Abstract
Production of CCR5 expression and MIP-1α, a ligand of CCR5, by CD4+ T cells from patients with rheumatoid arthritis (RA) were studied. We analysed further the influence of IL-15 stimulation, CD40/CD40 ligand (CD40L) interaction and CCR5 promotor polymorphism. One hundred and fifty-five RA patients and another 155 age- and sex-matched healthy individuals were enrolled. Peripheral CD4+ and double negative (DN) T cells from patients had lower portions of CCR5, whereas synovial CD4+ and DN T cells showed a much higher CCR5 expression. IL-15 significantly up-regulated the expression of CCR5 on purified CD4+ T cells. CD40L expression on synovial CD4+ T cells was increased greatly in CCR5+ portions by IL-15. MIP-1α production by synovial CD4+ T cells was also enhanced by IL-15. Co-culture of CD40 expressing synovial fibroblasts with IL-15-activated synovial CD4+ T cells significantly increased MIP-1α production. Expression of CCR5 on patients’ CD4+ T cells was not influenced by the promotor polymorphism of CCR5 gene. Taken together, these data suggest CCR5+CD4+ T cells infiltrate the inflamed synovium and IL-15 up-regulates CCR5 and CD40L expression further and enhance MIP-1α production in synovial CD4+ T cells. Production of MIP-1α by synovial fibroblasts is significantly increased by engagement of CD40 with CD40L. Synovial microenvironment plays a potential role in regulation of CCR5+CD4+ T cells in rheumatoid joints.
Keywords: CCR5, CD4+ T cells, MIP-1α, rheumatoid arthritis
INTRODUCTION
Chemokines are major factors that regulate important steps of lymphocytes trafficking by inducing cell motility and activating adhesion molecules [1]. In addition to the role of controlling leucocyte recruitment and activation, these molecules are involved in a number of other physiological processes including lymphocyte development, modulation of angiogenesis and cell compartmentalization within lymphoid tissues [2,3]. Chemokine receptors are G-protein-coupled seven-transmembrane receptors, and these molecules have been named CXCR1 to CXCR6 (bind CXC chemokines), CCR1 to CCR10 (bind CC chemokines), XCR1 and CX3CR1 [4]. Recent studies in expression of chemokine receptors on different lymphocyte subsets have revealed important mechanisms of regulated cell trafficking in the immune system [5,6].
Type 1 and type 2 helper T (Th1 and Th2) cells mediate different types of protective or pathogenetic responses by secreting different cytokines and interacting with different types of leucocytes [7,8]. Differential recruitment of Th1 and Th2 cells is controlled by Th1-associated expression of CXCR3/CCR5 and Th2-associated expression of CCR3/CCR4/CCR8 [6,9]. Selective recruitment of lymphocyte subsets by certain chemokines is defined mainly by the presence of their corresponding cognate receptors. Rheumatoid arthritis (RA) is an autoimmune disease characterized by T lymphocytes accumulation within the synovial compartment. Activated CD4+ T cells predominate in the infiltrating mononuclear cells (MNCs) of the rheumatoid joints [10]. Th1-type immune responses have been suggested to be important for the pathogenesis of organ-specific autoimmunity such as type I insulin-dependent diabetes mellitus, multiple sclerosis and RA [11]. In the collagen-induced arthritis (CIA) murine model, Th1 cells play a proinflammatory role, while Th2 cells appear to have an anti-inflammatory effect [12].
The relative contribution made by individual chemokines to the development or progression of RA is not known. Some members of the CC and CXC families of chemokines could be implicated in the pathogenesis of RA [13,14]. These molecules include regulated upon activation normal T expressed and secreted (RANTES), macrophage inflammatory protein-1α (MIP-1α), MIP-1β and monocyte chemoattractant protein-1 (MCP-1) in the CC family and IL-8, epithelial–neutrophil activating protein-78, growth-related gene product-α and connective tissue activating peptide-III in the CXC family. These findings are based on the ability of investigators to detect these proteins or steady-state expression of their mRNA in the joints of RA patients.
Patients with RA are encountered frequently with a high estimated prevalence rate in this area [15]. In this study, the regulation of CCR5 expression and MIP-1α production in CD4+ T cells from RA patients were investigated. We also analysed the influence of IL-15 stimulation and interaction of CD40 with CD40 ligand (CD40L). The promotor polymorphism of CCR5 gene might down-regulate CCR5 expression at the transcription level. We examined further the possibility that the polymorphism might affect the CCR5 expression on CD4+ T cells from RA patients.
MATERIALS AND METHODS
Patients and controls
One hundred and fifty-five RA patients, with diagnosis according to the diagnostic criteria set by the American College of Rheumatology [16], were enrolled into the study project. All these patients were classified with active disease when not fulfilling the ACR criteria for clinical remission [17]. Venous blood samples were drawn from these patients. Samples of synovial fluid were available from therapeutic aspiration of inflammatory joint. Synovial tissues were obtained during the operation. Peripheral blood samples were taken simultaneously when therapeutic aspiration or operation were performed. The control group included 155 sex- and age-matched healthy individuals. The Ethics Committee of the National Cheng-Kung University Hospital approved this study. Informed consents were obtained from all the individuals enrolled into this study.
Preparation and stimulation of MNCs
Peripheral MNCs were purified from heparin-anticoagulated venous samples by Histopaque (Sigma Diagnostics, St Louis, MO, USA) gradient centrifugation. Synovial fluid was incubated with 15 U/ml hyaluronidase (Sigma) for 30 min at 37°C and then passed through a nylon mesh. After spinning down and suspending in RPMI-1640, MNCs were separated by Histopaque gradient. The synovial tissue obtained from operation room was trimmed immediately to remove fatty tissue and minced into small pieces. The tissues were incubated in 0·5 mg/ml of collagenase (Sigma), 0·15 mg/ml of DNAse (Sigma) and 15 U/ml heparin for 1 h at 37°C. The digest was passed through a nylon mesh. The cells were then washed and purified further by the Histopaque gradient centrifugation × 2. For in vitro stimulation, MNCs were cultured in the presence of 30, 100 or 300 ng/ml recombinant human IL-15 (R&D Systems, Minneapolis, MN, USA) for 1, 3, 5, 7 or 9 days and were subjected further to flow cytometry analysis.
Preparation of synovial fibroblasts
Freshly prepared synovium tissue was cultured in Dulbecco's modified Eagle's medium (DMEM) with 2 mm l-glutamine, 20 mm HEPES buffer, 10% fetal calf serum and antibiotics in Petri dishes in a humidified 5% CO2 incubator. After cell adherence for 48 h, non-adherent cells were removed and adherent cells were cultured continuously until confluence. These cells were then passed after trypsin treatment. After four passages or older, synovial fibroblasts became a homogeneous population and were used for further studies. Synovial fibroblasts showed strong CD40 expression and addition of IFN-γ in fibroblasts culture enhanced further the CD40 expression as shown in previous experiments [18].
Purification of CD4+ T cells
The MNCs isolated by Histopaque gradient centrifugation were stained with antihuman CD4 (clone PRA-T4, PharMingen, San Diego, CA, USA) monoclonal antibody (MoAb). After washing, these cells were incubated with magnetic particles coated anti-IgG microbeads (Miltenyi Biotec, Germany), and were passed through a positive selection column in a Magnectic Cell Sorter (Miltenyi Biotec). The column was removed from the separator and the positive fraction of CD4+ T cells was flushed out. The purity of CD4+ T cells in this preparation was more than 95%.
Flow cytometry analysis
Triple fluorescence staining was used for the surface phenotype analysis. The MoAbs against human antigens including anti-CD3 (clone UCHT1), anti-CD4 (clone PRA-T4), anti-CD8 (clone PRA-T8), anti-CCR5 (clone 2D7), anti-CXCR4 (clone 12G5), anti-CD40 (clone 5C3) and CD40L (clone TRAP1) were purchased from PharMingen. Anti-CCR3 (clone 61828·111) were purchased from R&D Systems. Purified MNCs were stained with Cy-chrome- or phycoerythrin (PE)-conjugated anti-CD3, PE-conjugated anti-CD4 and/or anti-CD8, and fluorescein isothiocyanate (FITC)-conjugated anti-CCR3 or anti-CCR5. The cells were incubated with these MoAbs for 30 min on ice in the dark. After washing, the stained cells were analysed with a FACSort (Becton Dickinson, Mountain View, CA, USA) and CellQuest software programs (Becton Dickinson). The control isotype MoAbs (PharMingen) staining was included for each sample.
Intracellular cytokine staining
Purified CD4+ T cells were stimulated with phorbol myristate acetate (25 ng/ml) and ionomycin (1 µg/ml) for 4 h with the addition of Brefeldin A (Sigma) in the culture. Cells were harvested and stained with Cy-Chrome-conjugated anti-CD4 (clone PRA-T4, PharMingen) and FITC-conjugated anti-CCR5. After fixation with 1% paraformaldehye (Sigma) for 20 min, these MNCs were stained with PE-conjugated anti-IFN-γ (clone 4S.B3, PharMingen) or PE-conjugated anti-IL-4 (clone MP4-25D2, PharMingen) in staining buffer containing 0·1% saponin (Sigma). These cells were further subjected to flow cytometry analysis.
Quantification of MIP-1αand MIP-1β
MIP-1α and MIP-1β levels were quantified by enzyme-linked immunosorbant assay (ELISA) kits (R&D Systems). MNCs in density of 2 × 106 per well in 24-well flat-bottomed microplates or purified CD4+ T cells in density of 3 × 105 per well in 96-well flat-bottomed microplates were cultured with 100 ng/ml recombinant human IL-15 (R&D Systems) in RPMI-1640 supplemented with ’10% heat-inactivated fetal calf serum, 2 mm glutamine and antibiotics. Culture supernatants were taken serially at 12, 24 and 48 h and the contents of MIP-1α and MIP-1β were measured. Synovial fibroblasts 3 × 104 per well in 24-well flat-bottomed microplates were co-cultured with 0·5% paraformaldehyde fixed IL-15 activated synovial CD4+ T cells 106 per well in the presence of 1, 10 or 100 ng/ml IFN-γ and 1, 2 or 5 µg/ml anti-CD40L MoAb or isotype-matched control MoAb. After 24, 48 or 72 h, supernatants were harvested and tested for levels of MIP-1α and MIP-1β.
Reverse transcription polymerase chain reaction (RT-PCR)
The CD4+ T cells were stimulated with 100 ng/ml IL-15 (R&D Systems) for 24, 48, 72, 96 or 120 h. Total RNA was extracted from these cells by a PUREscript RNA isolation kit (Gentra, Minniapolis, MN, USA).
RT-PCR of CCR5 gene was performed with 1 µg of RNA, 20 pmol of oligo(dT)18 primer, 0·5 mm dNTP, 0·5 U of RNase inhibitor and 200 U of MLV RTase in 4 µl of 5× RT buffer. All reagents used in the cDNA synthesis were purchased from Clonetech (Palo Alto, CA, USA). The mixture was incubated at 42°C for 1 h and 95°C for 5 min. A total of 35 cycles of PCR amplification was performed with forward primer (5′-TGGTCCTGC CGCTGCTTGTC-3′) and reverse primer (5′-GTGTAAACT GAG CTTCCTCGC-3′), and yielded a 412-base pair (bp) PCR product [19]. For an internal control gene, β-actin was used with forward primer (5′-AGCGGGAAATCGTGCGTG-3′) and reverse primer (5′-CAGGGTACATGGTGGTGCC-3′).
PCR-restriction fragment length polymorphism (RFLP)
Genomic DNA was prepared from MNCs using a PUREgene DNA isolation kit (Gentra, Minniapolis, MN, USA) and purified DNA was dissolved in TE solution. Three hundred ng of genomic DNA was used in the routine PCR-RFLP genotyping of the pCCR5-59653 and pCCR5-59029 polymorphism as reported previously [19]. Primer sequences for pCCR5-59653 were forward (5′-ATGATTTAACTCCACCCTCC-3′) and reverse (5′-AACCGTCTGAAACTCATTCC-3′) and for pCCR5-59029 were forward (5′-CCCGTGAGCCCATAGTTAAAACTC-3′) and reverse (5′-TCACAGGGCTTTCAACAGTAAGG-3′). The amplification with the pCCR5-59653 specific primers yielded a 303-bp PCR product that was digested subsequently with restriction enzyme BbsI (New England Biolab, Beverly, MA, USA). The digestion yielded 212 and 91 bp DNA fragments as a diagnostic determinant for the mutant allele. The specific primers of pCCR5-59029 amplified a 273-bp PCR product. The digestion with restriction enzyme BSP12861 (New England Biolab) gave rise to 137 and 136 bp DNA fragments in the mutant allele. The PCR products for the genotyping of these alleles were selected for automated nucleotide sequence analysis (ABI Prism 377 DNA Sequencer; Perkin Elmer, Foster City, CA, USA) to ensure the accuracy of the PCR-RFLP determination.
Statistical analysis
Statistical analyses were carried out using non-parametric tests, including the Mann–Whitney rank sum test for comparisons between RA patients and healthy controls and the Wilcoxon signed-ranks test for comparisons between different groups of RA patients and healthy controls. The difference in frequencies of genotype in patients and controls was calculated using the χ2 test and Fisher's exact test for comparison. A P-value of less than 0·05 was considered to be statistically significant.
RESULTS
Increased infiltration of IFN-γ secreting CCR5+CD4+ T cells into synovium
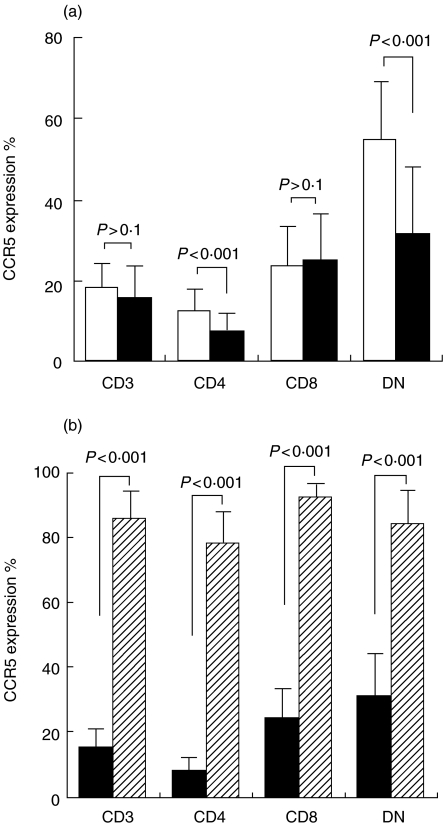
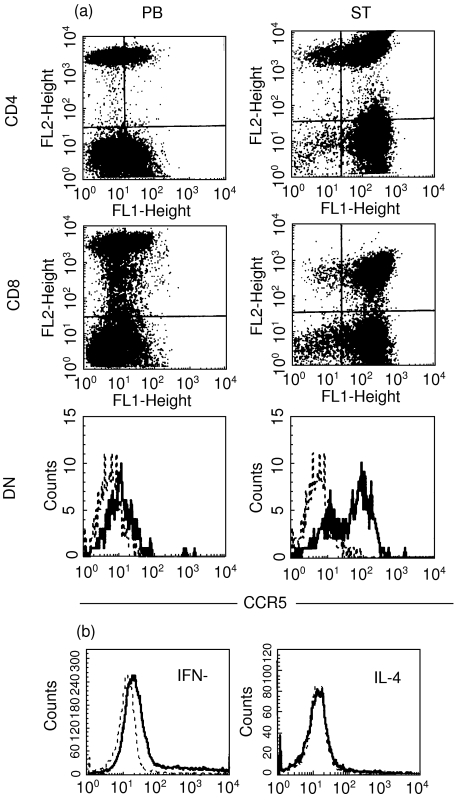
The expression of CCR5 molecules on CD3+ T cells from peripheral MNCs were divided into three subsets including CD4, CD8 and double negative (DN) by flow cytometry analysis. Peripheral MNCs from patients had a lower CCR5 expression on CD4+ and DN T cells but not on CD8+ T cells compared with those from healthy individuals (P < 0·001) (Fig. 1a). MNCs from synovial fluid of 30 RA patients showed a much higher CCR5 expression on CD4+ and DN T cells compared with those from peripheral blood (P < 0·001) (Fig. 1b). The staining pattern of CCR5 on T cells of synovial tissue was similar to those of synovial fluid. Representative figures with CCR5 expression on T cells of synovial tissue and peripheral blood from a RA patient are shown in Fig. 2a. There was no statistical difference (P > 0·1) in CCR3 expression on peripheral T cells between healthy individuals and RA patients (CD4, 1·009% ± 0·451%versus 0·934% ± 0·365%; CD8, 0·851% ± 0·373%versus 0·850% ± 0·325%; and DN, 0·902% ± 0·414%versus 0·873% ± 0·158%). MNCs from synovial fluid of 30 RA patients showed similar expression of CCR3 on T cells including CD4, CD8 and DN subsets compared with those from their peripheral blood. Purified CD4+ T cells from peripheral blood and synovial fluid of 18 patients were stimulated with PMA/ionomycin, and intracellular cytokines staining for IL-4 and IFN-γ were performed. CCR5+CD4+ T cells showed significant IFN-γ staining but no IL-4 staining. Figure 2b represents figures with IL-4 and IFN-γ expression on CCR5+ CD4+ T cells.
Fig. 1.
CCR5 expression percentage on peripheral CD3+ T cells, including CD4, CD8 and DN subsets in 155 RA patients and 155 healthy controls (a). CCR5 expression percentage on peripheral and synovial CD3+ T cells including CD4, CD8 and DN subsets in 30 RA patients (b). Bar heights represent mean values; brackets indicate standard deviation. (a) u, Normal; ▪, patient. (b) ▪, Peripheral blood;  , synovial fluid.
, synovial fluid.
Fig. 2.
Representative figures of CCR5 expression on T cells of synovial tissue (ST) and peripheral blood (PB) from a RA patient (a). Double or triple fluorescence staining shows CCR5 on the horizontal axis (FL1), CD4 or CD8 (FL2) on the vertical axis and CD3 on the FL3 axis. DN T cells are gated on the CD3+CD4−CD8− portion. Representative figures are shown in (b) with IL-4 and IFN-γ expressions on CCR5+CD4+T cells. The staining of isotype control MoAbs in (a) and (b) are shown by (––).
Increased CCR5 expression on CD4+ T cells by IL-15 stimulation
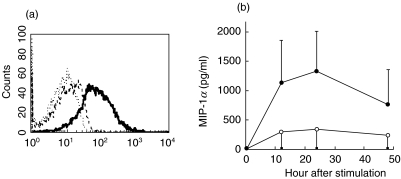
CCR5 expression on peripheral and synovial CD4+ T cells was enhanced greatly in the presence of IL-15. We further purified patients’ CD4+ T cells and stimulated these cells with IL-15. The cell surface expression of CCR5 was significantly up-regulated by addition of 100 ng/ml IL-15 on day 7 (Fig. 3a). The transcription of CCR5 reached its peak at 72 h after stimulation of IL-15 at the concentration of 100 ng/ml.
Fig. 3.
Expression of CCR5 on the horizontal axis (FL1) on purified peripheral CD4+ T cells from a patient after in vitro stimulation with 100 ng/ml IL-15 (—) or medium alone (––) for 7 days (a). Staining of the isotype control MoAb in (a) is shown by (···), a representative figure from 16 patients. Time-course of MIP-1α production by synovial CD4+ T cells spontaneously or with the addition of 100 ng/ml IL-15. Each point represents mean values ± standard deviation for 16 patients (b). (b) s, Medium; •, IL-15.
Increased MIP-1α production from synovial CD4+ T cells by IL-15 stimulation
High amount of MIP-1α were detected in synovial fluid from 30 patients (279·5 ± 183·1 pg/ml), but none was detected in their simultaneous blood samples (the lowest detection sensitivity 46·9 pg/ml). High amounts of MIP-1β were also found in synovial fluid from these patients (404·5 ± 282·0 pg/ml) and their blood samples contained lower levels (212·6 ± 182·1 pg/ml) (P < 0·005). MIP-1α and MIP-1β were released spontaneously by synovial MNCs. We tested further the ability of purified synovial CD4+ T cells to produce MIP-1α and MIP-1β. As shown in Fig. 3b, the production of MIP-1α by CD4+ T cells from 16 patients was enhanced markedly by the addition of IL-15. MIP-1β was not produced by synovial CD4+ T cells from these patients, and the production was not increased by the addition of IL-15.
Increased CD40L expression on CCR5+CD4+ T cells by IL-15 stimulation
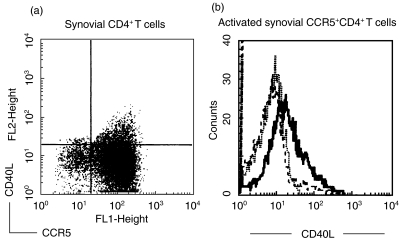
Expression of CD40L on CD4+ T cells was examined on paired synovial fluid and blood samples from 16 patients. Significantly higher percentages of expression were found on synovial CCR5+CD4+ T cells than those on CCR5−CD4+ T cells; however, no difference was found between peripheral CCR5+CD4+ T cells and CCR5−CD4+ T cells (synovial CCR5+CD4+ T cells versus CCR5−CD4+ T cells, 9·36 ± 4·25% vs. 2·29 ± 1·22%, P < 0·01; for peripheral blood, 3·59 ± 1·77%versus 3·18 ± 1·82%, P > 0·1). A representative figure of CD40L expression on synovial CCR5+CD4+ T cells versus CCR5−CD4+ T cells was shown in Fig. 4a. Furthermore, we stimulated purified peripheral and synovial CD4+ T cells with IL-15 100 ng/ml for 72 h and the expression of CD40L on CCR5+CD4+ T cells was enhanced significantly. Figure 4b shows a representative figure of CD40L expression on IL-15-treated synovial CCR5+CD4+ T cells.
Fig. 4.
Expression of CD40L on synovial CCR5+CD4+ T cells before (a) and after (b) IL-15 (100 ng/ml) stimulation for 72 h. Triple fluorescence staining shows CCR5 on the horizontal axis (FL1), CD40L (FL2) on the vertical axis and CD4 in the FL3 axis. A representative figure is shown in (b) with expression of CD40L on the gated CCR5+ portion of purified synovial CD4+ T cells after in vitro stimulation with 100 ng/ml IL-15 (—) or medium alone (––) for 72 h. (b) ···, Staining of the isotype control MoAb.
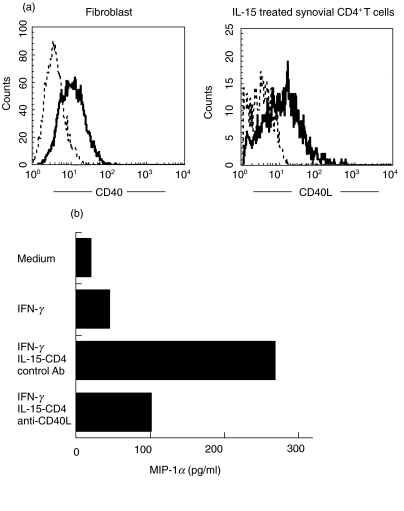
Increased MIP-1α production from synovial fibroblasts by CD40/CD40L engagement
We examined further the influence of synovial microenviroment on the production of MIP-1α. Synovial fibroblasts demonstrated high expression of CD40; CD40L was enhanced greatly in purified synovial CD4+ T cells by in vitro IL-15 stimulation (Fig. 5a). In the presence of 10 ng/ml IFN-γ, co-culture of synovial fibroblasts with IL-15-treated synovial CD4+ T cells increased the production of MIP-1α, which was inhibited significantly by the addition of 5 µg/ml anti-CD40L MoAb as shown in Fig. 5b. Synovial fibroblasts did not produce MIP-1β, and the stimulation with CD40L failed to up-regulate the production.
Fig. 5.
Influence of synovial fibroblasts on MIP-1α production. Expression of CD40 (FL2) on synovial fibroblasts and expression of CD40L (FL2) on activated synovial CD4+T cells are shown in (a). Stainings of CD40 and CD40L are demonstrated by (—) and stainings of isotype control MoAbs are shown by (––). (b) Results in MIP-1α production by co-culture of synovial fibroblasts with medium alone, IFN-γ or IL-15-treated purified synovial CD4+ T cells in the presence of IFN-γ. Addition of anti-CD40L MoAb (5 µg/ml) significantly blocked the enhancement of MIP-1α production by co-culture with IL-15-activated synovial CD4+ T cells. (b) Representative study of six experiments.
No association between CCR5 expression and the CCR5 promotor polymorphism
Statistical differences were found in polymorphism of CCR5 promotor 59653 but not in promotor 59029, as shown in Table 1. Difference in CCR5 expression on peripheral CD4+, CD8+ or DN T cells from controls or patients carrying the pCCR5-59653 T allele was not statistically significant compared with those carrying C allele. In addition, the Δ32 CCR5 mutant allele was analysed, but no such allele was found in Chinese patients, including 155 RA patients and 155 healthy controls.
Table 1.
pCCR5-59029 and pCCR5-59653 genotype distribution in 155 healthy controls and 155 RA patients
| Genotype | Control no. (%) | Patient no. (%) | P-value |
|---|---|---|---|
| pCCR5-59029 | |||
| G/G | 52 (33·6%) | 47 (30·3%) | >0·1 |
| G/A | 69 (44·5%) | 71 (45·8%) | >0·1 |
| A/A | 34 (21·9%) | 37 (23·9%) | >0·1 |
| pCCR5-59653 | |||
| C/C | 102 (65·8%) | 97 (62·6%) | >0·1 |
| C/T | 47 (30·3%) | 39 (25·2%) | >0·1 |
| T/T | 6 (3·9%) | 19 (12·2%) | <0·01 |
DISCUSSION
Chemokines and their receptors have received increasing attention due to their intimate participation in many pathological conditions, such as RA [6,9]. A discrete network of cells within the synovial cavity interact through their specific production of chemokines or expressing a highly specific pattern of chemokine receptors [20]. Ligands for the CCR5, including MIP-1α, RANTES and MIP-1β, have been shown to be co-secreted in vitro with IFN-γ by Th1 cells [3,5]. The mRNA of RANTES is expressed in synovial fluid T cells and in fibroblasts and sites of extensive lymphocytic infiltration of synovium from RA patients; however, this chemokine is at a low protein level in synovial fluid [13,21]. Synovial concentration of MIP-1β is increased significantly in osteoarthritis patients but not in RA patients [22]. CD4+ T cells from synovial fluid and synovium fibroblasts did not produce MIP-1β, as shown in results of this study. Human recombinant MIP-1α was reported to be a potent chemoattractant of human activated T cells in the in vitro microchemotactic activity assay [23]. Synovial fluid MIP-1α has been shown to be bioactive and accounting for about one-third of synovial fluid-derived chemotactic activity for MNCs [24]. However, the relevance of these findings in a functional role of MIP-1α with CCR5 remains to be established in RA patients. An abundant amount of MIP-1α has been found in synovial fluid of RA patients, which has also been demonstrated in this study [13,24]. MIP-1α has been shown to be derived from activated lymphoid cells including T cells, B cells and monocytes and non-lymphoid cells from tissue [24,25]. From results in this study, MIP-1α was produced spontaneously in protein level by synovial CD4+ T cells and fibroblasts. Moreover, the addition of IL-15 up-regulated MIP-1α production significantly by synovial CD4+ T cells. Cross-linking of CD40 by anti-CD40 MoAb or engagement of CD40 with CD40L-transfected L cells has been shown to increase MIP-1α production by fibroblasts [26]. The production of MIP-1α by fibroblasts has been reported to be increased further by IL-1, TNF-α or IFN-γ stimulation [13,26]. Previous studies have shown that IL-15 increases CD40L expression on CD4+ T cells [27,28]. Our results indicated that IL-15 up-regulated the expression of CD40L on synovial CCR5+CD4+ T cells. Engagement of CD40 on fibroblasts, in the presence of IFN-γ, by CD40L on IL-15-activated synovial CD4+ T cells significantly enhanced MIP-1α production. IL-15 could modulate the MIP-1α production by synovial CD4+ T cells and fibroblasts within the microenvironment of rheumatoid joints. Secretion of MIP-1α at sites of synovitis by fibroblasts and leucocytes establishes a chemokine concentration gradient. This gradient brings the rolling leucocytes to firm adherence with vascular endothelium and to extravasate into inflamed tissue. MIP-1α might have a role in recruitment of Th1 cells into rheumatoid joint and in perpetuation of synovitis response.
Effort has been directed toward the functional characterization of subsets of immune cells defined by chemokine receptor expression. The receptors expressed preferentially on Th1 cells are CCR5 and CXCR3 [1,3]. On the other hand, CCR3, CCR4 and CCR8 have been reported to have higher expression levels on Th2 cells. Although the expression of a given chemokine receptor does not necessarily imply its involvement in the pathogenesis, increased numbers of highly activated Th1 cells predominantly expressing CCR5 have been found in the joints of patients with RA and juvenile RA [20,29]. In the results of this study, peripheral CD4+ T cells from patients showed a lower CCR5 expression and a much higher CCR5 expression was found on synovial CD4+ T cells. In addition, CCR5+CD4+ T cells from patients produced IFN-γ but not IL-4 upon activation. These results also suggest a preferential accumulation of CCR5+ Th1 cells in rheumatoid joints. We examined the CCR5 expression on peripheral blood from autoimmune diseases other than RA such as systemic lupus erythematosus (SLE) and polymyositis (PM)/dermatomyositis (DM). Compared with healthy individuals, there was no difference in CCR5 expression on CD4+ T cells from 121 patients with SLE and 20 patients with PM/DM (unpublished observation). The onset of murine lupus disease is delayed by anti-IL-10 antibody treatment; in lupus patients, serum level of Th2 cytokines, such as IL-4 and IL-10, are elevated, while a decrease in production of Th1 cytokines, including IL-2 and IFN-γ, is observed [30,31]. These in vivo data suggest the involvement of Th2 cells in SLE. Results available from lupus patients may support further the concept that CCR5+CD4+ T cells play a role in Th1- but not Th2-mediated autoimmune diseases.
Upon activation, IL-4 and IFN-γ are detected in supernatants of human DN T cells [32]. A subset of human DN T cells is CD1-restricted and shows preferential Vα 24 T cell receptor usage analogous to Vα 14 usage by the murine NK 1·1+ T cells [33]. These DN T cells might play an important role in regulation of immune responses. No difference in the number of DN T cells from peripheral blood was found between RA patients and healthy controls [34]. However, this paper is the first study to demonstrate decreased expression of CCR5 on peripheral DN T cells and increased CCR5 expression on synovial DN T cells from RA patients. Increased infiltration of CCR5+ DN T cells into the joint cavity might play a role in rheumatoid synovitis.
We evaluated the effect of anti-T cell receptor antibody, mitogens (PHA or ConA) and calcium ionophore/PKC activator in CCR5 expression on CD4+ T cells. However, all these stimulations resulted in CCR5 down-regulation (data not shown). In the previous observation, the addition of IL-2 in the culture gradually increased surface CCR5 expression on T lymphocytes [35]. Although several studies support a role for IL-2 in the synovitis response of RA, the amount of IL-2 presence in the synovial fluid from RA patients is much lower compared to that of other cytokines [36]. Unlike IL-2, IL-15 is found routinely in the synovial fluid of RA patients [37]. IL-15 is produced by a variety of non-T cells including monocytes, fibroblasts and endothelial cells, and these cells are important constituents of the synovial microenvironment in RA [36–38]. IL-15 has been shown to increase CCR5 expression and MIP-1α production by peripheral blood T lymphocytes from healthy individuals [39]. We analysed the effect of IL-15 in CCR5 expression on CD4+ T cells from RA patients. It was found that CCR5 expression, both in protein and mRNA levels, was enhanced significantly in the presence of IL-15. In addition, production of MIP-1α by synovial CD4+ T cells was also enhanced greatly by the addition of IL-15. The synovial microenvironment may have important effects on the activation of CCR5 expression and production of its ligands.
In conclusion, our data suggest infiltration of IFN-γ secreting CCR5+CD4+ T cells into the joint cavity of RA patients. IL-15 up-regulates CCR5 and CD40L expression and enhances MIP-1α production in synovial CD4+ T cells. Engagement of CD40 with CD40L increases MIP-1α production by synovial fibroblasts. Expression of CCR5 on patients’ CD4+ T cells was not influenced by the gene polymorphism of CCR5 promotor. Synovial microenvironment plays a potential role in regulation of CCR5+CD4+ T cells in rheumatoid joints.
Acknowledgments
This work was supported by grants NSC 89–2314-B-006–098, 89–2314-B-006–142, 90–2314-B-006–094 and 91–2314-B-006–019 from the National Science Council, Taiwan, ROC. We thank professor Chyun-Yu Yang, Chief of Department Orthopedics, Medical College, National Cheng-Kung University for providing synovium specimens from RA patients. The authors are indebted to Professor Ching-Li, Department Microbiology and Immunology, Chung San Medical University, for providing PCR primers for CCR5 promoters. We also thank Dr Pei-Chih Chen, Ms Chiung-Ru Wu, Ms Li-Ling Fang, Ms Wan-Ching Cathy Lin, Ms Li-Ching Lin and Ms Shan-Chien Chao for technical assistance.
References
- 1.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–35. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 2.Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mice lacking expression of the chemokines CCL21-Ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med. 2001;193:207–17. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luther A, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–7. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A, Rossi D. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 2000;19:1–47. [PubMed] [Google Scholar]
- 6.Proudfoot-Amanda EI, Power CA, Wells-Timothy NC. The strategy of blocking the chemokine system to combat disease. Immunol Rev. 2000;177:246–56. doi: 10.1034/j.1600-065x.2000.17721.x. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 9.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 10.Panayi GS. T cell-dependent pathways in rheumatoid arthritis. Curr Opin Rheumatol. 1997;9:236–40. doi: 10.1097/00002281-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Liblau RS, Singer SM, McDevit HO. Th1 and Th2 CD4+ T cells in pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 12.Mauri C, Williams RO, Walmsley M. Relation between Th1 and Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–8. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 13.Szekanecz Z, Strieter RM, Kunkel SL, Koch AE. Chemokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:115–32. doi: 10.1007/BF00832002. [DOI] [PubMed] [Google Scholar]
- 14.Koch AE, Volin MV, Woods JM, et al. Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheum. 2001;44:31–40. doi: 10.1002/1529-0131(200101)44:1<31::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Chou CT, Pei L, Chang DM, Lee CF, Schumacher HR, Liang MH. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural difference. J Rheumatol. 1994;21:302–6. [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, et al. American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar ETH, Voskuyl AE, Familian A, vanMierlo GJ, Dijkmans BAC, Hack CE. Complement activation in patients with rheumatoid arthritis mediated in part by C-reactive protein. Arthritis Rheum. 2001;44:997–1002. doi: 10.1002/1529-0131(200105)44:5<997::AID-ANR178>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Liu MF, Chao SC, Wang CR, Lei HY. Expression of CD40 and CD40 ligand among cell populations within rheumatoid synovial compartment. Autoimmunity. 2001;34:107–13. doi: 10.3109/08916930109001958. [DOI] [PubMed] [Google Scholar]
- 19.Shieh BH, Liau YE, Yan YP, et al. Alleles that may influence HIV-1 disease progression in Chinese subjects. AIDS. 1999;13:421–4. doi: 10.1097/00002030-199902250-00018. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int Immunol. 1999;11:553–9. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 21.Badolato R, Oppenheim JJ. Role of cytokines, acute phase proteins, and chemokines in the progression of rheumatoid arthritis. Semin Arthritis Rheum. 1996;26:526–38. doi: 10.1016/s0049-0172(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 22.Robinson E, Keystone EC, Schall TJ, Gillett N, Fish EN. Chemokines expression in rheumatoid arthritis. evidence of RANTES and macrophage inflammatory protein (MIP)-1 beta production by synovial T cells. Clin Exp Immunol. 1995;101:398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD 8T+ cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–8. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 24.Koch AE, Kunkle SL, Shah MR, et al. Macrophage inflammatory protein-1 alpha, a novel chemotactic cytokine for macrophage in rheumatoid arthritis. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka Y, Koichi F, Hubscher S, et al. Heparan sulfate proteoglycan on the endothelium efficiently induces integrin-mediated T cells adhesion by immobilizing chemokines in patients with rheumatoid synovitis. Arthritis Rheum. 1998;41:1365–77. doi: 10.1002/1529-0131(199808)41:8<1365::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Rissoan MC, Kooten CV, Chomarat P, et al. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol. 1996;106:481–90. doi: 10.1046/j.1365-2249.1996.d01-858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mottonen M, Isomaki P, Luukkainen P, Toivanen P, Lassila O. IL-15 up-regulates the expression of CD154 on synovial fluid T cells. Immunology. 2000;100:238–44. doi: 10.1046/j.1365-2567.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skov S, Bonyhadi M, Odum N, Ledbetter JA. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J Immunol. 2000;164:3500–5. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- 29.Ruth JH, Rottman JB, Katschke KJ, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44:2750–60. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 31.Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunol Rev. 1995;144:157–93. doi: 10.1111/j.1600-065x.1995.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 32.Niehues T, Eichelbauer D, Schneider EM. Functional characteristics of human peripheral blood α/β TCR+, CD4−, CD8− double negative T cells. Microbiol Immunol. 1999;43:153–9. doi: 10.1111/j.1348-0421.1999.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 33.Godfrey DI, Hammond-Kirsten JL, Poulton LD, Smyth MJ, Baxter AGNKT. cells: facts, functions and fallacies. Immunol Today. 2000;21:573–83. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 34.Liu MF, Yang CY, Chao SC, Weng TH, Lei HY. Distribution of double-negative T subsets in blood and synovial fluid from patients with rheumatoid arthritis. Clin Rheumatol. 1999;18:227–31. doi: 10.1007/s100670050089. [DOI] [PubMed] [Google Scholar]
- 35.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brennan FM, Maini RN, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:133–47. doi: 10.1007/BF00832003. [DOI] [PubMed] [Google Scholar]
- 37.McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R. The role of interleukin-15 in T cells migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheimer-Markers N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin-15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse–human rheumatoid arthritis model in vivo. J Clin Invest. 1998;101:1261–72. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carson DA. Unconventional T-cell activation by IL-15 in rheumatoid arthritis. Nat Med. 1997;3:148–9. doi: 10.1038/nm0297-148. [DOI] [PubMed] [Google Scholar]