Abstract
FcγRIII (CD16) is found in two alternative forms, a transmembrane FcγRIIIa expressed on NK cells and macrophages, and a glycosylphosphatidylinositol-linked FcγRIIIb present on neutrophils. Previously, we measured soluble FcγRIIIa (sFcγRIIIa) in plasma of NA(1 +, 2-) phenotyped donors with the anti-FcγRIII monoclonal antibody (MoAb) GRM1, which recognizes NA2-FcγRIIIb and FcγRIIIa. The level of sFcγRIIIa, as well as the total sFcγRIII (sFcγRIIIa plus sFcγRIIIb) in patients with rheumatoid arthritis (RA) was significantly higher than that in healthy controls. In this study, we measured sFcγRIIIaMφ in plasma with a newly developed anti-FcγRIII MoAb, MKGR14 (mIgM), which recognizes FcγRIIIaMφ specifically. From the recovery of purified sFcγRIIIaMφ, the amount of sFcγRIIIaMφ present was about half that of sFcγRIIIaNK, and that of sFcγRIIIa was about 50 times lower than that of sFcγRIIIb in pooled plasma from healthy NA(1 +, 2-) phenotyped donors. The level of sFcγRIIIaMφ in RA patients was about four times higher than that in healthy controls. In RA patients, both the sFcγRIIIaMφ and sFcγRIIIa levels were increased as proportionally as the Lansbury Index. The sFcγRIIIa, but not sFcγRIIIaMφ levels, were increased directly proportional to C-reactive protein. sFcγRIIIaMφ may be a novel marker of disease activity in RA.
Keywords: Fc receptors, human, monocytes/, macrophages, rheumatoid arthritis
INTRODUCTION
FcγRIII (CD16) exists in two alternative forms. FcγRIIIa is an integral membrane protein expressed on natural killer (NK) cells, on a subset of T lymphocytes and on a subpopulation of monocytes and macrophages [1], and shows a cell type-specific glycosylation pattern [2]. FcγRIIIb is a glycosylphosphatidylinositol-linked protein expressed exclusively on neutrophils, and it can be induced on eosinophils [1]. Both FcγRIIIs are released from the cell surface, possibly by the activation of cells. FcγRIIIa is released by the action of a metalloprotease upon in vitro activation of NK cells and macrophages [3,4]. FcγRIIIb is released upon activation and during apoptosis of neutrophils by proteolytic activity [5,6]. The release of FcγRIIIb is inhibited by the serine protease inhibitors and metalloprotease inhibitors, depending on the stimulus used to activate the cells [7].
Rheumatoid arthritis (RA) is a chronic inflammatory disease, affecting the joints and extra-articular tissues. Increased protein expression of a series of matrix metalloproteases, which are involved in the breakdown of extracellular matrix molecules, has been demonstrated in patients with RA [8]. Several reports [9,10] have described a reduced expression of FcγRIIIa on NK cells isolated from the synovial fluid of affected joints of RA patients. It is possible that the activated metalloprotease may cleave FcγRIIIa on NK cells. Soluble FcγRIIIa (sFcγRIIIa) is detected in plasma from RA patients and in very low amounts in plasma from healthy donors [2]. In addition to NK cells, mononuclear cells, such as macrophages, lymphocytes and plasma cells, are the very common cells in the synovium of the rheumatoid joint. Macrophage FcγRIIIa is distributed in restricted tissues [11], being expressed at high levels only in the synovial intimal tissue and other tissues, such as pericardium. Three studies that examined the changes in synovial membrane pathology resulting from treatment with gold injections showed a significant reduction in the number of cells of the macrophage lineage in the lining and subintimal regions of these patients, which correlated with an improvement of their clinical courses [12–14].
Although sFcγRIII has been detected in saliva, synovial and seminal fluid, serum and plasma [2,5,15–17], none of the assays used discriminates sFcγRIIIa from sFcγRIIIb. Plasma sFcγRIII was shown to be derived mainly from neutrophils and to a lesser extent from NK cells [2,5,16]. sFcγRIIIa derived from macrophages has not yet been detected in plasma. Previously, we measured sFcγRIIIa in plasma of NA(1 +, 2-) phenotyped donors with the anti-FcγRIII monoclonal antibody (MoAb) GRM1 [18], which recognizes NA2-FcγRIIIb and sFcγRIIIa. The level of sFcγRIIIa, as well as the total sFcγRIII (sFcγRIIIa plus sFcγRIIIb), in RA patients was significantly higher than that in healthy controls. In this study, we measured sFcγRIIIaMφ in plasma with immuno-polymerase chain reaction (PCR) with a newly developed anti-FcγRIII MoAb, MKGR14 (mIgM), which specifically recognizes FcγRIIIaMφ.
METHODS
FcγRIIIaMφ specific anti-FcγRIII MoAb, MKGR14
FcγRIIIaMφ were prepared from Nonidet P-40 lysates of 4-day-cultured monocytes. Briefly, monocytes were isolated from a buffy coat prepared from citrated blood of healthy donors by Percoll density centrifugation and subsequent counterflow centrifugal elutriation of the mononuclear leucocytes [19]. The purified monocytes were cultured for 4 days in Iscove's minimal essential medium supplemented with 10% fetal calf serum. The cultured monocytes were lysed at 4°C by treatment for 15 min with 1% Nonidet P-40 (Sigma, St Louis, MO, USA) in 110 mM NaCl and 50 mM Tris (pH 7·5) in the presence of 50 µg of phenylmethylsulphonyl fluoride (PMSF) per ml, 1 mM Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK) and 40 µg of soybean trypsin inhibitor per ml. After the lysates were precleared with bovine serum albumin (BSA)-coated Sepharose CL-4B beads (Amersham Pharmacia Biotech AB, Uppsala, Sweden), FcγRIIIaMφ were purified by affinity chromatography with lentil lectin-sepharose (Amersham Pharmacia Biotech AB) and anti-FcγRIII MoAb CLBFcRgranI (Table 1) bound to Sepharose CL-4B beads. Anti-FcγRIII MoAb CLBFcRgranI was provided generously by Dr M. de Haas, CLB, Amsterdam, the Netherlands.
Table 1.
Anti-FcγRIII MoAbs used in this study
| MoAbs | Specificity | Source |
|---|---|---|
| CLBFcRgranI | FcγRIIIa and FcγRIIIb | M. de Haas |
| 3G8 | FcγRIIIa and FcγRIIIb | MEDREX, INC |
| CLB-LM6·30 | FcγRIIIa and FcγRIIIb | M. de Haas |
| CLBFcRgranII | NA1-FcγRIIIb | M. de Haas |
| GRM1 | FcγRIIIa and NA2-FcγRIIIb | F. Garrido |
| MKGR14 | FcγRIIIaMφ | M. Masuda |
We developed a new anti-FcγRIII MoAb, MKGR14, by immunization of mice with purified FcγRIIIaMφ. The MoAb was tested simultaneously with the anti-FcγRIII MoAb CLBFcRgranI for reactivity with NA1NA2-neutrophils, NK cells, monocytes, cultured monocytes, peritoneal macrophages and THP-1 by indirect immunofluorescence. The peritoneal cells were collected from the peritoneal fluid of a patient with renal disease treated with continuous ambulatory peritoneal dialysis. The cross-blocking experiments were performed with fluorescein isothiocyanate (FITC)-labelled anti-FcγRIII MoAbs, MKGR14, CLBFcRgranI, 3G8, CLBFcRgranII, GRM1 and CLB-LM6·30 (Table 1). Anti-FcγRIII MoAb CLBFcRgranII and CLB-LM6·30 or GRM1 were provided generously by Dr M. de Haas, CLB, Amsterdam, the Netherlands, or Dr F. Garrido, Hospital Virgen de las Nieves, Granada, Spain, respectively.
Anti-FcγRIII immunoblot analysis
Neutrophils were isolated from blood of healthy NA(1 +, 2 +) phenotyped donors by Percoll density (1·077 g/cm3) centrifugation and subsequent lysis of erythrocytes with ammonium chloride [5]. Monocytes and large granular lymphocytes (LGL) were isolated from a buffy coat prepared from citrated blood of healthy donors [19]. The purified NA1NA2-neutrophils, LGL or 4-day-cultured monocytes were lysed with 1% Nonidet P-40 and immunoprecipitated with anti-FcγRIII MoAb MKGR14 or CLBFcRgranI. Purified anti-FcγRIII MoAb MKGR14 or CLBFcRgranI had been coupled to sepharose CL-4B beads or protein G-Sepharose 4 fast flow beads (Amersham Pharmacia Biotech AB), respectively.
Anti-FcγRIII immunoprecipitates were analysed by one-dimensional SDS-PAGE under non-reducing conditions on an acrylamide gel and transferred to a nitrocellulose membrane. After blotting with anti-FcγRIII MoAb CLB-LM6·30 [18], followed by a peroxidase-conjugated goat anti-mouse IgG antibody (Jackson Immuno Research, West Grove, PA, USA), the FcγRIIIaMφ, FcγRIIIaNK and FcγRIIIb were detected by enhanced chemiluminescence (Boehringer Mannheim GmbH, Germany).
Analysis of immunoprecipitates by gel electrophoresis
Four-day-cultured monocytes were surface-labelled with biotin and lysed in 1% Nonidet P-40, as described before. The cell lysates were incubated with Sepharose-coupled MKGR14 or CLBFcRgranI, and the immunoprecipitates were then analysed by SDS-PAGE under non-reducing conditions and transferred to a nitrocellulose membrane. The biotin-labelled protein was detected by blotting with peroxidase-conjugated streptavidin.
Patients
Patients were recruited from the Division of Rheumatology at our hospital, and were followed-up from April 1994 to April 1996. All patients met the American Rheumatism Association (ARA) criteria for RA [20]. Seventy-six patients with a NA(1 +, 2-) phenotype were selected from 178 patients with RA. Although the measurement of sFcγRIIIaMφ in plasma from any donors is possible, we selected NA(1 +, 2-) phenotyped donors to compare the levels of sFcγRIIIa. Four of these 76 patients had nephritis and four had hepatic diseases, and were excluded because sFcγRIII are probably catabolized by the liver, as well as excreted from the kidney [5,17]. As pathological controls, eight patients with a NA(1 +, 2-) phenotype were selected from 40 patients with osteoarthritis (OA). Laboratory findings in these patients are shown in Table 2. Informed consent was obtained from all patients, and the trial was approved by the ethical committee in our hospital.
Table 2.
Laboratory findings in the patients
| Controls | RA patients | OA patients | |
|---|---|---|---|
| n | F: 28, M: 12 | F: 54, M: 14 | F: 7, M: 1 |
| Age (years) | 53·4 ± 13·4 | 56·1 ± 11·9 | 58·6 ± 7·4 |
| RF (IU/ml) | < 10·1 | 131·6 ± 318·4 | < 10·1 |
| IgG (g/l) | 15·4 ± 2·2 | 21·5 ± 6·5*† | 14·9 ± 2·7 |
| IgA (g/l) | 3·1 ± 1·1 | 4·4 ± 1·9* | 3·5 ± 1·6 |
| IgM (g/l) | 1·8 ± 0·7 | 2·0 ± 1·0 | 2·0 ± 1·7 |
| CRP (mg/l) | < 1·0 | 34·2 ± 34·4† | 7·1 ± 17·4 |
| ESR (mm) | – | 48·1 ± 33·7 | – |
| Lansbury Index | – | 35·8 ± 18·5 | – |
| Neutrophils (µl) | 3684 ± 998 | 5316 ± 1877* | 4500 ± 2552 |
| NK cells (µl) | 253 ± 112 | 263 ± 152 | 335 ± 146 |
| Platelets (104/µl) | 24·1 ± 6·0 | 31·2 ± 9·9*† | 21·7 ± 5·4 |
RF indicates rheumatoid factor; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate. The data are presented as the mean ± s.d. Significant differences versus healthy controls
P < 0·01) or versus OA patients
P < 0·01).
Three hundred and forty-two healthy volunteers were recruited randomly from the hospital staff. One hundred individuals were selected for an NA(1 +, 2-) phenotype to constitute the pooled plasma. Forty-one age-matched individuals were selected as healthy controls. None of the control individuals had any evidence of renal, hepatic, infectious or inflammatory disease or diabetes mellitus, and none were taking any medication.
FcγRIIIB-NA(1, 2) genotyping assays
Genomic DNA (gDNA) was extracted from leucocytes by standard techniques. Genotyping for the FcγRIIIB-NA1/2 polymorphism was performed according to Koene et al. [21]. In brief, two sets of primers specifically annealing to either an NA1-FcγRIIIB or an NA2-FcγRIIIB fragment were used. NA1-FcγRIIIB- and NA2-FcγRIIIB-specific fragments were amplified separately from gDNA in a PerkinElmer GeneAmp PCR System 9600 (Foster City, CA, USA).
ELISA for total sFcγRIII
The total sFcγRIII concentrations were measured by enzyme-linked immunosorbent assay (ELISA) according to Koene et al. [17]. Briefly, a 96-well ELISA plate (Nunc Immunoplate Maxisorp, Roskilde, Denmark) was coated with anti-FcγRIII MoAb CLBFcRgranI. After unbound sites had been blocked with 2% milk in phosphate-buffered saline (PBS), diluted EDTA plasma in high-performance ELISA buffer (HPE buffer; CLB, Amsterdam, the Netherlands) was incubated in the wells for 1 h at room temperature. After washing with PBS containing 0·02% (v/v) Tween-20, the plates were incubated with a biotin-labelled rabbit anti-FcγRIII antibody. After incubating with horseradish-peroxidase-labelled streptavidin, the amount of sFcγRIII was detected with tetramethylbenzidine and H2O2. A calibration curve was constructed with pooled plasma from 100 healthy NA(1 +, 2-) phenotyped donors. The concentration of total sFcγRIII in this pool was set at 100 arbitrary units (AU).
Immuno-PCR method for sFcγRIIIa and sFcγRIIIaMφ
The sFcγRIIIa and sFcγRIIIaMφ concentrations were measured with immuno-PCR according to Furuya et al. [22] with minor modifications. Briefly, thin-walled 96-well polypropylene plates suitable for thermocycling (Bio Medical Equipment, Tokyo, Japan) were coated with anti-FcγRIII MoAb GRM1 for sFcγRIIIa or MKGR14 for sFcγRIIIaMφ. After unbound sites were blocked with 1 g/l salmon sperm DNA, 1% fetal calf serum, 5% milk and 1% gelatin in PBS, diluted EDTA-plasma in HPE buffer was incubated in the wells for overnight at 4°C. After washing with PBS containing 0·02% (v/v) Tween-20, the plates were incubated with the biotin-labelled CLBFcRgranI for sFcγRIIIa or GRM1 for sFcγRIIIaMφ. After incubating with Neutravidin (ImmunoPure grade, from Pierce, Rockford, IL, USA) (1 mg/l in HPE buffer), the plates were incubated with the biotinylated DNA (5 nM in HPE buffer containing 1 g/l salmon sperm DNA). Biotinylated DNA has been produced from plasmid Bluescript by PCR amplification with biotinylated M13 primer and non-biotinylated Rev primer, as described previously [22]. The amount of sFcγRIII was detected by real-time PCR in an ABI PRISM® 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). A calibration curve was constructed with the plasma pool and the concentration of sFcγRIIIa or sFcγRIIIaMφ in the plasma pool was set at 100 AU.
Recovery of sFcγRIIIaMφ
In our study, sFcγRIII concentrations were presented as the percentage of sFcγRIII compared with the amount of sFcγRIII in a standard plasma pool. To determine the relative levels of sFcγRIIIaMφ, sFcγRIIIaNK and sFcγRIIIb in plasma, we added purified sFcγRIIIaMφ to pooled plasma, measured sFcγRIIIaMφ, sFcγRIIIa and total sFcγRIII, and then calculated how much AU had been added. sFcγRIIIaMφ was prepared from culture supernatant of monocytes by affinity chromatography with Lentil Lectin-Sepharose and anti-FcγRIII MoAb CLBFcRgranI-Sepharose, as described before. Contaminating MoAb was removed by filtration with a 100-kDa cut-off membrane (OMEGA™ disk, Pall Filtron Co., Northborough, MA, USA), followed by concentration of FcγRIIIaMφ (OD280: 0·457). Purified sFcγRIIIaMφ or PBS (1/10 volume) was supplemented to pooled plasma from healthy NA(1 +, 2-) phenotyped donors and then sFcγRIIIaMφ, sFcγRIIIa and total sFcγRIII were measured 10 times each.
Effects of IgG complex in RA samples
A γ-globulin fraction was prepared from pooled RA patient's serum by precipitation with 30% saturated ammonium sulphate. After dialysis against PBS, the fraction was passed through a column of anti-FcγRIII MoAb CLBFcRgranI bound Sepharose CL-4B beads (Amersham Pharmacia Biotech AB) to remove sFcγRIII.
The concentrated γ-globulin fraction with or without sFcγRIIIaMφ was added to the pooled plasma or to the RA patient's plasma, and subsequently the amount of the three types of sFcγRIIIa were measured.
Statistical analysis
Differences in the sFcγRIII levels or laboratory data among the groups were tested by analysis of variance (ANOVA) with the Fisher's PLSD post hoc test. Correlations were tested by Bartlett's test and multiple comparisons by analysis of variance.
RESULTS
FcγRIIIaMφ-specific anti-FcγRIII MoAb, MKGR14
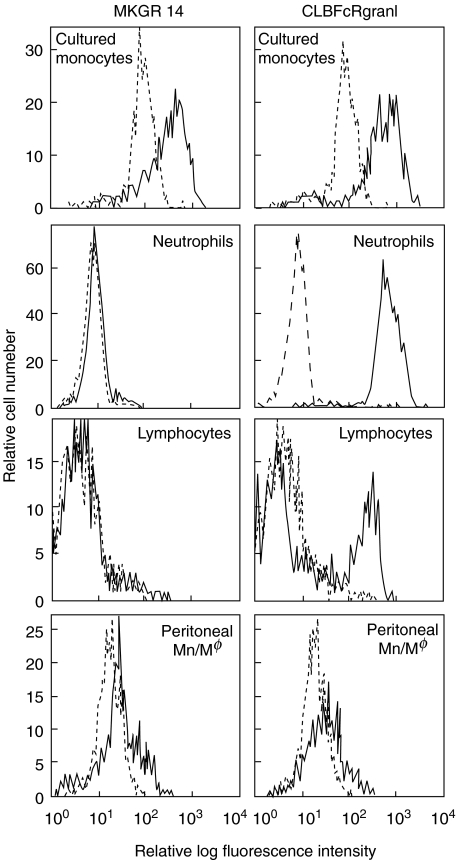
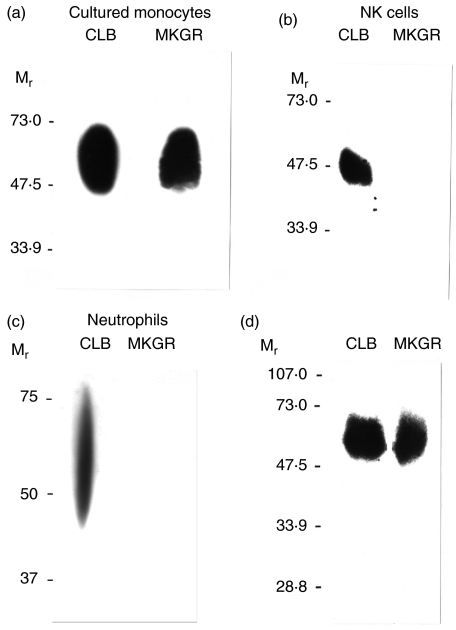
We developed a new anti-FcγRIII MoAb, MKGR14 (mIgM), by immunizing mice with purified human FcγRIIIaMφ. As shown in Fig. 1, MKGR14 bound to cultured monocytes, a subpopulation of monocytes and peritoneal monocytes/macrophages, but not to NA1NA2-neutrophils or NK cells. In contrast, CLBFcRgranI bound to all cell types. Both MoAbs did not bind to THP-1 (data not shown). The cross-blocking experiments showed that MKGR14 slightly inhibited the binding of the CLBFcRgranI, but not the other tested MoAbs (data not shown). Immunoblotting assay showed that MKGR14 precipitated FcγRIIIaMφ from the lysate of cultured monocytes, but did not precipitate FcγRIIIb or FcγRIIIaNK from the lysate of NA1NA2-neutrophils or LGL, respectively (Fig. 2). In contrast, CLBFcRgranI precipitated all three types of FcγRIII. As shown in Fig. 2d, MKGR14, as well as CLBFcRgranI, precipitated only 50–60 kDa protein from the lysate of biotin-labelled cultured monocytes. All these results show that MKGR14 specifically recognizes FcγRIIIaMφ.
Fig. 1.
Histograms of anti-FcγRIII MoAb MKGR14 binding cells. Four-day-cultured monocytes, peripheral blood leucocytes (NA (1 +, 2 +) donor) or peritoneal cells were incubated on ice with anti-FcγRIII MoAb MKGR14 (left) or CLBFcRgranI (right). Neutrophils, lymphocytes or peritoneal monocytes/macrophages were identified by their characteristic forward and side scatter properties, and the cell population exhibiting these characteristics was selected by flow cytometric gating for analysis. The black lines indicate anti-FcγRIII MoAb binding cells and the grey lines indicate negative control.
Fig. 2.
Analysis of immunoprecipitates of MKGR14 by anti-FcγRIII immunoblot (a, b, c) and gel electrophoresis (d). (a, b, c) Immunoprecipitates of MKGR14 (MKGR) or CLBFcRgranI (CLB) prepared from 4-day-cultured monocytes (a), LGL (b: NK cells) or NA1NA2-neutrophils (c) were analysed by SDS-PAGE under non-reducing conditions and transferred to a nitrocellulose membrane. The FcγRIII were detected by immunoblotting with an anti-FcγRIII MoAb CLB-LM6·30. (d) Four-day-cultured monocytes were surface-labelled with biotin and lysed in 1% Nonidet P-40. The cell lysates were incubated with Sepharose-coupled MKGR14 (MKGR) or CLBFcRgranI (CLB), and the immunoprecipitates were then analysed by SDS-PAGE under non-reducing conditions and transferred to a nitrocellulose membrane. The biotin-labelled protein was detected by blotting with streptavidin. Mr, relative molecular mass × 10−3.
Measurement of sFcγRIIIaMφ in plasma
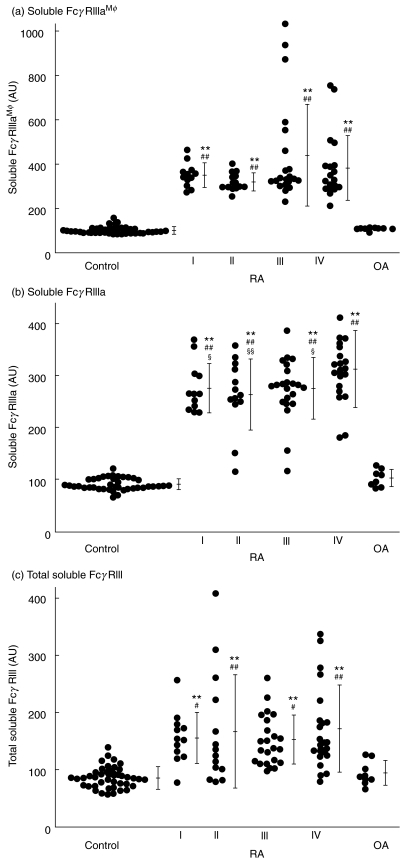
The FcγRIIIa is released from NK cells and/or macrophages, and FcγRIIIb is released from neutrophils [3–7,16]. Using FcγRIIIaMφ-specific anti-FcγRIII MoAb, MKGR14, we measured sFcγRIIIaMφ in plasma. As shown in Fig. 3, the level of sFcγRIIIaMφ in RA patients was about four times higher than that in healthy controls. In particular, there were extraordinarily higher levels in patients in stage III or IV. In contrast, the level of total sFcγRIII was only about two times and the level of sFcγRIIIa was about three times higher. As pathological controls with arthritis, the sFcγRIII concentrations were measured in OA patients. All the sFcγRIII levels in OA patients were not different from those in healthy controls and were significantly lower than those in RA patients.
Fig. 3.
Concentration of sFcγRIIIaMφ, sFcγRIIIa and total sFcγRIII in plasma from RA patients. The sFcγRIIIaMφ (upper), sFcγRIIIa (centre) or total sFcγRIII (lower) concentrations were measured with immuno-PCR or with ELISA, respectively, and are presented as the percentage of sFcγRIII compared with the amount of sFcγRIII in the plasma pool. Significant differences versus healthy control (*P < 0·05, **P < 0·01), versus OA patients (#P < 0·05, ##P < 0·01) or versus stage 4 (§P < 0·05, §§P < 0·01).
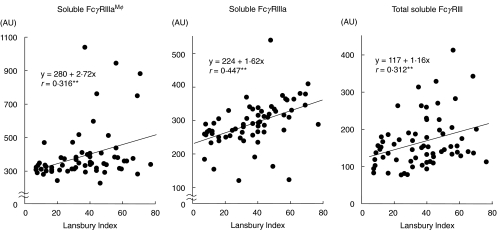
As shown in Table 3, the sFcγRIIIaMφ levels in plasma correlated with age, the sFcγRIIIa levels correlated with the number of NK cells in peripheral blood and total sFcγRIII levels correlated with the number of neutrophils in peripheral blood in healthy controls, but not in RA patients. All the sFcγRIII levels correlated with each other (sFcγRIIIaMφ to sFcγRIIIa: r = 0·335**, sFcγRIIIaMφ to total sFcγRIII: r = 0·274*, sFcγRIIIa to total sFcγRIII: r = 0·447**) in RA patients, but not in healthy controls. In RA patients, there was a significant correlation between the sFcγRIIIaMφ levels and the Lansbury Index, and between the sFcγRIIIa levels and C-reactive protein or the Lansbury Index, and between total sFcγRIII levels and the concentrations of IgG (Table 3, Fig. 4).
Table 3.
Multiple comparisons of correlation among sFcγRIII levels and laboratory findings in patients
| Correlation coefficient | ||||||
|---|---|---|---|---|---|---|
| sIIIaMφ | P | sIIIa | P | TsIII | P | |
| (a) Controls | ||||||
| Age | 0·801 | < 0·0001† | − 0·068 | 0·738 | 0·030 | 0·882 |
| IgG | − 0·071 | 0·726 | 0·301 | 0·129 | − 0·042 | 0·834 |
| Neutrophils | 0·120 | 0·553 | − 0·152 | 0·451† | 0·231 | 0·249 |
| Platelets | 0·218 | 0·276 | − 0·282 | 0·157 | − 0·079 | 0·694 |
| (b) RA patients | ||||||
| Age | − 0·066 | 0·604 | − 0·029 | 0·821 | − 0·187 | 0·134 |
| RF | − 0·103 | 0·419 | − 0·093 | 0·463 | 0·034 | 0·785 |
| IgG | 0·033 | 0·797 | 0·120 | 0·342 | 0·282 | 0·022* |
| CRP | − 0·204 | 0·106 | 0·247 | 0·047* | − 0·099 | 0·429 |
| Lansbury Index | 0·310 | 0·013* | 0·254 | 0·042* | 0·239 | 0·054 |
| Neutrophils | − 0·148 | 0·244 | 0·005 | 0·971 | 0·064 | 0·608 |
| NK cells | 0·221 | 0·080 | 0·056 | 0·660 | 0·032 | 0·799 |
| Platelets | 0·212 | 0·094 | − 0·198 | 0·115 | − 0·029 | 0·814 |
sIIIaMφ indicates level of sFcγRIIIaMφ; sIIIa, level of sFcγRIIIa; TsIII, level of total sFcγRIII; RF, rheumatoid factor; CRP, C-reactive protein.
Fig. 4.
Correlation between sFcγRIII levels and the Lansbury Index in RA patients. The sFcγRIIIaMφ (left), sFcγRIIIa (centre) or total sFcγRIII (right) concentrations were measured with immuno-PCR or with ELISA, respectively, and are presented as the percentage of sFcγRIII compared to the amount of sFcγRIII in the pooled plasma. Significant correlations (**P< 0·01).
Previous papers have shown that plasma sFcγRIII is derived mainly from neutrophils and to a lesser extent from NK cells [2,5,16], and the sFcγRIIIa is derived mainly from NK cells [2]. To determine the relative levels of sFcγRIIIaMφ, sFcγRIIIaNK and sFcγRIIIb in plasma, we added purified FcγRIIIaMφ to pooled plasma, measured sFcγRIIIaMφ, sFcγRIIIa and total sFcγRIII, and then calculated how much AU had been added. The supplemented FcγRIIIaMφ were 263·78 ± 9·61 AU in sFcγRIIIaMφ assay, 95·78 ± 7·38 AU in sFcγRIIIa assay and 1·93 ± 0·93 AU in total sFcγRIII assay.
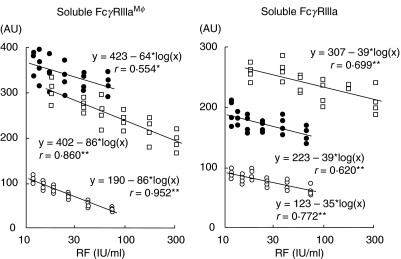
Previously, we evaluated the effects of IgG complex in RA samples on measured sFcγRIII concentrations. To evaluate the effect of IgG complexes in RA samples, we composed ‘RA-like’ samples with pooled plasma from healthy volunteers, sFcγRIIIaMφ prepared from culture supernatant of monocytes and the γ-globulin fraction prepared from RA patients’ serum. The levels of sFcγRIIIaMφ, as well as the total sFcγRIII or sFcγRIIIa, decreased with increasing amounts of supplemented RA γ-globulin fraction (Fig. 5).
Fig. 5.
Effects of IgG complex on measurement of sFcγRIIIaMφand sFcγRIIIa in plasma from RA patients. The RA γ-globulin fraction was added to the plasma pool, with (•) or without (○) sFcγRIIIaMφ, or RA patient's plasma (R56) (□), and then the amount of sFcγRIIIaMφ(left) or sFcγRIIIa (right) was measured. The amount of supplemented RA γ-globulin fraction is presented as the concentration of rheumatoid factor.
DISCUSSION
FcγRIII (CD16) exists in two alternative forms. NK cells and macrophages express FcγRIIIa and neutrophils express FcγRIIIb [1]. Both FcγRIIIs are released from the cell surface with proteolytic cleavage [3–7,16] and these soluble forms are present in plasma [2,5,16,17]. Previously, we measured sFcγRIIIa in plasma of NA(1 +, 2-) phenotyped donors with anti-FcγRIII MoAb GRM1 [18], which recognizes NA2-FcγRIIIb and FcγRIIIa. The level of sFcγRIIIa, as well as the total sFcγRIII, in RA patients was significantly higher than that in healthy control.
Thus, to determine unequivocally the plasma level of sFcγRIIIa, an assay that discriminates sFcγRIIIaNK from sFcγRIIIaMφ should be developed. We have succeeded in raising a new anti-FcγRIII MoAb which specifically recognizes FcγRIIIaMφ, by immunization of mice with purified human FcγRIIIaMφ. This MoAb, MKGR14 (mIgM), bound to monocytes/macrophages, but not to neutrophils or NK cells. MKGR14 precipitated FcγRIIIaMφ, but not FcγRIIIb, FcγRIIIaNK or other proteins from the surface of the cultured monocytes. Because in NK cells and macrophages the FcγRIIIA gene is expressed [1] and FcγRIIIa shows a cell type-specific glycosylation [2], MKGR14 probably recognize the oligosaccharide portion.
Plasma sFcγRIII was shown to be derived mainly from neutrophils and to a lesser extent from NK cells [2,5,16], and the sFcγRIIIa is derived mainly from NK cells [2]. In our study, sFcγRIII concentrations were presented as the percentage of sFcγRIII compared with the amount of sFcγRIII in the pooled plasma. It is interesting to clarify the differences in the amounts of sFcγRIIIaMφ, sFcγRIIIaNK and sFcγRIIIb. From the recovery of purified sFcγRIIIaMφ, we calculated these difference. The amount of sFcγRIIIaMφ present was about half that of sFcγRIIIaNK, and that of sFcγRIIIa was about 50 times lower than that of sFcγRIIIb in pooled plasma from healthy NA(1 +, 2-) phenotyped donors.
Previously, we evaluated the interfering effects of IgG complex in RA samples on the measured levels of sFcγRIII. To detect the effect of IgG complexes in RA plasma samples, we mimicked RA samples by adding sFcγRIIIaMφ and the γ-globulin fraction prepared from RA patients’ serum to pooled normal plasma. The measured levels of sFcγRIIIaMφ, as well as the total sFcγRIII or sFcγRIIIa, decreased with increasing amounts of supplemented RA γ-globulin fraction (Fig. 4). High levels of IgG complex apparently decreased the sFcγRIII levels by about 10–15% in our assay.
Several reports [9,10] have described a reduced expression of FcγRIIIa on NK cells in RA patients. Macrophage FcγRIIIa is expressed at high levels only in the synovial intimal tissue and other tissues, such as the pericardium, that are involved in RA [11]. We showed that the increased sFcγRIIIa in RA patients is caused by activation of NK cells and/or macrophages, and the ratio of sFcγRIIIaNK to sFcγRIIIaMφ varied between RA patients. In RA patients, both the sFcγRIIIa and sFcγRIIIaMφ levels correlated with the Lansbury Index (Fig. 4, Table 3) and thus with the disease severity. However, there was a difference between the two levels; i.e. sFcγRIIIa levels, but not sFcγRIIIaMφ levels were increased directly proportional to C-reactive protein (Table 3) and thus to inflammation. sFcγRIIIaMφ correlated independently with the severity of RA. In conclusion, sFcγRIIIaMφ may serve as a new marker of the disease activity in RA.
Acknowledgments
We thank Dr Masja de Haas and Dr Federico Garrido for their generous gifts of antibodies and Mr Takashi Kizu, Mr Hideaki Nakatuji and Mr Hiroki Bandoh for their assistance with the genotyping assay. This work was supported partly by grants from the Ministry of Education, Grant-in-Aid for Scientific Research (C) (nos 07672505 and 10672192), from the Charitable Trust Clinical Pathology Research Foundation of Japan and from the TERUMO Life Science Foundation (no. 99–319).
REFERENCES
- 1.Ravetch JV, Perussia BV. Alternative membrane forms of FcγRIII (CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Haas M, Kleijer M, Minchinton RM, et al. Soluble FcγRIIIa is present in plasma and is derived from natural killer cells. J Immunol. 1994;152:900–7. [PubMed] [Google Scholar]
- 3.Harrison D, Phillips JH, Lanier LL. Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated FcγRIII (CD16-II) J Immunol. 1991;147:3459–65. [PubMed] [Google Scholar]
- 4.Levy PC, Utell MJ, Fleit HB, et al. Characterization of human alveolar macrophages Fc receptor III. A transmembrane glycoprotein that is shed under in vitro culture conditions. Am J Resp Cell Mol Biol. 1991;5:307–14. doi: 10.1165/ajrcmb/5.4.307. [DOI] [PubMed] [Google Scholar]
- 5.Huizinga TW, de Haas M, van Oers MH, et al. The plasma concentration of soluble FcγRIII is related to production of neutrophils. Br J Haematol. 1994;87:459–63. doi: 10.1111/j.1365-2141.1994.tb08298.x. [DOI] [PubMed] [Google Scholar]
- 6.Homburg CHE, de Haas M, von dem Borne AEGKr, et al. Human neutrophils lose their surface FcγRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–40. [PubMed] [Google Scholar]
- 7.Middelhoven PJ, van Buul JD, Hordijk PL, Roos D. Different proteolytic mechanisms involved in FcγRIIIb shedding from human neutrophils. Clin Exp Immunol. 2001;125:169–75. doi: 10.1046/j.1365-2249.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinckerhoff CE. Joint destruction in arthritis: metalloproteases in the spotlight. Arthritis Rheum. 1991;34:1073–5. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- 9.Hendrich C, Kuipers JG, Kolanus W, et al. Activation of CD16+ effector cells by rheumatoid factor complex: role of natural killer cells in rheumatoid arthritis. Arthritis Rheum. 1991;34:423–31. doi: 10.1002/art.1780340407. [DOI] [PubMed] [Google Scholar]
- 10.Tak PP, Kummer JA, Hack CE, et al. Granzyme-positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994;37:1735–43. doi: 10.1002/art.1780371205. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia A, Blades S, Cambridge G, Edwards JCW. Differential distribution of FcγRIIIA in normal human tissues and colocalisation with DAF and fibrillin-1. Immunology. 1998;94:56–63. doi: 10.1046/j.1365-2567.1998.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkham BW, Navarro FJ, Corkill MM, Panayi GS. In vivo analysis of disease modifying drug therapy activity in RA by sequential immunohistological analysis of synovial membrane interleukin 1β. J Rheumatol. 1994;21:1615–9. [PubMed] [Google Scholar]
- 13.Yanni G, Nabil M, Farahat MR, et al. Intramuscular gold decreases cytokine expression and macrophage numbers in the rheumatoid synovial membrane. Ann Rheum Dis. 1994;53:315–22. doi: 10.1136/ard.53.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MD, Kraan MC, Slavotinek J, et al. Treatment-induced remission in rheumatoid arthritis patients is characterized by a reduction in macrophage content of synovial biopsies. Rheumatol. 2001;40:367–74. doi: 10.1093/rheumatology/40.4.367. [DOI] [PubMed] [Google Scholar]
- 15.Sautès C, Teillaud C, Mazieres N, et al. Soluble Fcγ receptors (sFcγR). Detection in biological fluids; production of a murine recombinant sFcγR biologically active in vitro and in vivo. Immunobiology. 1992;185:207–21. doi: 10.1016/s0171-2985(11)80642-x. [DOI] [PubMed] [Google Scholar]
- 16.Galon J, Moldovan I, Galinha A, et al. Identification of the cleavage site of plasma soluble Fcγ receptor type III (CD16) Eur J Immunol. 1998;28:2101–7. doi: 10.1002/(SICI)1521-4141(199807)28:07<2101::AID-IMMU2101>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Koene HR, de Haas M, Kleijer M, et al. NA-phenotype-dependent differences in neutrophil FcγRIIIb expression cause differences in plasma levels of soluble FcγRIII. Br J Haematol. 1996;93:235–41. doi: 10.1046/j.1365-2141.1996.4971038.x. [DOI] [PubMed] [Google Scholar]
- 18.de Haas M, Kleijer M, von Roos D, von dem Borne AEGKr. Characterization of MAbs of the CD16 cluster and six new generation CD16 MAbs. In: Schlossmann SF, et al., editors. Leukocyte typing V. Oxford: Oxford University Press; 1995. pp. 811–7. [Google Scholar]
- 19.Masuda M, Nakamura S, Murakami T, et al. Association of tissue factor with a γ-chain homodimer of the IgE receptor type I in cultured human monocytes. Eur J Immunol. 1996;26:2529–32. doi: 10.1002/eji.1830261037. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Koene HR, Kleijer M, Roos D, et al. FcγRIIIB gene duplication. Evidence for presence and expression of three distinct FcγRIIIB genes in NA (1+, 2+) SH (+) individuals. Blood. 1998;91:673–9. [PubMed] [Google Scholar]
- 22.Furuya D, Yagihashi A, Yajima T, et al. An immuno-polymerase chain reaction assay for human interleukin-18. J Immunol Meth. 2000;238:173–80.4. doi: 10.1016/s0022-1759(00)00143-5. [DOI] [PubMed] [Google Scholar]