Abstract
In experimental animals inhibition of T cell co-stimulation immediately after organ transplantation effectively prevents rejection. We investigated whether the expression of co-stimulatory molecules is enhanced in cadaveric liver transplants, whether their expression is influenced by the transplantation procedure, and whether variation in expression between liver transplants is related to the occurrence of acute rejection. Expression of CD80, CD86 and the macrophage marker CD68 were determined by immunohistochemistry in biopsies from 40 clinical liver transplants obtained at different time-points during the transplantation procedure, and in normal liver tissue obtained from 10 human livers. Expression of CD80 and CD86 on Kupffer cells was graded by comparison with CD68-staining. In a subgroup CD80 and CD86 mRNA was quantified by real-time detection polymerase chain reaction. CD86 was expressed in all liver transplants and normal livers on the majority of Kupffer cells. CD80 was absent or sporadically expressed in normal liver tissue, but in 18 of 40 liver transplants at least one-quarter of Kupffer cells expressed CD80. CD80- and CD86-mRNA and protein expression in liver transplants did not change during the warm ischaemic and reperfusion phases of the transplantation procedure. CD80-expression on Kupffer cells varied strongly between individual donor livers; this variation was, however, not significantly related to the occurrence of acute rejection after transplantation. In conclusion, in nearly half of cold-preserved cadaveric liver transplants an increased proportion of Kupffer cells express CD80 at the time of transplantation in comparison with normal liver tissue. The expression was not further induced by warm ischaemia and reperfusion. However, the observed variation in CD80-expression between liver transplants is not a accurate predictive measure for acute rejection.
Keywords: acute rejection, CD80, CD86, Kupffer cells, liver transplantation
INTRODUCTION
Standardized immunosuppressive regimens after liver transplantation are associated with increased risk of postoperative infections. If acute rejection could be predicted during or shortly after transplantation, individualized immunosuppressive therapy might become possible. However, accurate predictive risk factors have not been identified until now. The effect of HLA-mismatching on acute rejection after liver transplantation is unclear: the extents of MHC class I or II mismatching between donor and recipient have been reported to be either slightly [1], not [2,3], or even inversely [4] related to the occurrence of acute rejection. Genetic polymorphisms in cytokine genes are related to rejection of liver grafts [5,6], but do not provide enough predictive accuracy to be used as a basis for patient-specific immunosuppression. The same holds true for factors regarding the donor liver which are related to acute rejection, namely the age of the donor and cold ischaemic storage time of the liver-transplant [4].
Acute rejection is a T cell-mediated inflammatory response to donor antigen. Activation of T cells requires, in addition to the interaction between the T cell receptor (TCR) and the MHC-complex on the antigen-presenting cell (APC), a second signal, which is provided through co-stimulatory pairs of molecules. The main co-stimulatory signals for the activation of T cells are delivered by binding of CD28 on the T cell to CD80 (B7-1) or CD86 (B7-2) on the APC. Blocking of this interaction by treatment with cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) Ig or anti-B7 antibodies leads to prolonged or permanent rejection-free survival of allogeneic organ allografts, including liver, in rodents [7,8]. Because this co-stimulatory signal, in contrast to TCR-signalling, is not suppressed by calcineurin inhibitors [9,10], it is probably fully functional in the clinical transplant setting.
Nothing is known about expression of co-stimulatory molecules in clinical liver grafts. In general, CD86 is expressed constitutively on APC, but CD80 only on activated APC [11]. Murine Kupffer cells express CD86, but not CD80 [12,13]. In normal human liver tissue few Kupffer cells were found to express CD80 and CD86, but both molecules were strongly induced on Kupffer cells during fulminant hepatic failure and hepatitis C infection [14,15].
Given the importance of these molecules in initiation of allograft rejection, we studied the expression of CD80 and CD86 in clinical liver transplants. Three specific questions were addressed: (1) whether expression of these molecules in cadaveric donor livers is increased in comparison with normal liver tissue. Cadaveric organ-grafts may be in an immunologically activated state due to the effects of brain death [16,17], endotoxin absorption from the gut [18,19], and ischaemic storage. (2) Whether expression of these molecules is affected by the transplantation procedure, specifically by warm ischaemia and reperfusion. In liver, the Kupffer cells in particular may become activated by reperfusion after ischaemic storage [20,21]. (3) Whether the extent of expression of these molecules at the time of transplantation is related to acute rejection after transplantation.
MATERIALS AND METHODS
Patients
Forty liver transplant patients, from whom biopsies were obtained during the transplantation procedure, were included retrospectively in this study. Eighteen of these had experienced one or more acute rejection-episodes after transplantation and 22 had not. Acute rejection was defined as biopsy-proven rejection (RAI-score ≥ 6), occurring within 2 months after transplantation, and responsive to treatment with methylprednisolone. Immunosuppressive therapy consisted of calcineurin inhibitor (either cyclosporin A or tacrolimus) and low-dose methylprednisolone. Eight patients in the rejector group and 12 in the non-rejector group received anti-CD25 MoAb (Basiliximab, Novartis, Basle, Switzerland) in addition to baseline immunosuppression.
Liver biopsies
During the transplantation procedure, wedge biopsies were taken from the donor liver at the end of the cold ischaemic storage, at the end of the warm ischaemic phase and 1 h after reperfusion. One part of the biopsies was processed for diagnosis of ischaemia and reperfusion damage, and a second part was embedded in Tissue Tek OCT Compound (Sakura Finetek, Zoeterwoude, the Netherlands), frozen in a mixture of ethanol/dry-ice, and stored at −80°C. The third part was immersed immediately in RNA-lysis buffer (Qiagen, Hilden, Germany) and stored at −80°C. Non-tumour-bearing liver-wedge biopsies obtained from eight patients during the resection of hepatic malignancies and two liver biopsies from patients undergoing surgery for oesophagus carcinoma served as normal controls. Needle biopsies from liver grafts obtained for diagnostic reasons during episodes of acute rejection (RAI-score 6–7) after transplantation were used to investigate expression of CD28 and CD152 on infiltrating cells. The use of the tissues for research purposes was approved by the local medical ethical committee and patients had given informed consent.
Immunohistochemistry
Immunohistochemical detection was performed on 5-µm cryostat sections. The slides were fixed in 4% paraformaldehyde/phosphate-buffered saline pH 7·3, fixed in staining chambers (Shandon, Amsterdam, the Netherlands), and incubated with 10% normal rabbit serum (Gibco BRL Life Technologies, Breda, the Netherlands) and 10% normal human plasma (NHP; Bloodbank ZWN, Rotterdam, the Netherlands) in Tris-buffered saline (TBS) pH 7·4. Thereafter, the slides were incubated overnight at 4°C with primary MoAb CD28 (BD Biosciences, Erembodegem, Belgium), CD68 (clone PG-M1, DAKO, Glostrup, Denmark), CD80, CD152 (Beckman Coulter, Mijdrecht, the Netherlands) or CD86 (BD Pharmingen, Heidelberg, Germany) in TBS pH 7·4 supplemented with 1% NHP. All stainings were performed in duplicate. Primary antibodies CD68, CD80 and CD86 were detected with rabbit-antimouse immunoglobulins (D) followed by alkaline-phosphatase–anti-alkaline-phosphatase complex (APAAP, Serotec, Oxford, UK). Primary antibodies CD28 and CD152 were detected with biotinylated rabbit-antimouse F(ab′)2 immunoglobulins and alkaline-phosphatase-conjugated streptavidin (both from Dako), after blocking endogenous biotin with Dako Biotin Blocking System. Binding of antibodies was visualized by incubation in fast blue/naphtol-ASBI phosphate solution supplemented with levamisole (all from Sigma-Aldrich Chemie, Steinheim, Germany). Counterstaining was performed with nuclear fast red (Fluka Chemie, Zwÿndrecht, the Netherlands).
For immunohistochemical double-stainings endogenous peroxidase activity in sections was blocked by incubation in citric acid/phosphate buffer-solution (pH = 5·8) with 0·05% H2O2 and 0·2% NaN3 (15 min, 20°C). CD80 MoAb were applied for 18 h at 4°C and detected with rabbit-antimouse antibodies followed by APAAP. Next the sections were incubated with 10% normal mouse serum (Dako, 30 min, 20°C) and thereafter with CD68-FITC (clone PGM-1, Dako; 1 h, 20°C), followed by rabbit-anti-FITC-peroxidase (PO) (Dako). AP was visualized by fast blue/ASBI phosphate substrate (blue) and PO with amino-ethyl-carbazole (AEC; red).
Optimal dilutions of the primary MoAb were established by titrating on tonsil tissue. On every slide used in the study, a tonsil section was included as positive control tissue. For every liver biopsy a negative control was performed by replacement of the primary MoAb by an isotype-matched irrelevant MoAb (IgG1 (Dako) or IgG2a (CLB, Amsterdam, the Netherlands).
Analysis of immunohistochemical stainings
All stainings were performed in duplicate. The slides were examined blindly by two observers. In biopsies from 20 donor livers the proportions of perivascular cells in portal fields expressing CD68, CD80 or CD86 were semiquantified according to the following grades: 0 = no expression, 1 = expression on <25%, 2 = on <50%, 3 = on <75% and 4 = expression on 75% to 100% of perivascular cells. For each biopsy the mean expression grade in portal fields was calculated from these scores. In all liver biopsies, the proportions of Kupffer cells expressing CD80 or CD86 were semiquantified by comparison with the CD68-staining on consecutive sections according to the same grading system.
Quantitative real-time detection polymerase chain reaction (PCR)
After thawing, the pieces of liver biopsy were homogenized in RNA lysis-buffer by using a small Potter pestle and by passing the homogenate through an injection needle. Residual tissue fragments were spun down and total RNA was extracted from the supernatant using the Rneasy Midi kit (Qiagen), according to the manufacturer's protocol. RNA was dissolved in 50 µl H2O and reverse transcribed in a volume of 120 µl using random hexamers (Promega, Madison, WI, USA). The mRNA levels of CD80, CD86 and housekeeping gene β-actin were measured by real-time detection PCR based upon Taqman chemistry on an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). PCR products were detected using dual-fluorescent non-extendable probes containing a 6-carboxyfluorescein (FAM) reporter and a TAMRA quencher for all reactions, except for the β-actin reaction in which FAM was replaced by VIC. PCR-primers and probes were designed using Primer Express software (Applied Biosystems). Probes were chosen on the transition of two exons to prevent detection of genomic DNA. For CD80 forward primer 5′-TGGTGCTGGCTG GTCTTTC (nucleotides 443–461), reverse primer 5′-CTGTGCCACTTCTTTCACTTCC (nucleotides 495–515) and probe 5′-CACTTCTGTTCAGGTGT TATCCACGTGACCA (nucleotides 463–493; transition exon 2/exon 3) were used. CD86 was amplified using forward primer 5′-ACATTCTCTTTGTGATGGCCTTC (nucleotides 161–183) and reverse primer 5′-TGCAGTCTCATTGAA ATAAGCTTGA (nucleotides 213–237) and detected with probe 5′-TGCTCTCTGGTGCTGCTACCTCTGAAGA (nucleotides 185–211; transition exon 3/exon 4). β-Actin mRNA levels were measured using predeveloped Taqman Assay Reagents (Applied Biosystems). The CD80 and CD86 primer and probe concentrations were optimized in experiments with cDNA of an Epstein–Barr virus (EBV)-transformed B cell line with high surface expression of CD80 and CD86. Duplicate reactions were performed in 50 µl reaction mixtures containing 10 µl cDNA and Taqman Universal Master Mix (Applied Biosytems) supplemented with 45 pmoles (CD80) or 15 pmoles of each primer (CD86) and 5 pmoles of probe. Samples were first incubated for 2 min at 50°C to enable AmpErase uracil N-glycosylase, present in the Master Mix, to inactivate possible contaminating PCR carryover products. Thereafter samples were heated 10 min at 95°C and amplified subsequently for 45 cycles of 15 s at 95°C and 60 s at 60°C. As a positive control a serial dilution in water of cDNA from an EBV-transformed B cell line with high surface expression of CD80 and CD86 was used. All PCR reactions were performed with comparable efficiencies. The relative expression levels of CD80 and CD86 mRNA were calculated using the comparative threshold cycle (Ct) method [22]. Briefly, the mean of duplicate target PCR CT values, i.e. the cycle number at which the emitted fluorescence exceeds the 10 times standard deviation of baseline emissions as measured between cycles 3–15, was normalized by subtracting the mean CT value of β-actin. The relative expression level of CD80 and CD86 mRNA was calculated by the equation: 2−(Ct target − Ct β-actin).
Statistical analysis
Differences between normal livers and liver transplants and between the rejector and non-rejector groups were analysed by the Mann–Whitney test. Differences in expression between biopsies taken at different time-points from the same liver transplants were tested by the Wilcoxon signed-ranks test.
RESULTS
Expression pattern of CD80 and CD86 protein in normal liver and clinical liver transplants
Normal liver tissue and the biopsies from 20 liver transplants (10 of the rejector group and 10 of the non-rejector group) were examined for CD80- and CD86-expression in portal areas. CD80 was expressed in 7/20 (35%) and CD86 in 17/20 (85%) of the liver transplants on perivascular cells within one or more portal fields. This was similar to normal liver tissue: 3/8 normal livers contained CD80-positive cells and 7/8 CD86-positive cells in portal fields.
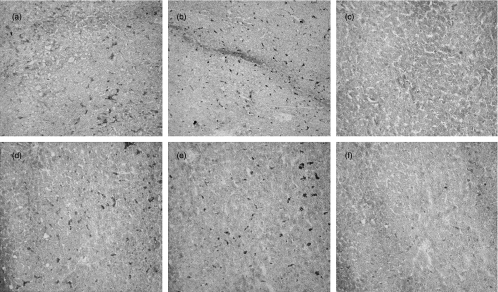
Biopsies from all 40 liver transplants and from normal livers were examined for lobular CD80- and CD86-expression. Both in liver transplants and normal liver tissue CD86 was expressed in a pattern resembling that of the Kupffer cells in CD68-stainings (Fig. 1a,b,d,e). In normal liver tissue (Fig. 1c) and in 22/40 donor livers no or only few cells within the parenchyma were found to express CD80. However, in 18/40 donor livers more extensive lobular expression of CD80 was observed (Fig. 1f). Immunohistochemical double-staining demonstrated that lobular CD80 expression in these livers was confined to CD68+-Kupffer cells (Fig. 2).
Fig. 1.
Cryostat sections of a normal human liver tissue (a–c) and cadaveric donor liver tissue (d–f) immunohistochemically stained for CD86 (a,d), CD68 (b,e) or CD80 (c,f).
Fig. 2.
Immunohistochemical double-staining of CD68 (grey) and CD80 (black) on cadaveric donor liver tissue, demonstrating that CD80 is expressed on Kupffer cells. (A colour version of can be requested from the corresponding author.)
CD80 and CD86 protein and mRNA expression does not change during the liver transplantation procedure
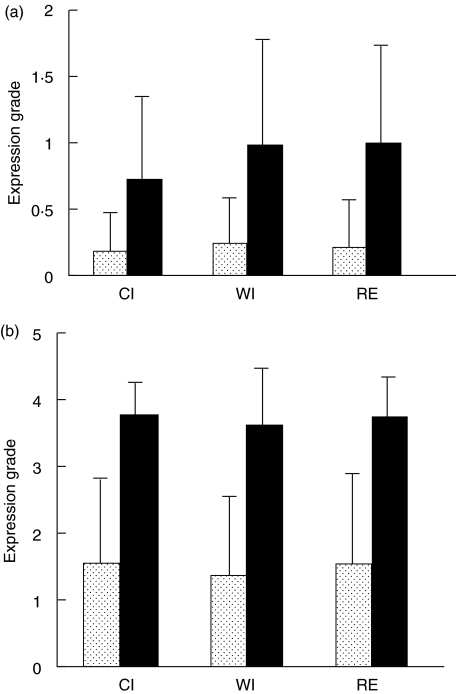
Expression of CD80 and CD86 on perivascular cells in portal fields and on Kupffer cells was graded into five categories as described in the Materials and methods section. Figure 3 shows that the grades of portal and lobular expression of CD80 in all three liver transplant biopsies was lower than those of CD86, and that expression of both molecules was not enhanced during the transplantation procedure. To investigate the effects of the transplantation procedure on co-stimulatory gene transcription, CD80 and CD86 mRNA was quantified by real-time PCR in biopsies taken from 19 donor livers at the end of the cold ischaemic storage and at the end of the reperfusion phase. As is shown in Table 1, the relative expression of CD86 mRNA in comparison to the housekeeping gene β-actin was about 10 times higher than that of CD80-mRNA, in accordance with the difference in protein expression. As for protein expression, there was no significant change in the levels of both transcripts during the transplantation procedure.
Fig. 3.
Effect of the transplantation procedure on the expression of CD80 and CD86 in cadaveric donor liver. Expression of CD80 and CD86 was graded in biopsies taken from human cadaveric liver transplants at the end of the cold ischaemic storage (CI), at the end of the warm ischaemic period (WI) and 1 h after reperfusion (RE). (a) Mean grade of expression (± s.d.) on perivascular cells in portal fields in biopsies from 20 donor livers. (b) Mean grade of expression (± s.d.) on Kupffer cells in biopsies from 40 donor livers.  , CD80; ▪, CD86.
, CD80; ▪, CD86.
Table 1.
Effect of warm ischaemia and reperfusion on CD80 and CD86 mRNA-expression in liver transplants
| Cold ischaemia biopsies (n = 19) | Reperfusion biopsies (n = 19) | ||
|---|---|---|---|
| molecule | Median relative expression (range) (2−(Ct target – Ct β-actin) × 105) | P-valuea | |
| CD80 | 17 (3–100) | 28 (0–414) | 0·09 |
| CD86 | 220 (39–1100) | 320 (139–1000) | 0·37 |
Relative CD80 and CD86-mRNA levels in relation to the housekeeping gene β-actin mRNA were quantified by real-time detection PCR in cold-ischaemia and 1 h after reperfusion biopsies from 19 liver transplants. Expression levels of co-stimulatory molecule mRNAs were calculated by the comparative Ct method and depicted as medians with ranges.
Differences between cold ischaemia and reperfusion biopsies were statistically tested by the Wilcoxon signed-ranks test.
Part of clinical liver transplants have enhanced CD80 protein expression on Kupffer cells compared with normal liver tissue
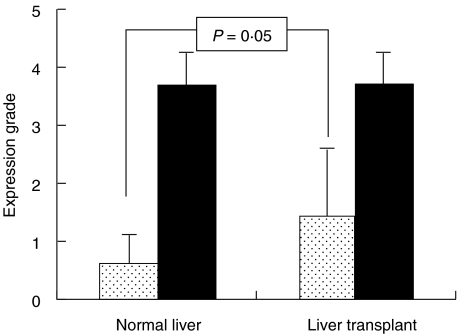
To compare lobular expression of CD80 and CD86 between liver transplants and normal liver tissue, mean grade of expression on Kupffer cells for each donor liver was calculated from those found in the three different biopsies, as the expression grades did not change during the transplantation procedure. As shown in Fig. 4, significantly higher proportions of Kupffer cells expressed CD80 in the liver transplants compared to normal liver tissue. In all normal livers the grade of lobular CD80 expression was ≤1, while 18 of 40 donor livers showed expression on at least one-quarter of Kupffer cells (>grade 1). In contrast, CD86 was expressed both in liver transplants and in normal liver on the majority of the Kupffer cells.
Fig. 4.
Mean grades (± s.d.) of expression of CD80 and CD86 on Kupffer cells in 10 normal livers and in 40 cadaveric liver transplants. Grades of CD80 expression were significantly higher in liver transplants.  , CD80; ▪, CD86.
, CD80; ▪, CD86.
To investigate whether this difference in CD80 protein expression was due to increased CD80 transcription rate, relative CD80 mRNA content was determined in normal liver tissue and compared with that found in cold ischaemia biopsies from donor livers. However, the relative CD80 mRNA contents of donor liver biopsies (median 17; range: 3–100; n = 19) and those of normal liver tissues (median 20; range: 5–28; n = 5) appeared to be similar (P = 0·62).
As is shown in Table 2, the enhanced lobular CD80 protein expression observed in 18 of 40 donor livers was not related to donor age or sex, nor to the time of cold or warm ischaemic periods of the grafts.
Table 2.
Lobular CD80-expression in relation to donor and liver transplant variables
| Lobular CD80- expression grade | Donor age (years; mean ± s.d.) | Donor sex (f/m) | Cold ischaemia time (min; mean ± s.d.) | Warm ischaemia time (min; mean ± s.d.) |
|---|---|---|---|---|
| Normal (≤1) (n = 22) | 35·7 ± 13·0 | 10/17 (59%) | 568 ± 159 | 59 ± 21 |
| Enhanced (>1) (n = 17) | 39·9 ± 13·7 | 12/22 (55%) | 521 ± 118 | 71 ± 27 |
Donor livers were divided into those with similar lobular CD80-expression as normal liver tissue (≤grade 1), and those with enhanced CD80-expression. From one liver transplant with enhanced CD80-expression, donor variables could not be ascertained.
No relation between CD80 and CD86 expression in liver transplants and acute rejection
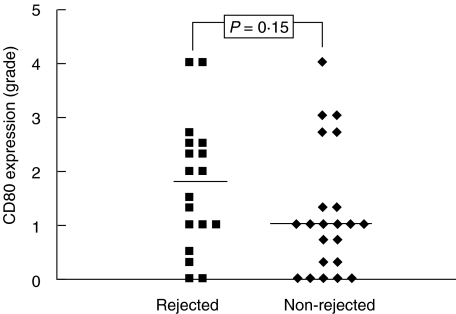
Eighteen of the liver transplants included in this study were rejected after transplantation and 22 were not rejected. Grades of CD80 and CD86 expression in portal fields and of lobular CD86 expression at the time of transplantation were similar in both groups of liver transplants (data not shown). There was a tendency to higher lobular CD80 expression in liver transplants which were later on rejected (Fig. 5), but this did not reach statistical significancy. There were no significant differences in CD80 or CD86 relative mRNA contents between donor livers which were rejected and which were not (data not shown).
Fig. 5.
Comparison of the grades of CD80 expression on Kupffer cells at the time of transplantation in 18 individual cadaveric liver transplants which were rejected after transplantation, and in 22 which were not rejected. Each point represents mean expression grade in one liver transplant, obtained from grading of three different biopsies. 0 = no expression on Kupffer cells; 4 = expression on 75% to all Kupffer cells.
Expression of B7 ligands on infiltrating cells during acute rejection
To investigate whether liver-graft infiltrating T cells are able to receive stimulatory signals, the expression of CD28 and CD152 (CTLA-4) in eight liver transplant biopsies obtained during an episode of acute rejection after transplantation was determined. CD152 is expressed on activated T cells and is a second receptor for CD80 and CD86, but unlike CD28 transmits an inhibitory signal into the T cell [23,24]. CD28 was found to be expressed on about three-quarters of cells in portal infiltrates. In contrast, less than one-quarter of the cells in portal infiltrates expressed CD152. Scattered throughout the liver parenchyma, CD28-positive cells were observed, but almost no CD152-positive cells.
DISCUSSION
In this study we observed that nearly half of cadaveric liver transplants had a higher proportion of Kupffer cells expressing the co-stimulatory molecule CD80 compared with normal human liver tissue. Almost no CD80-positive Kupffer cells were present in normal liver tissue biopsies, whether these were derived from livers containing malignancies or from healthy livers from patients with oesophagus carcinoma. The enhanced expression was present at the end of cold ischaemia, suggesting that activation of the Kupffer cells had occurred in situ in the organ donor or during the cold ischaemic storage. Because there was no difference in cold ischaemic storage times between the liver transplants with enhanced lobular CD80 expression and those with low expression, we favour the possibility that CD80 expression was induced in situ in the organ donor.
In contrast to the difference in proportions of Kupffer cells expressing CD80, the relative levels of CD80 mRNA were not enhanced in cold ischaemia biopsies of donor livers compared to normal liver tissue. One should be cautious in interpreting this result, as an indication that the increased CD80 protein expression on Kupffer cells in donor livers was not the result of increased transcription. Probably CD80 mRNA was partly degraded during the cold ischaemic storage of the donor livers before the biopsies were taken. Moreover, part of the CD80 mRNA detected in normal liver tissues may have been derived from CD80 positive cells in portal fields, which were present in about one-third of normal livers.
The first factor that may have induced CD80 expression is brain death. In rats, brain death has been shown to induce CD80 expression on interstitial macrophages and endothelium in kidney [16]. In rat liver, brain death induces immune activation with induced adhesion molecule expression and leucocyte infiltration [17,25]. This activation may be induced by a crisis of sympathetic activity occurring in the early phase of brain death, resulting in a peak of circulating catecholamines, or by increased blood levels of cytokines in the brain-dead donor [26,27]. A second possible cause of Kupffer cell activation may be bacterial translocation and endotoxin absorption from the gut, which occurs frequently among clinical organ donors [18]. Endotoxins are potent Kupffer cell stimulators [19].
No induction of co-stimulatory molecule mRNA or protein expression occurred during the transplantation procedure. This does not mean that ischaemia and reperfusion do not influence expression of these molecules: such effects may become apparent at a later time-point after transplantation. In vitro, it takes 24 h before CD86 protein expression increases and 48 h before CD80 protein expression increases after activation of APC [28]. In rats, cold ischaemia and reperfusion resulted in increase of CD80 expression in kidney after 24 h [29].
Although liver transplants that were rejected after transplantation showed a tendency to have higher CD80 expression on Kupffer cells at the time of transplantation than liver grafts that were not rejected, this was not statistically significant. Therefore, the extent of lobular CD80 expression cannot be used to predict the probability of acute rejection. Probably other stimulatory molecular mechanisms are more important in the early events leading to rejection activity. It has been suggested that natural killer (NK) cells in the liver have a crucial role in T cell recruitment through a multistep cytokine and chemokine cascade [30]. Activation of NK cells is mediated by interactions between other stimulatory molecules, such as CD160 with HLA-C [31], than T cells.
In contrast to recipient lymphocytes infiltrating in clinical heart allografts [32], the lymphocytes in rejection infiltrates in liver grafts express CD28 abundantly, indicating that these cells can receive a stimulatory signal from the B7 molecules in the donor liver. Moreover, only a small proportion of the infiltrating cells expressed the inhibitory receptor CD152.
In conclusion, nearly half of cold-preserved cadaveric donor livers show enhanced expression of the co-stimulatory molecule CD80 on Kupffer cells compared to normal liver tissue. This expression was not induced further during warm ischaemia and reperfusion of the grafts. The extent of lobular CD80 expression at the time of transplantation cannot be used as a predictive parameter for acute rejection.
Acknowledgments
The authors acknowledge Dr P.G.M. Mulder from the Department of Epidemiology and Biostatistics for his help with statistical analysis, S. de Rave of the Department of Gastroenterology and Hepatology for providing organ donor variables, and Dr E. Wiemer from the Department of Hematology for his advices on real-time PCR technology.
References
- 1.Markus BH, Duquesnoy RJ, Gordon RD, et al. Histocompatibility and liver transplant outcome. Does HLA exert a dualistic effect? Transplantation. 1988;46:372–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson P, Underhill J, Doherty D, et al. Influence of human leukocyte antigen matching on liver allograft survival and rejection: ‘the dualistic effect’. Hepatology. 1993;17:1008–15. [PubMed] [Google Scholar]
- 3.Nikaein A, Backman L, Jennings L, et al. HLA compatibility and liver transplant outcome. Improved patient survival by HLA and cross-matching. Transplantation. 1994;58:786–92. [PubMed] [Google Scholar]
- 4.Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection. incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638–45. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 5.Bathgate AJ, Pravica V, Perrey C, et al. The effect of polymorphisms in tumor necrosis factor-alpha, interleukin-10, and transforming growth factor-beta1 genes in acute hepatic allograft rejection. Transplantation. 2000;69:1514–7. doi: 10.1097/00007890-200004150-00054. [DOI] [PubMed] [Google Scholar]
- 6.Warle MC, Farhan A, Metselaar HJ, et al. Cytokine gene polymorphisms and acute human liver graft rejection. Liver Transplant. 2002;8:603–11. doi: 10.1053/jlts.2002.33967. [DOI] [PubMed] [Google Scholar]
- 7.Sayegh MH, Turka LA. The role of T cell co-stimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 8.Tu Y, Rehman A, Flye MW. CTLA4-Ig treatment prolongs rat orthotopic liver graft survival. Transplant Proc. 1997;29:1036–7. doi: 10.1016/s0041-1345(96)00358-2. [DOI] [PubMed] [Google Scholar]
- 9.Van Gool SW, de Boer M, Ceuppens JL. CD28 ligation by monoclonal antibodies or B7/BB1 provides an accessory signal for the cyclosporin A-resistant generation of cytotoxic T cell activity. J Immunol. 1993;150:3254–63. [PubMed] [Google Scholar]
- 10.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7 : CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–82. [PubMed] [Google Scholar]
- 11.Reiser H, Stadecker MJ. Co-stimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med. 1996;335:1369–77. doi: 10.1056/NEJM199610313351807. [DOI] [PubMed] [Google Scholar]
- 12.Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–60. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohse AW, Knolle PA, Bilo K, et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 1996;110:1175–81. doi: 10.1053/gast.1996.v110.pm8613007. [DOI] [PubMed] [Google Scholar]
- 14.Leifeld L, Trautwein C, Dumoulin FL, Manns MP, Sauerbruch T, Spengler U. Enhanced expression of CD80 (B7-1), CD86 (B7-2), and CD40 and their ligands CD28 and CD154 in fulminant hepatic failure. Am J Pathol. 1999;154:1711–20. doi: 10.1016/S0002-9440(10)65427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgio VL, Ballardini G, Artini M, Caratozzolo M, Bianchi FB, Levrero M. Expression of co-stimulatory molecules by Kupffer cells in chronic hepatitis of hepatitis C virus etiology. Hepatology. 1998;27:1600–6. doi: 10.1002/hep.510270620. [DOI] [PubMed] [Google Scholar]
- 16.Takada M, Nadeau KC, Hancock WW, et al. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65:1533–42. doi: 10.1097/00007890-199806270-00001. [DOI] [PubMed] [Google Scholar]
- 17.van der Hoeven JA, Ploeg RJ, Postema F, et al. Induction of organ dysfunction and up-regulation of inflammatory markers in the liver and kidneys of hypotensive brain dead rats: a model to study marginal organ donors. Transplantation. 1999;68:1884–90. doi: 10.1097/00007890-199912270-00012. [DOI] [PubMed] [Google Scholar]
- 18.van Goor H, Rosman C, Grond J, Kooi K, Wubbels GH, Bleichrodt RP. Translocation of bacteria and endotoxin in organ donors. Arch Surg. 1994;129:1063–6. doi: 10.1001/archsurg.1994.01420340077014. [DOI] [PubMed] [Google Scholar]
- 19.Arai M, Peng XX, Currin RT, Thurman RG, Lemasters JJ. Protection of sinusoidal endothelial cells against storage/reperfusion injury by prostaglandin E2 derived from Kupffer cells. Transplantation. 1999;68:440–5. doi: 10.1097/00007890-199908150-00017. [DOI] [PubMed] [Google Scholar]
- 20.Carles J, Fawaz R, Hamoudi NE, Neaud V, Balabaud C, Bioulac-Sage P. Preservation of human liver grafts in UW solution. Ultrastructural evidence for endothelial and Kupffer cell activation during cold ischemia and after ischemia-reperfusion. Liver. 1994;14:50–6. doi: 10.1111/j.1600-0676.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 21.Lemasters JJ, Bunzendahl H, Thurman RG. Reperfusion injury to donor livers stored for transplantation. Liver Transpl Surg. 1995;1:124–38. doi: 10.1002/lt.500010211. [DOI] [PubMed] [Google Scholar]
- 22.Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluestone JA. Is CTLA-4 a master switch for peripheral T cell tolerance? J Immunol. 1997;158:1989–93. [PubMed] [Google Scholar]
- 24.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001;2:58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 25.van der Hoeven JA, Ter Horst GJ, Molema G, et al. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg. 2000;232:804–13. doi: 10.1097/00000658-200012000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amado JA, Lopez-Espadas F, Vazquez-Barquero A, et al. Blood levels of cytokines in brain-dead patients: relationship with circulating hormones and acute-phase reactants. Metabolism. 1995;44:812–6. doi: 10.1016/0026-0495(95)90198-1. [DOI] [PubMed] [Google Scholar]
- 27.Novitzky D, Cooper DK, Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43:852–4. [PubMed] [Google Scholar]
- 28.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 29.Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL. The role of the B7 co-stimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest. 1997;100:1199–203. doi: 10.1172/JCI119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crispe IN. Hepatic T cells and liver tolerance. Nature Immunol Rev. 2003;3:51–61. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 31.Le Bouteiller P, Barakonyi A, Giustiniani J, et al. EngAgement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc Natl Acad Sci USA. 2002;99:16963–8. doi: 10.1073/pnas.012681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Hoffen E, Van Wichen DF, Leemans JC, et al. T cell apoptosis in human heart allografts. association with lack of co-stimulation? Am J Pathol. 1998;153:1813–24. doi: 10.1016/S0002-9440(10)65696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]