysts. A second panel of sera included 71 samples obtained in Rio Grande do Sul State (Southern Brazil) from CHD patients diagnosed clinically (by radiology or ultrasonography) and immunologically (by immunoelectrophoresis for the detection of arc 5 or by ELISA and/or immunoblot using crude antigen preparations from hydatid cyst fluid) (group II). Eighteen sera from toxocariasis (Toxocara spp.) patients and 47 sera from cysticercosis (Taenia solium) patients, either from São Paulo State (South-eastern Brazil) or from Rio Grande do Sul, were tested. Sera from additional 203 healthy individuals were also included in this study as negative controls; the donors had no recent history of any parasitic disease and were from Rio Grande do Sul and Uruguay urban areas (not endemic for CHD). All procedures had the approval of the Ethical Committees of the institutions involved and all donors authorized the use of their sera for research purposes.
Native antigens
Hydatid cyst fluid antigen (HCFA) was obtained by aspiration from fertile bovine cysts. Each extract was prepared according to the protocol described by Maddison et al. [24]. The AgB, kindly provided by G. González (Cátedra de Inmunología, Montevideo, Uruguay), was purified as described previously [25].
Recombinant antigens
Six recombinant antigens were used in this study: a cytosolic malate dehydrogenase isoform (EgcMDH) [20,21], two 8 kDa subunits of antigen B (AgB8/1) [19] and (AgB8/2) [18], an EF-hand calcium-binding protein (EgCaBP2) [23], a full-length (EgAFFPf) and a truncated form (EgAFFPt, aa 261–370) of an actin filament fragmenting protein [22]. Each antigen encoding cDNA was cloned previously and expressed in Escherichia coli BL21 (Amersham, Uppsala, Sweden) and/or BL21 Codon Plus Ril (Stratagene, La Jolla, CA, USA) strains using plasmid vectors of the pGEX series (Amersham). The recombinant antigens, expressed as fusion proteins with glutathione S-transferase (GST), were purified by affinity chromatography according to Smith and Johnson [26]. The fusion protein antigen moieties were recovered by thrombin cleavage (10 U/mg of bound fusion protein) for 14 h at room temperature. Yields of 2–18 mg of recombinant antigen per litre of culture were obtained depending on the clone. The concentrations of the recovered proteins were determined by spectrophotometry [27].
Elisa
Total IgG
The assays were performed as described by Rott et al.[19], with some modifications. Briefly, microtitration plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with 0·4 µg/well of each recombinant antigen, 0·25 µg/well of AgB or 2 µg/well of crude HCFA diluted in 0·1 m carbonate/bicarbonate buffer (pH 9·6). The sera samples were diluted 1 : 100 in blotto [phosphate buffered saline (PBS) containing 5% milk powder] and tested in duplicate. Positive and negative sera and conjugate controls were included in each plate. Goat antihuman IgG conjugated to horseradish peroxidase (Sigma, St. Louis, USA), diluted 1 : 1000, was used as a second antibody. OPD (0·4 mg/ml o-phenilendiamine dihydrochloride in 0·1 m phosphate/citrate buffer, pH = 5) and H2O2 were used to visualize the antigen–antibody reaction. Optical density (O.D.) was registered at 492 nm (A492) after the addition of stop solution (H2SO4 1 N). The considered value of A492 represents the mean of two readings for each serum with less than 10% of variation between them. For the interpretation of seropositivity, threshold values (cut-off) were calculated for each antigen based on receiver-operating-characteristic (ROC) curves [28], as described below.
IgG subclasses
The assays were performed as described by Hernández et al.[29], with modifications. Briefly, microtitration plates (Maxisorp, Nunc) were coated with 0·4 µg/well of each recombinant antigen diluted in 0·1 m carbonate/bicarbonate buffer (pH 9·6). The sera samples were diluted 1 : 100 in PBS-T-BSA (PBS with 0,05% Tween and 1% bovine serum albumin) and tested in duplicate. Positive, negative and no serum controls were included in each plate. Goat antihuman IgG1, IgG2, IgG3 and/or IgG4 with biotin (Sigma) were diluted 1 : 1600, 1 : 5000, 1 : 4000 and 1 : 20000, respectively, in PBS-T-BSA and incubated at 37°C for 90 min. Extravidin-peroxidase (Sigma), diluted 1 : 4000, was utilized for binding with the biotin conjugate by incubating it for 40 min at 37°C. OPD was used to visualize the antigen-antibody reaction, as described for total IgG. The cut-off values were calculated as described below.
Statistical analysis
A ROC analysis [28] was performed to determine a cut-off value for each recombinant antigen. Levels of sensitivity were plotted against the levels of one minus specificity at each cut-off point on a ROC curve. Threshold values used were those that gave the highest sum of sensitivity (%) and specificity (%), as described by Amagai et al. [30]. The area under the ROC curve (AUC) was the parameter used to define the antigens’ discriminatory values (between subjects with and without the disease). Student's t-test for paired observations with statistical significance level of P = 0·05 was used to test differences between AUCs [31]. All statistical calculations were performed using the Statistical Program for Social Science (SPSS) [32].
RESULTS
ELISA for total IgG
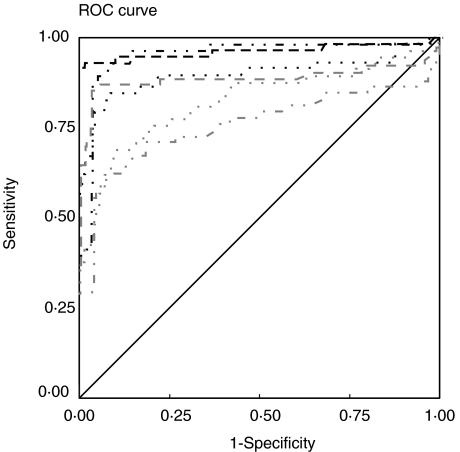
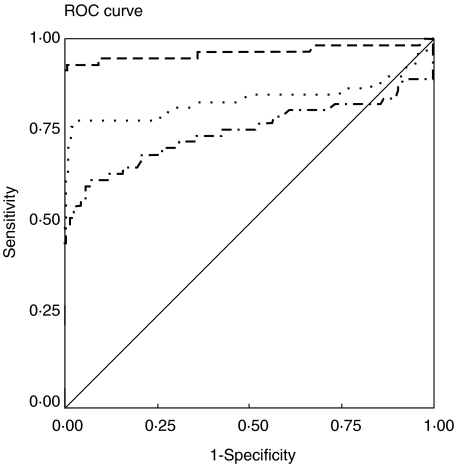
The AgB, the HCFA and the six recombinant antigens from E. granulosus (AgB8/2, AgB8/1, EgcMDH, EgCaBP2, EgAFFPt and EgAFFPf) were tested in an anti-IgG ELISA with sera from CHD patients and from clinically normal sera. ROC analyses were performed to determine the cut-off values that differentiate positive and negative reactions most effectively. Figure 1 shows the ROC curves obtained for the recombinant antigens with the group I sera (confirmed by surgery). The highest AUC value was that for AgB8/2 (0·964), although it was not significantly different (Z = 0·44, P = 0·05) from that for EgcMDH (0·953). Nevertheless, AgB8/2 AUC was significantly higher than any of the AUCs calculated for the other recombinant and native antigens tested (Z-values between 1·97, P = 0·05 and 4·41, P = 0·001). As shown in Fig. 2, AgB8/2 discriminatory value was also significantly better than those of HCFA (AUC = 0·831) and AgB (AUC = 0·735) (Z = 3·02, P = 0·01 and Z = 4·77, P = 0·001, respectively). The other AgB recombinant subunit, AgB8/1, presented an AUC of 0·897, followed by recombinants EgCaBP2 (0·879), EgAFFPf (0·824) and EgAFFPt (0·757). The sensitivity and specificity data and relevant parameters derived from ROC analysis for each antigen are summarized in Table 1. Among the 58 positive sera, only one showed no reaction with any of the recombinant antigens.
Fig. 1.
ROC curves used to determine cut-off and AUC values for the six recombinant antigens tested in total IgG ELISA against sera from group I and from healthy individuals. The calculated cut-offs and AUCs are presented in Table 1. Source of the curve: Black lines: —, reference line; – – –, AgB8/2; ·· ·, AgB8/1; ·· – – · ·, EgcMDH; Grey lines: –– –, EgCaBP2; ·· ·, EgAFFPf; ·· – – · ·, EgAFFPt.
Fig. 2.
ROC curves used to determine cut-off and AUC values for HCFA and AgB tested in total IgG ELISA against sera from group I and from healthy individuals. The AgB8/2 ROC curve was included for comparison. The calculated cut-offs and AUCs are presented in Table 1. Source of the curve: —, reference line; – ––, AgB8/2; - - -, HFCA; –· − · –, AgB.
Table 1.
Summary of parameters derived from ROC analysis of recombinant and native antigens tested in ELISA against sera from group I and from healthy individuals
| Recombinant antigen | (AUC) ± s.e. | CI (95%) | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| AgB8/2 | 0·964 ± 0·021 | 0·923–1·000 | 0·283 | 93·1 | 99·5 |
| AgB8/1 | 0·897 ± 0·033 | 0·833–0·961 | 0·248 | 84·5 | 91·2 |
| EgcMDH | 0·953 ± 0·019 | 0·916–0·990 | 0·155 | 89·7 | 95·1 |
| EgCaBP2 | 0·879 ± 0·039 | 0·802–0·955 | 0·108 | 84·5 | 96·6 |
| EgAFFPt | 0·757 ± 0·047 | 0·666–0·849 | 0·133 | 58·6 | 95·6 |
| EgAFFPf | 0·824 ± 0·038 | 0·750–0·898 | 0·215 | 69·0 | 89·7 |
| AgB | 0·735 ± 0·050 | 0·637–0·833 | 0·237 | 60·3 | 92·6 |
| HCFA | 0·831 ± 0·044 | 0·745–0·918 | 0·256 | 77·6 | 96·6 |
AUC = area under the curve; s.e. = standard error; CI = confidence interval.
A considerable reduction in AUC value was observed for some antigens in the tests performed with group II sera (patients diagnosed clinically and/or immunologically) (Table 2, compare to data in Table 1). For instance, AgB8/2, AgB8/1, EgcMDH and EgAFFPf had their AUC values significantly reduced from 0·964 to 0·850 (Z = 2·07, P = 0·05), from 0·897 to 0·705 (Z = 2·70, P = 0·01), from 0·953 to 0·749 (Z = 3·92, P = 0·001) and from 0·824 to 0·492 (Z = 4·95, P = 0·001), respectively. The AUC value reductions observed for HCFA (from 0·831 to 0·733), AgB (from 0·735 to 0·689), EgCaBP2 (from 0879 to 0·775) and EgAFFPt (from 0·757 to 0·714) were not statistically significant for P = 0·05 (Z = 1·34, Z = 0·58, Z = 1·65 and Z = 0·60, respectively).
Table 2.
Summary of parameters derived from ROC analysis of recombinant and native antigens tested in ELISA against sera from group II and from healthy individuals
| Recombinant antigen | (AUC) ± s.e. | CI (95%) | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| AgB8/2 | 0·850 ± 0·051 | 0·750–0·950 | 0·099 | 73·2 | 80·0 |
| AgB8/1 | 0·705±0·063 | 0·581–0·828 | 0·115 | 73·2 | 61·1 |
| EgcMDH | 0·749 ± 0·049 | 0·654–0·844 | 0·095 | 53·5 | 86·5 |
| EgCaBP2 | 0·775 ± 0·049 | 0·679–0·871 | 0·108 | 74·6 | 78·4 |
| EgAFFPt | 0·714 ± 0·053 | 0·611–0·818 | 0·102 | 56·3 | 78·4 |
| EgAFFPf | 0·492 ± 0·055 | 0·385–0·600 | 0·086 | 44·4 | 73·0 |
| AgB | 0·689±0·062 | 0·567–0·810 | 0·255 | 59·2 | 75·9 |
| HCFA | 0·733 ± 0·058 | 0·619–0·847 | 0·121 | 83·1 | 64·9 |
AUC = area under the curve; s.e. = standard error; CI = confidence interval.
Specificity values were also calculated considering sera from cysticercosis and toxocariasis patients. Table 3 summarizes the results obtained for each recombinant or native antigen using the cut-offs calculated for group I and healthy subjects’ sera (Table 1). EgAFFPf presented no cross-reactions (100% specificity), while the other recombinant antigens presented a limited number of positive reactions with sera from cysticercosis or toxocariasis (specificities between 84·6% and 97·0%). Native antigen preparations showed lower specificities than those showed by any of the recombinant antigens (78·4% for HCFA and 81·5% for AgB).
Table 3.
Summary of the results of specificity with sera from cysticercosis and toxocariasis patients obtained in total IgG ELISA against the recombinant antigens
| No. of positive reactions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group (no. of sera tested) | AgB8/2 | AgB8/1 | EgcMDH | EgCaBP2 | EgAFFPt | EgAFFPf | AgB | HCFA |
| Cysticercosis (47) | 3 | 2 | 5 | 8 | 1 | 0 | 8 | 11 |
| Toxocariasis (18) | 1 | 0 | 0 | 2 | 1 | 0 | 4 | 3 |
| Total (65) | 4 | 2 | 5 | 10 | 2 | 0 | 12 | 14 |
| Specificity (%) | 94·0 | 97·0 | 92·3 | 84·6 | 97·0 | 100·0 | 81·5 | 78·4 |
Specificity was calculated by rate between the absolute number of positive sera among the 65 sera from bearers of the helminthic infections.
ELISA for IgG subclasses
The recombinant antigens were alternatively tested in ELISA for specific IgG subclasses (Table 4) with group I sera. The observed sensitivity values for the CHD patients’ sera varied between 40·3% (IgG1 for AgB8/2) and 98·3% (IgG1 for EgcMDH). IgG1, IgG3 or IgG4 were the predominant IgG isotypes in the responses detected for each different recombinant antigen. Specificities ranging from 41·7 (IgG2 for EgAFFPt) to 95·8% (IgG1 for EgCaBP2, EgAFFPt and EgAFFPf), as determined in tests with healthy individuals sera samples (n = 24), were obtained. The only serum that showed no reaction in the total IgG assay was positive in different subclasses assays (IgG1, IgG3, or IgG4) for all recombinant antigens, except for AgB8/2 (data not shown).
Table 4.
Sensitivity (SE) and specificity (SP) derived from ROC analysis of recombinant antigens tested in IgG class-specific ELISA against sera from group I and from healthy individuals
| IgG1 | IgG2 | IgG3 | IgG4 | |||||
|---|---|---|---|---|---|---|---|---|
| Recombinant antigen | SE (%) | SP (%) | SE (%) | SP (%) | SE (%) | SP (%) | SE (%) | SP (%) |
| AgB8/2 | 40·3 | 79·2 | 66·2 | 79·2 | 77·5 | 87·5 | 69·0 | 87·5 |
| AgB8/1 | 79·0 | 79·2 | 76·1 | 87·5 | 86·0 | 91·7 | 91·4 | 91·7 |
| EgcMDH | 98·3 | 50·0 | 95·0 | 54·2 | 41·7 | 91·7 | 91·7 | 92·0 |
| EgCaBP2 | 45·0 | 95·8 | 63·3 | 87·5 | 68·3 | 75·0 | 93·3 | 91·7 |
| EgAFFPt | 66·7 | 95·8 | 66·7 | 41·7 | 57·7 | 62·5 | 89·9 | 75·0 |
| EgAFFPf | 65·0 | 95·8 | 75·0 | 54·2 | 75·0 | 79·2 | 87·1 | 83·3 |
DISCUSSION
The cloning and expression of E. granulosus antigen-encoding genes in E. coli is an important alternative for the production of antigens for the immunodiagnosis of human CHD. In this work we assessed the immunodiagnostic potential in ELISA of six recombinant antigens of E. granulosus for the detection of circulating specific antibodies in CHD cases. These antigens were considered previously to be of diagnostic value with reasonable sensitivities (between 25% and 83%) and with a low degree of cross-reaction when analysed individually against a limited sampling of homologous and heterologous sera [19,21,23].
The definitive confirmation of an E. granulosus infection in a human host can be given only by the direct examination of a surgically removed hydatid cyst [33]. Therefore, the effective evaluation of antigen specificity depends on the availability of a good set of sera from patients with surgical confirmation of CHD. The reduction in discriminatory value (expressed by AUC) for all recombinant antigens when considering sera from group II indicates that some of the sera samples may correspond to false positive cases, the major problem associated with the use of sera from patients without surgical confirmation of CHD. However, we cannot exclude formally the possibility that at least some of the group II sera correspond to patients that do not respond to any of the tested antigens, a situation already described for CHD cases [34].
The recombinant AgB8/2 presented the best discriminatory value in total IgG ELISA in comparison to the other recombinants and the native antigens tested. As expected, HCFA an AgB showed relatively poor specificities, due to their content of epitopes shared with other helminth parasites, evidenced in previous studies [13,15,16,19]. The lower diagnostic sensitivity of AgB in relation to both AgB recombinant subunits (AgB8/1 and AgB8/2) is due probably to the presence of other subunits, already identified by González et al. [25] and Chemale et al. [35], that may be less sensitive.
The AgB small subunit (8 kDa) was described previously as specific for the genus Echinococcus[24], but some cross-reactions were demonstrated later by other authors [19,36]. However, the AgB8/2 is more specific and was proposed as E. granulosus species-specific, because it discriminates sera from patients infected with other Echinococcus species [19]. Extending these previous results, we have now demonstrated an AgB8/2 high specificity in relation to both normal sera and sera from patients with cysticercosis and toxocariasis. EgAFFPf showed a higher specificity in tests with these potentially cross-reactive sera, but it was associated with a significantly lower sensitivity. Cross-reaction with T. solium is a major problem in CHD immunodiagnosis [9,37,38] and this disease is also prevalent in E. granulosus endemic areas, such as the Andean region and the South Cone of South America [39–45]. In the same regions, toxocariasis (Toxocara spp.) is also a frequent source of cross-reactions in immunological assays, as it is one of the most common urban helminthiases [46–50], although it is often overlooked in serological surveys.
AgB8/1, EgcMDH, and EgCaBP2 are considerably less sensitive than AgB8/2. However, their inclusion in an immunodiagnostic test could improve the sensitivity of the assay because, of the four positive sera that do not recognize AgB8/2, three were positive for at least one of these antigens. Furthermore, any of the recombinant antigens has the potential to increase the level of confidence of the test, as 89% of the group I sera that recognize AgB8/2 also recognize at least another antigen.
Among the 58 sera of the group I utilized in this study, only one showed no reaction in ELISA for total IgG antibodies with any of the antigens, recombinant or native. However, this serum was positive in tests for specific IgG subclasses with different recombinant antigens. It has been described that the immunodiagnosis of CHD might be improved by the use of distinct immunoglobulin classes, and the problem of cross-reaction with sera from patients with other parasitic diseases appears to be reduced with the use of IgG subclass detection [8–10]. The use of IgG subclasses (specially IgG4) improved sensitivity for all recombinant antigens tested, except for AgB8/2. Therefore, for this set of recombinant proteins, IgG4 would be the subclass of choice to be assessed in an immunodiagnostic test of complementary value in relation to a total IgG assay, because it has the potential to detect a few sera from CHD patients that give negative results in total IgG ELISA.
Different patterns of antibody response involving specific IgG subclasses have been reported in parasitic infections [51,52], IgG1 and particularly IgG4 being the most dominant antibody G isotypes, despite the fact that IgG4 represents only 3–4% of the IgG total in normal serum [9,53]. Distinct IgG subclasses seem to be more important for the response to E. granulosus infections. General rises in the title of all subclasses, especially in IgG3 have been described [6,54], but specific responses to E. granulosus antigens are more often associated with IgG1 and IgG4 [5,9,10,55].
Sambesh et al. [56] have noted a difference in the IgG subclass response between asymptomatic CHD patients and more advanced cases who had undergone surgery. Asymptomatics preferentially induced IgG1 and surgical cases IgG4, suggesting a class switch with the disease progress. In agreement with that, our sera from group I patients, obtained moments before surgery, showed a considerable IgG4 response, being the predominant class for four of the antigens (AgB8/1, EgAFFPt, EgAFFPf and EgCaBP2).
Our results indicate that the recombinant AgB8/2 present a very good performance in total IgG ELISA for the detection of antibodies in sera from CHD patients. It is a potential replacement for native AgB that might be considered for the development of a single antigen-based test for CHD diagnosis. The other recombinant antigens tested are of complementary and confirmatory value in assays for total IgG. This type of recombinant antigen-based assay may be useful for confirmation of clinical diagnosis or for epidemiological surveys. Specific IgG4 ELISA may represent a complementary assay, useful as secondary confirmatory tests for patients with suspect of CHD negative for the total IgG ELISA.
Acknowledgments
We thank Dr Guita Rubinsky Elefant and Dr Márcia Imenes for the kind gift of toxocariasis and cysticercosis patients’ sera, respectively. We thank Dr Gualberto González for the kind gift of native AgB, and Dr Sotero Mengue for his support in the statistical analysis. We also thank the LACEN/RS and the PUC/RS for the kind gift of sera from clinically normal individuals. This work was supported in Brazil by PADCT/CNPq, CNPq, CAPES, FAPERGS and RTPD Network (SIDA/SAREC), and in Uruguay by PEDECIBA and CSIC.
References
- 1.Thompson RCA. Biology and systematics of Echinococcus. In: Thompson RCA, Limbery A, editors. Echinococcus and hydatid disease. Wallingford: CAB International; 1995. pp. 1–50. [Google Scholar]
- 2.González-Sapienza G, Lorenzo C, Nieto A. Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein, Echinococcus granulosus antigen B. J Clin Microbiol. 2000;38:3979–83. doi: 10.1128/jcm.38.11.3979-3983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riganò R, Profumo E, Bruschi F, et al. Modulation of human immune response by Echinococcus granulosus antigen B and Its possible role in evading host defenses. Infect Immun. 2001;69:288–96. doi: 10.1128/IAI.69.1.288-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biava MF, Dao A, Fortier B. Laboratory diagnosis of cystic hydatid disease. World J Surg. 2001;25:10–4. doi: 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 5.Grimm F, Maly FE, Lü J, Llano R. Analysis of specific immunoglobulin G subclass antibodies for serological diagnosis of Echinococcosis by a standard enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1998;5:613–6. doi: 10.1128/cdli.5.5.613-616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aceti A, Pennica A, Teggi A, et al. IgG subclasses in human hydatid disease: prominance of the IgG4 response. Int Arch Allergy Immunol. 1993;102:347–51. doi: 10.1159/000236582. [DOI] [PubMed] [Google Scholar]
- 7.Riganò R, Profumo E, Ioppolo S, et al. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–5. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortona E, Riganò R, Margutti P, et al. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 2000;22:553–9. doi: 10.1046/j.1365-3024.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 9.Wen H, Craig PS. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:741–8. doi: 10.4269/ajtmh.1994.51.741. [DOI] [PubMed] [Google Scholar]
- 10.McVie A, Ersfeld K, Rogan MT, Craig PS. Expression and immunological characterisation of Echinococcus granulosus recombinant antigen B for IgG4 subclass detection in human cystic echinococcosis. Acta Trop. 1997;67:19–35. doi: 10.1016/s0001-706x(97)00056-9. [DOI] [PubMed] [Google Scholar]
- 11.Lightowlers MW, Gottstein B. Thompson RCA, Lymbery AJ. Echinococcus and hydatid disease. Wallingford: CAB International; 1995. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis; pp. 355–410. [Google Scholar]
- 12.Parija SC. A rewiew of some simple immunoassays in the serodiagnosis of cystic hydatid disease. Acta Trop. 1998;70:17–24. doi: 10.1016/s0001-706x(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 13.Irabuena O, Nieto A, Ferreira A, et al. Characterization and optimization of bovine Echinococcus granulosus cyst fluid to be used in immunodiagnosis of hydatid disease by ELISA. Rev Inst Med Trop S Paulo. 2000;42:255–62. doi: 10.1590/s0036-46652000000500004. [DOI] [PubMed] [Google Scholar]
- 14.Paul M, Stefaniak J. Comparison of the dot immunobinding assay and two enzyme-linked immunosorbent assay kits for the diagnosis of liver cystic echinococcosis. Hepatol Res. 2001;21:14–26. doi: 10.1016/s1386-6346(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 15.Liance M, Janin V, Bresson-Hadni S, et al. Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by new commercial Western blot. J Clin Microbiol. 2000;38:3718–21. doi: 10.1128/jcm.38.10.3718-3721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira HB, Rodrigues JJS, Raupp RM, Zaha A. Molecular cloning and characterization of antigen encoding genes from Echinococcus granulosus. Montevidéo: Biology of Parasitism Ediciones Trilce; 1994. pp. 205–16. [Google Scholar]
- 17.Martin RM, Colebrook AL, Gasser RB, Lightowlers MW. Antibody responses of patients with cystic hydatid disease to recombinant myophilin of Echinococcus granulosus. Acta Trop. 1996;61:307–14. doi: 10.1016/0001-706x(96)00012-5. [DOI] [PubMed] [Google Scholar]
- 18.Fernández V, Ferreira HB, Fernández C, et al. Molecular characterisation of a novel 8 kDa subunit of Echinococcus granulosus antigen B. Mol Biochem Parasitol. 1996;77:247–50. doi: 10.1016/0166-6851(96)02602-3. [DOI] [PubMed] [Google Scholar]
- 19.Rott MB, Fernández V, Farias S, et al. Comparative analysis of two different subunits of antigen B from Echinococcus granulosus: gene sequences, expression in Escherichia coli and serological evaluation. Acta Trop. 2000;75:331–40. doi: 10.1016/s0001-706x(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues JJS, Ferreira HB, Zaha A. Molecular cloning and characterization of an Echinococcus granulosus cDNA encoding malate dehydrogenase. Mol Biochem Parasitol. 1993;60:157–60. doi: 10.1016/0166-6851(93)90040-5. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira HB, Zaha A. Molecular cloning and analysis of the diagnostic value of an Echinococcus granulosus antigen gene clone. Int J Parasitol. 1994;24:863–70. doi: 10.1016/0020-7519(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 22.Cortez-Herrera E, Yamamoto RR, Rodrigues JJS, et al. Echinococcus granulosus: cloning and functional in vitro characterization of an actin filament fragmenting protein. Exp Parasitol. 2001;97:215–25. doi: 10.1006/expr.2001.4605. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira HB, Rodrigues JJS, Zaha A. Echinococcus granulosus recombinant antigens. Ciência e Cultura J Brazil Assoc Adv Sci. 1996;48:370–6. [Google Scholar]
- 24.Maddison SR, Slemenda SB, Schantz PM, et al. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDa. Am J Trop Med Hyg. 1989;40:377–83. doi: 10.4269/ajtmh.1989.40.377. [DOI] [PubMed] [Google Scholar]
- 25.González-Sapienza G, Nieto A, Fernández C, et al. Two different 8 kDa monomers are involved in the oligomeric organization of the native Echinococcus granulosus antigen B. Parasite Immunol. 1996;18:587–96. doi: 10.1046/j.1365-3024.1996.d01-38.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith DB, Johnson KS. Single-step purification polypeptides expressed in Escherichia coli as fusions with gluthatione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone A, Thorpe R. Immunochemistry in practice. Oxford: Blackwell Scientific Publications; 1982. Basic techniques; pp. 1–17. [Google Scholar]
- 28.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 29.Hernández-Pomi A, Borras-Salvador R, Mir-Gisbert A. Analysis of cytokine and specific antibody profiles in hydatid patients with primary infection and relapse of disease. Parasite Immunol. 1997;19:553–61. doi: 10.1046/j.1365-3024.1997.d01-173.x. [DOI] [PubMed] [Google Scholar]
- 30.Amagai M, Komai A, Hashimoto T, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–7. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- 31.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 32.Hambach L, Eder M, Dammann E, et al. Diagnostic value of procalcitonin serum levels in comparison with C-reactive protein in allogeneic stem cell transplantation. Haematology. 2002;87:643–51. [PubMed] [Google Scholar]
- 33.Despommier DD, Gwadz RW, Hotez PJ. Parasitic diseases. 3rd. New York: Springer-Verlag; 1994. Larval tapeworms; pp. 89–100. [Google Scholar]
- 34.Rogan MT, Craig PS. Immunology of Echinococcus granulosus infections. Acta Trop. 1997;67:7–17. doi: 10.1016/s0001-706x(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 35.Chemale G, Haag KL, Ferreira HB, Zaha A. Echinococcus granulosus antigen B is encoded by a gene family. Mol Biochem Parasitol. 2001;116:233–7. doi: 10.1016/s0166-6851(01)00316-4. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira HB, Zaha A. Analysis of different antigen sources in the diagnosis of human hydatid disease by immunoblot. Montevidéo: Biology of Parasitism Ediciones Trilce; 1990. pp. 189–201. [Google Scholar]
- 37.Rickard MD, Lightowlers MW. Immunodiagnosis of hydatid disease. In: Thompson RCA, editor. The biology of echinococcus and hydatid disease. London: George Allen & Unwin; 1986. pp. 217–49. [Google Scholar]
- 38.Kharebov A, Nahmias J, El-On J. Cellular and humoral immune responses of hydatidosis patients to Echinococcus granulosus purified antigens. Am Soc Trop Med Hyg. 1997;57:619–25. doi: 10.4269/ajtmh.1997.57.619. [DOI] [PubMed] [Google Scholar]
- 39.Arambulo P., III Public health importance of cystic echinococcosis in Latin America. Acta Trop. 1997;67:113–24. doi: 10.1016/s0001-706x(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 40.Lamberti R, Calvo C, Pombar A, et al. Hydatidosis in the province of La Pampa, Argentina, 1998. Bol Chil Parasitol. 1999;54:110–2. doi: 10.4067/s0365-94021999000300013. [DOI] [PubMed] [Google Scholar]
- 41.Cruz ME, Preux PM, Debrock C, et al. Epidemiology of cerebral cysticercosis in an Andean community in Ecuador. Bull Soc Pathol Exot. 1999;92:38–41. [PubMed] [Google Scholar]
- 42.Rigatti M, Trevisol-Bittencourt PC. Causes of late-onset epilepsy in a epilepsy clinic of Santa Catarina − Southern Brazil. Arq Neuropsiquiatr. 1999;57:787–92. doi: 10.1590/s0004-282x1999000500009. [DOI] [PubMed] [Google Scholar]
- 43.Garcia HH, Talley A, Gilman RH, et al. Epilepsy and neurocysticercosis in a village in Huaraz. Peru Clin Neurol Neurosurg. 1999;101:225–8. doi: 10.1016/s0303-8467(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 44.Silva JE, Diefenthaler AP, Palma JK. Frequency of suspected cases of neurocysticercosis detected by computed skull tomography in Santa Maria, RS. Brazil Rev Inst Med Trop São Paulo. 2000;42:57–8. doi: 10.1590/s0036-46652000000100010. [DOI] [PubMed] [Google Scholar]
- 45.Carrique-Mas J, Iihoshi N, Widdowson MA, et al. An epidemiological study of Taenia solium cysticercosis in a rural population in the Bolivian Chaco. Acta Trop. 2001;80:229–35. doi: 10.1016/s0001-706x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 46.Lescano SAZ, Chieffi PP, Peres BA, et al. Soil contamination and human infection by Toxocara sp. in the urban area of Lima. Peru Mem Inst Oswaldo Cruz. 1998;93:733–4. doi: 10.1590/s0074-02761998000600005. [DOI] [PubMed] [Google Scholar]
- 47.Araújo FR, Crocci J, Rodrigues RGC, et al. Contamination of public squares of Campo Grande, Mato Grosso do Sul, Brazil, by eggs of Toxocara and Ancylostoma in dog feces. Rev Soc Bras Med Trop. 1999;32:581–3. doi: 10.1590/s0037-86821999000500017. [DOI] [PubMed] [Google Scholar]
- 48.Radman NE, Archelli SM, Fonrouge RD, et al. Human toxocarosis: its seroprevalence in the City of La Plata. Mem Inst Oswaldo Cruz. 2000;95:281–5. doi: 10.1590/s0074-02762000000300001. [DOI] [PubMed] [Google Scholar]
- 49.Castillo D, Paredes C, Zañartu C, et al. Contaminación ambiental por huevos de Toxocara sp. en algunas plazas y parques públicos de Santiago de Chile, 1999. Bol Chil Parasitol. 2000;55:86–91. [PubMed] [Google Scholar]
- 50.Coelho LMPS, Dini CY, Milman MHSA, Oliveira SM. Toxocara spp. eggs in public squares of Sorocaba, São Paulo State. Brazil Rev Inst Med Trop São Paulo. 2001;43:189–91. doi: 10.1590/s0036-46652001000400002. [DOI] [PubMed] [Google Scholar]
- 51.Maizels RM, Sartoro E, Kurniawan A, et al. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11:50–6. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 52.Sterla S, Sato H, Nieto A. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 1999;21:27–34. doi: 10.1046/j.1365-3024.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- 53.Schur PH. IgG subclass − a review. Ann Allergy. 1987;58:89–99. [PubMed] [Google Scholar]
- 54.Hira PR, Bahr GM, Shweiki HM, et al. Diagnostic value of anti-arc5 IgG antibody and analysis of the IgG subclass in sera of patients with cystic hydatid disease. Serodiag Immuno Infect Dis. 1990;4:285–93. [Google Scholar]
- 55.Ioppolo S, Notargiacomo S, Profumo E, et al. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 1996;18:571–8. doi: 10.1046/j.1365-3024.1996.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 56.Sambesh MK, Craig PS, Wen H, et al. IgG1 and IgG4 serum antibody responses in asymptomatic and clinically expressed cystic echinococcosis patients. Acta Trop. 1997;64:53–63. doi: 10.1016/s0001-706x(96)00637-7. [DOI] [PubMed] [Google Scholar]