Abstract
Helicobacter pylori induces symptomatic chronic gastritis in a subpopulation of infected individuals. The mechanism(s) determining the development and severity of pathology leading to symptoms are not fully understood. In a mouse model of H. pylori infection we analysed the influence of immunoregulatory CD4+CD25+ T cells on H. pylori colonization and gastritis. Athymic C57BL/6 nu/nu mice were reconstituted with (a) lymph node (LN) cells (b) LN cells depleted of CD25+ T cells (CD25– LN) or (c) not reconstituted at all. Mice were then infected orally with 3 × 108H. pylori SS1 bacteria. At 2 and 6 weeks after the inoculation there was a significant (P < 0·001) reduction in H. pylori colonization in athymic mice transferred with CD25– LN cells compared to mice transferred with LN cells. Colonization was still reduced at 12 weeks after inoculation. Mice transferred with CD25– LN cells showed an earlier onset and increased severity of gastritis as compared to mice receiving LN cells. Splenic cells isolated from mice receiving CD25– LN cells produced the highest level of IFN-γ on stimulation with H. pylori antigens in vitro, had a higher H. pylori-specific DTH response and increased infiltration of CD4+ T cells and macrophages in the gastric mucosa. Athymic mice not transferred with T cells had persistent high H. pylori colonization and displayed a normal gastric epithelium without inflammatory cells. In conclusion, CD4+CD25+ cells reduce immunopathology in H. pylori infection, possibly by reducing the activation of IFN-γ producing CD4+ T cells, even at the expense of a higher H. pylori load in the gastric mucosa.
Keywords: CD25+ regulatory T cells, Helicobacter pylori, mouse model, gastritis
INTRODUCTION
Elimination of a peripheral CD4+ T cell subpopulation expressing the IL-2 receptor α-chain (IL-2Rα) (CD25) and subsequent transfer of remaining CD4+ T cells to nu/nu mice results in a wide spectrum of organ-specific autoimmune diseases, while reconstitution with CD4+CD25+ T cells prevents these diseases [1]. Further, removal of the thymus at day 3 after birth (d3Tx) results in autoimmune diseases in BALB/c mice which is prevented by reconstitution with spleen cells including CD25+ cells [1,2]. These CD4+CD25+ regulatory T cells (Treg) originate in the thymus indicating that the thymic selection mechanisms may not only delete self-reactive T cells but also give rise to Treg specific for self-antigens [1]. A similar subset of Treg has been identified in human peripheral blood and thymus [3]. In addition to the well established role in preventing autoimmune diseases, Treg play an important role in preventing inflammatory bowel disease, as well in the suppression of tumour immunity and induction of dominant transplantation tolerance [1]. However, we are not aware of studies testing the influence of CD4+CD25+ T cells in Helicobacter pylori infection.
H. pylori is a spiral Gram-negative bacterium, that colonizes the human stomach and causes chronic active gastritis, gastric atrophy and peptic ulcers [4]. Although half the world's population is infected with this bacterium only approximately 10–15% of those colonized develop disease [5]. It can be speculated that the activation of Treg in asymptomatic carriers keeps the pathology mild enough to avoid symptoms. Further, it has been suggested that H. pylori-induced gastritis could lead to an autoimmune process since H. pylori lipopolysaccharide (LPS) contain Lewis blood group antigens that are structurally similar to Lewis antigens expressed on gastric epithelial cells in mice, and some humans infected with H. pylori develop parietal cell specific anticanalicular and H/K ATPase-reactive autoantibodies [6]. To study the course of H. pylori infection after removal of Treg, we used a mouse model of H. pylori infection and addressed the following questions:
Are Treg involved in suppression of immune responses to infectious antigens?;
Does H. pylori induce an earlier onset of gastritis in the absence of Treg?;
Does H. pylori-induced gastritis lead to autoimmune gastritis?.
We show that in the absence of Treg, H. pylori colonization is reduced but at the cost of inducing an earlier onset and increased severity of gastritis. However, at least in C57BL/6 mice, the H. pylori-induced gastritis does not seem to induce bona fide autoimmunity.
MATERIALS AND METHODS
Animals
Female 6–8-week-old specific pathogen free C57BL/6 wild-type and female C57BL/6 nu/nu mice of similar age were purchased from M & B (Denmark). They were housed in microisolators at the Laboratory for Experimental Biomedicine, Göteborg University during the study. At the start of each experiment the nu/nu recipient mice were treated with a combination of omeprazole (Losec®, Astra, Sweden 0·4 mg/dose in physiological saline), Metronidazole (Dumex, Denmark, 1·35 mg/dose) and Amoxycillin (Scand Pharm, Sweden, 5 mg/dose) daily for 5 days to ensure the absence of Helicobacter infection in the stomach and small intestine. In separate experiments, we have determined that the C57BL/6 wild-type mice from this breeder are culture negative for H. pylori in the stomach, possess no signs of gastritis and have low serum IgG antibodies to H. pylori antigens (1/100 titre). All experiments were approved by the ethics committee of the National Board for Laboratory animals.
Preparation of lymphocytes for injection
Total lymph node (axillary, popliteal, mesenteric and mandibular) cells and lymph node cells depleted of CD25+ T cells were prepared from C57BL/6 wild-type mice using 7D4 anti-CD25 mAb and complement as previously described [2]. Flow cytometric analysis of cells stained with anti-CD4-FITC and anti-CD25-PE (PC61) showed that the percentage of CD4+CD25+ T cells was reduced from 16 to 20% to 1–2% of the CD4+ T cells after depletion, with only CD25dim cells remaining. Recipient nu/nu mice were divided into three groups and (a) reconstituted with total lymph node (LN) cells (b) reconstituted with LN cells depleted of CD25+ T cells (CD25– LN) or (c) not reconstituted. The numbers of cells were adjusted so that each mouse obtained 2 × 106 CD4+ cells and cells were injected intraperitoneally. For most experiments mice were infected with H. pylori after 3 weeks; however, some mice receiving CD25– LN cells were not infected and followed for 13 weeks.
Primary infection with H. pylori strain SS1
The mouse-adapted H. pylori strain SS1 [7] stored at −70°C was used as the stock culture for all experiments. Three weeks after the cell transfer all the groups of mice were orally infected with 3 × 108H. pylori SS1 bacteria as previously described [8]. Mice were killed 2, 6 or 12 weeks after infection and the number of bacteria colony forming units (cfu) in the stomach of each mouse was determined after culture on blood Skirrow agar plates as previously described [8].
Cellular immune responses to H. pylori antigen
Measurement of DTH reaction.
Mice were injected with 20 µg membrane preparation of H. pylori SS1 (MP SS1) in the right foot pad and PBS in the left foot pad 5 weeks after infection with H. pylori SS1. Swelling of the footpads was measured after 24 h using an Oditest caliper and the specific footpad swelling was determined for each animal as the difference between the thickness before and after injection of antigen in one foot or PBS in the other.
Cell ELISA for the quantification of IFNγ.
Measurement of cytokine production was performed using a modified version of a cell ELISA method as described [9]. Briefly, after erythrocyte lysis, pooled spleen cells from each group (n = 5) were plated on anti-IFNγ coated plates (4 µg/ml) at a concentration of 5 × 105 cells per well with or without MP SS1 antigen (20 µg/ml). CD4+ T cells from the mesentric lymph node (MLN) were enriched from 10 to 12% to 55–65% by negative selection as previously described [10]. The CD4+ cells were plated with or without antigen on the anti-IFNγ coated plates at a cell concentration of 2 × 105 cells/well together with 5 × 105 irradiated T cell depleted spleen cells. The concentration of the cytokine was determined by extrapolation from a standard curve obtained using recombinant IFNγ (DuoSet IFN-γ ELISA Development kit, R & D systems).
Immunohistochemical staining.
Five micrometre-thick frozen sections were fixed with acetone and stained using rat antimouse CD4 (H129) (Pharmingen), rat antimouse F4/80, and isotype control rat antimouse IgG2a (Serotec) antibodies as described [11]. Sections were evaluated using the Leica analysis software to calculate the percent area (of mucosa and submucosa) stained positive for F4/80 in 5 independent fields at 200× magnification (n = 8), while the number of CD4+ T cells was counted by visually identifying positive cells at 400× magnification which were exclusively located in the mucosa (n = 8).
Detection of H. pylori antibodies and parietal cell autoantibodies
Serum antibody titres to MP SS1 were determined by ELISA as previously described [12]. Antibody titres were expressed as the reciprocal sample dilution giving an absorbance of 0·4 above the background. Parietal cell antibodies were detected by immunoflorescence on cryostat sections of normal BALB/c stomach as described [10].
Histopathology
Formalin fixed strips of the entire long curvature of the stomach were cut and stained with haematoxylin and eosin. The slides were ‘blinded’ and examined by two independent investigators and the extent of gastritis was graded as described previously [10]. Briefly, the cellular infiltration and tissue damage was read as follows: Grade 0–1, normal gastric mucosa that contained few lymphocytes scattered throughout the submucosa; Grade 2, small aggregates containing three to four layers of cells in the mucosa or sparse infiltrates of cells in the submucosa covering ∼5% of the section; Grade 3, frequent and larger infiltrates extending into the mucosa; Grade 4, infiltrates spanning half to the entire width of the mucosa; Grade 5, partial or complete (grade 6) obliteration of parietal and chief cells with hyperplasia of mucous and epithelial cells.
Statistical analysis
Mann–Whitney test was used for comparisons between groups using Graphpad Prism® software.
RESULTS
Repopulation of T cells in the reconstituted mice
The LN of nude mice transferred with LN cells or CD25– LN cells showed equal repopulation containing 10 ± 3% and 9 ± 1% CD4+ cells, respectively, at all examined time points (2, 6 and 12 weeks) following infection (n = 15 mice). However, in the mice receiving LN cells 41 ± 9% of the CD4+ T cells coexpressed CD25, while in the group transferred with CD25– LN cells a lower percentage (28 ± 7%) of the CD4+ cells were CD25+. Since the inoculum in the latter group was depleted of CD25+ cells this still relatively high percentage of CD25+ cells must represent a de novo expression of CD25 by previously ‘naïve’ T cells transferred. This expression of CD25 is induced as a result of both homeostatic proliferation (since even before H. pylori infection, 3 weeks after cell transfer, these mice contained up to 20% CD25+ cells among CD4+ T cells; data not shown and [13]) and activation of H. pylori-induced effector T cells, analogous to the induction of effector cells in the autoimmune gastritis model [14]. In the mice receiving LN cells the absolute percentages of CD25+ T cells was higher due to the additional presence of Treg (P < 0·001).
Multiplication of H. pylori is suppressed in mice transferred with CD25− LN cells
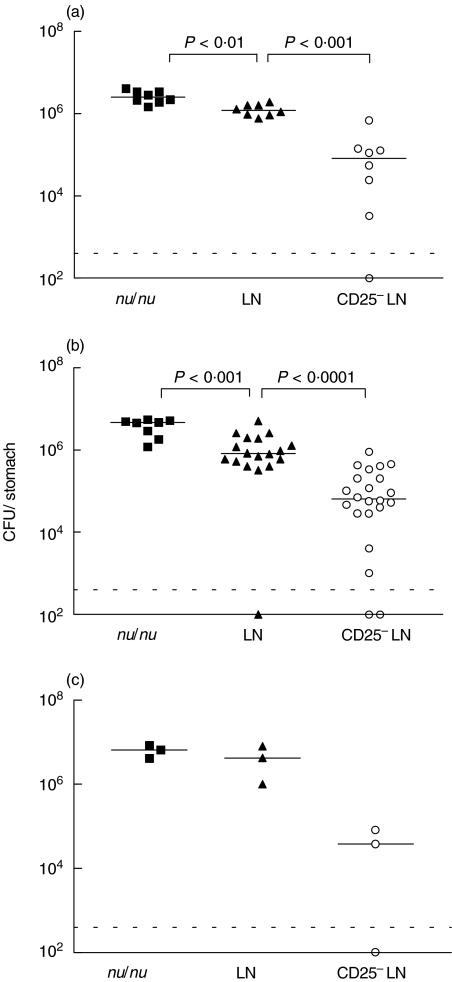
Since previous experiments in C57BL/6 wild-type mice showed maximal and consistent gastric colonization 2–8 weeks after infection with ∼108 CFU of H. pylori[15], we sacrificed the animals 2, 6 and 12 weeks after infection. When analysed 2 weeks after infection, nu/nu mice reconstituted with LN cells had a 2-fold reduction of H. pylori colonization compared to untreated nu/nu mice. Interestingly, a further 9-fold decrease in the bacterial load was seen in mice receiving CD25– LN cells (Fig. 1a). Those early established levels of colonization remained relatively constant over the entire observation period (Fig. 1b,c).
Fig. 1.
Colonization with H. pylori is reduced in mice depleted of Treg. Results of individual mice not transferred with T cells (▪) or transferred LN cells (▴) or CD25– LN cells (○) are shown. Mice were sacrificed (a) 2 weeks (b) 6 weeks or (c) 12 weeks after infection. Values are expressed as colony forming units with the dotted line indicating the detection limit and horizontal lines the geometric mean for each group. The data at 2 weeks are pooled from two and at 6 weeks from three independent experiments.
H.pylori-infected mice transferred with CD25−LN cells develop severe gastritis
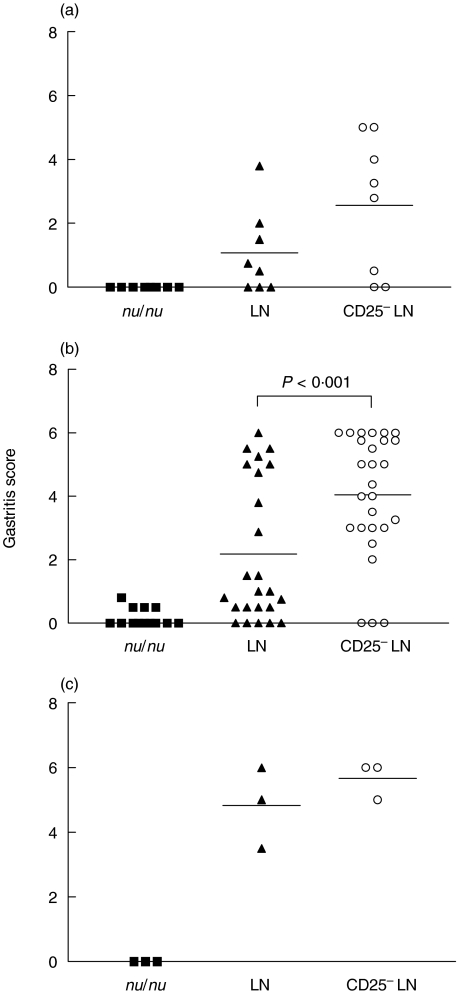
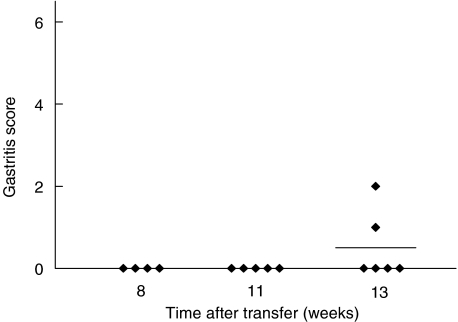
Histological examination of the stomachs revealed an early onset of gastritis, evident already at 2 weeks after infection, in the mice transferred with CD25– LN cells, i.e. lacking Treg (Fig. 2a). The degree of inflammation, parietal cell and chief cell destruction as well as concomitant hyperplasia of the mucosa was more pronounced in the mice depleted of Treg cells than in mice transferred with total LN cells, resulting in a further increase in gastritis score at 6 weeks after colonization (Figs 2b and 3c). While gastritis was more severe in the mice transferred CD25– LN cells, than in the mice transferred with total LN cells at all time points analysed, mice that received total LN cells also developed gastritis, albeit more slowly and with lesser severity (Figs 2a,b,c and 3b). The development of less severe gastritis was not related to insufficient colonization as all mice were colonized to the same levels. Consistent with the role of CD4+ T cells in Helicobacter clearance and gastritis development [16], athymic mice infected with H. pylori SS1 did not develop gastric inflammation (Figs 2a,b,c and 3a). Mice transferred with CD25– LN cells did not develop gastritis in the absence of H. pylori infection. Firstly, stomach histology showed no signs of inflammation at 8–11 weeks after cell transfer and only 2/6 mice developed mild inflammation at 13 weeks after cell transfer (Fig. 4). Secondly, the sera of these mice did not contain anti parietal cell antibodies as measured by indirect immunofluorescence (data not shown).
Fig. 2.
The severity of gastritis is enhanced in the absence of Treg. Gastritis scores of individual mice not transferred with T cells (▪) or transferred LN cells (▴) or CD25– LN cells (○) are shown. Mice were sacrificed (a) 2 weeks (b) 6 weeks or (c) 12 weeks after infection. The data at 2 weeks are pooled from two and at 6 weeks from four independent experiments, with horizontal lines indicating the mean of each group.
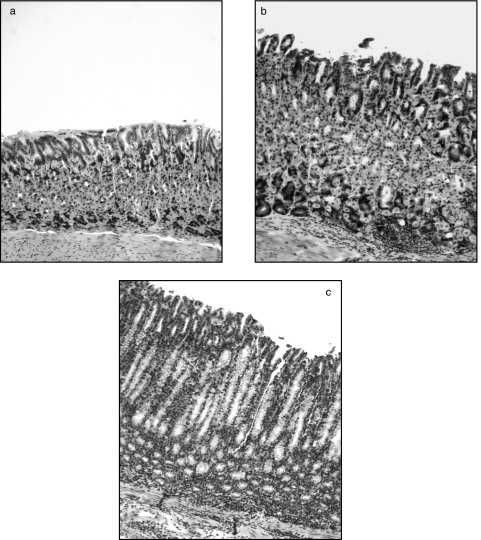
Fig. 3.
Atrophy and inflammation is seen in mice transferred CD25– LN cells after infection with H. pylori. (a) Athymic mouse infected with H. pylori but not transferred with T cells showing no signs of inflammation (gastritis score grade 0–0·5). (b) Athymic mouse transferred with LN cells and infected with H. pylori with moderate inflammation (gastritis score grade 1–2). (c) Athymic mouse transferred with CD25– LN cells and infected with H. pylori with severe inflammation, destruction of parietal and chief cells and hyperplasia of the mucosa (gastritis score grade 5–6). All photographs are at 1 : 100 magnification.
Fig. 4.
Transfer of CD25– LN cells to nu/nu mice in the absence of H. pylori infection does not result in autoimmune gastritis. Mice were sacrificed 8 weeks, 11 weeks or 13 weeks after transfer of T cells.
Enhanced CD4+ T cell activation in mice transferred with CD25– LN cells
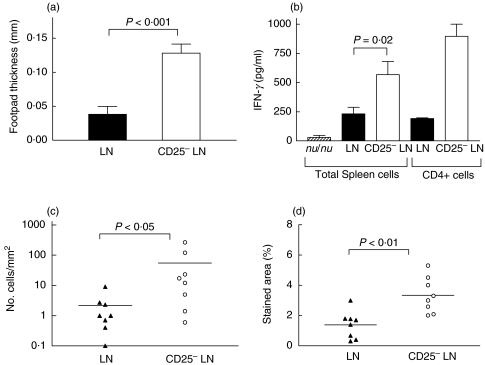
Since the development of postinfection gastritis and the suppression of Helicobacter expansion has been shown to be dependent on T cells [16], we speculated that the mice that received CD25– LN cells had lesser colonization but more severe gastritis because of enhanced activation of CD4+ (CD25–) T cells specific for H. pylori antigens in the absence of Treg. This seems to be indeed the case as judged from 3 different assays that depend on CD4+ T cell activation. First, a significantly higher DTH reaction after footpad injection of H. pylori MP SS1 was seen in mice transferred with CD25– LN cells as compared to mice transferred with total LN cells 6 weeks after infection (P < 0·001; Fig. 5a).
Fig. 5.
Cellular immune responses against H. pylori antigen are enhanced in the absence of Treg: (a) DTH reaction against H. pylori MP SS1 in mice transferred with LN cells (closed bar) or CD25– LN cells (open bar) at 5 weeks after infection. The increase in footpad swelling in PBS injected control mice was between 0 and 0·04 mm in mice transferred LN cells and 0–0·05 mm in mice transferred CD25– LN. (b) IFN-γ production of spleen cells or purified CD4+ T cells in response to H. pylori MP SS1 in mice transferred with LN cells (▪) or CD25– LN cells (□) 6 weeks after infection. The data shows the mean IFN-γ production and SD of 3 independent experiments using spleen cells and of 2 experiments using purified CD4+ T cells. (c,d) Immunohistochemical staining of the gastric mucosa of mice transferred LN cells (▴) or CD25– LN (○). Horizontal lines represent geometric mean (c) or mean (d). (c) Numbers of CD4+ T cells in the mucosa. (d) Percentage stained area of the mucosa and submucosa for the macrophage and dendritic cell marker F4/80.
Second, we detected an enhanced in vitro production of IFN-γ in spleen cells from mice repopulated with CD25– LN cells (P = 0·02; Fig. 5b). The culture of enriched CD4+ T cells from MLN confirmed that IFN-γ production was mainly CD4+ T cell derived and it was again enhanced in mice receiving CD25– LN cells (Fig. 5b). Third, we detected an increased number of CD4+ cells in the gastric mucosa of the mice receiving CD25– LN cells at 2 weeks after infection (P < 0·05; Fig. 5c). This increased T cell migration and activation also led to increased numbers of macrophages and dendritic cells (as detected by staining with F4/80 antibody) in the gastric mucosa (P < 0·01; Fig. 5d).
In contrast, the enhanced T cell activation in the mice receiving CD25– LN cells was not followed by any increase in antibody titres against H. pylori SS1 antigen. Serum antibody titres (IgG and IgM) in nu/nu mice were 326 ± 43, and in mice receiving LN cells 2229 ± 1196, while in mice receiving CD25– LN cells the titres were 487 ± 401 six weeks after infection (P < 0·05; LN versus CD25–LN). Specific IgG1 and IgG2a were similarly reduced in the latter mice. The reason for the lower antibody responses in CD25– LN transferred mice could be a Th1 promoting response as evidenced by an enhanced DTH reaction and IFN-γ production. It also corroborates the fact that there was no correlation between antibody titres and gastritis score or H. pylori colonization in individual mice in our experiments (data not shown).
DISCUSSION
This study shows that regulatory T cells reduce the immune responses to H. pylori. Depletion of CD4+CD25+ T cells (Treg) led to an enhanced H. pylori-specific CD4+ T cell activation with a consequent reduction in bacterial load in the stomach. However, this increased T cell activation resulted in more severe gastritis. These results are in accordance to a recently reported study showing that lethal lung pathology after transfer of CD4+ cells into Pneumocystis carinii-infected Rag2–/– mice was inhibited by CD4+CD25+ cells [17] and highlights the fact that Treg can not only prevent autoimmmune diseases but also dampen immune responses to infectious agents and thus reduce pathological tissue destruction.
In a study by Eaton & Mefford [18] SCID mice that received spleen cells showed a reduction in H. pylori load compared to nonreconstituted SCID mice. In this as well as in our study lowering of the bacterial load was associated with increased gastritis. We can further demonstrate that depletion of CD25+ cells leads to a significantly lower level of colonization (approx 9-fold) and H. pylori load further decreases gradually over the 12 week study period. Further long-term observation is needed to determine if mice lacking Treg can eradicate the bacteria and recover the mucosal architecture faster than mice receiving unfractionated LN cells or purified CD4+ T cells. Conversely, gastritis development was accelerated in nu/nu mice receiving CD25– LN cells compared to mice receiving total LN cells at 2 weeks, such that only in the former group the majority of the mice had severe gastritis with tissue destruction, while only a few mice in the latter group (receiving total LN cells) showed mild inflammatory cell infiltration into the stomach mucosa. Six weeks after infection 88% of the mice receiving CD25– LN cells had severe gastritis in comparison to only 33% of the mice receiving total LN cells. In an another study, Eaton et al. [19] have shown that both total CD4+ T cells and CD4+CD45RBhi cells were equally effective in reducing H. pylori SS1 colonization in SCID mice and that the gastritis was also similar in both groups. Since the CD45RBhi population lacks CD25+ T cells [20] one might have expected a reduced colonization in the CD4+CD45RBhi group compared to the CD4+ T cell-reconstituted group, similar to our results. The lack of this effect could be due to the administration of T cells after established H. pylori colonization and the use of lower T cell numbers which would reduce the likelihood for activation of H. pylori-specific Treg. In addition, it should also be noted that our experiments differed from the earlier study by Eaton et al. [19] in that we used nu/nu mice as recipients and LN cells as source for T cells while Eaton used SCID recipients and splenocytes as a T cell source. Most importantly though, in line with our results that CD25+ cells dampen the T cell activation, Eaton et al. [19] show that CD4+CD45RBlow cells (that are enriched in CD25+ cells [20]) do not reduce bacterial load and also do not induce gastritis.
Since the depletion of Treg leads to autoimmune gastritis in BALB/c mice, it could be argued that the increase in gastritis in the mice receiving CD25– LN cells could be due to an autoimmune process in addition to inflammation induced by H. pylori. We do not believe this to be the case. Firstly, C57BL/6 nu/nu mice did not develop any signs of gastritis during the first 11 weeks after transfer of CD25– LN cells in the absence of H. pylori SS1 infection, confirming that they are relatively resistant to autoimmune gastritis as determined previously after neonatal thymectomy [21]. Secondly, we could not detect parietal cell autoantibodies in any of the experimental groups and preliminary experiments did not reveal T cell proliferation against H/K ATP’ase (the dominant autoantigen in autoimmune gastritis) in vitro (data not shown). In addition, we have found that the gastric mucosa of C57BL/6 wild-type mice that had developed severe gastritis after colonization and immunization with H. pylori recovered to a normal histological appearance with intact parietal and chief cells after eradication of the infection with antibiotics [8]. Further studies, including other mouse strains, need to be performed to verify that H. pylori induced gastritis does not end up in autoimmune gastritis.
Although we injected total LN cells and not purified CD4+ T cells we conclude that the effects of reduced colonization as well as induction of gastritis are mediated by CD4+ T cells and that the CD25+ depletion specifically affected CD4+ T cell activation. Firstly, several studies have shown that CD4+ (but not CD8+ or B cells) are needed for the immune response against H. pylori and also to induce gastritis [10, 18, 22, 23]. Secondly, we have shown that the DTH reaction was enhanced only in the group receiving CD25 depleted cells, indicating enhanced CD4+ T cell activation. This correlated well with elevated IFN-γ production in vitro, by spleen cells as well as by purified CD4+ T cells in response to H. pylori membrane antigens. Finally, the number of CD4+ T cells that had infiltrated the gastric mucosa early after infection was significantly increased in the group lacking Treg. Concomitantly, macrophage and dendritic cell infiltration that is dependant on the activation of CD4+ T cells (data not shown), was significantly increased in the absence of down regulation by Treg.
A recent report has shown human CD25+ Treg specific for self (heat shock protein) and foreign antigens (cow milk antigen, tetanus toxoid and PPD) [24] and organ specific activation of diabetes preventing CD4+CD25+ cells has been reported in mice [25]. In addition, T regulatory 1 (Tr1) cells possessing regulatory function similar but not identical to CD25+ Treg, have been described for specific antigens [26]. Such Tr1 cells have recently been shown to be activated in vivo after Bordetella pertussis infection [27]. Therefore, we speculate that in our model CD4+CD25+ cells were activated by H. pylori antigen. These H. pylori antigen-specific Treg may then suppress not only H. pylori specific but also gastric self antigen specific effector T cells in the gastric mucosa and the gastric lymph nodes through bystander suppression [28]. As antigen-specific Treg can be induced in adult mice [29] we will test in further experiments if activation of H. pylori-specific Treg in C57BL/6 wild-type mice (that develop pathology slower than mice in our transfer model) can further reduce or even prevent H. pylori-induced pathology alone or in combination with IL-4 and IL-10 therapy.
In summary, we conclude that the activation of Treg in response to H. pylori reduces pathology and is therefore beneficial to the host. In this respect it is interesting to note that human asymptomatic H. pylori carriers have a lower H. pylori-specific T cell response than uninfected volunteers and this may be due to suppression mediated by CD4+CD25+ T cells [30]. Thus, we speculate that asymptomatic H. pylori carriers may have been infected with H. pylori during early childhood, which leads to efficient activation of Treg that dampens H. pylori-specific T cell responses and therefore protects the mucosa. Activation of Treg may therefore be of an advantage not only in autoimmune diseases and transplantation tolerance but also in chronic infectious diseases associated with tissue damaging inflammation such as H. pylori infection.
Acknowledgments
We thank the Swedish Medical Research Council for project support (projects 16x-3382, 16x-9084, 71x-13487–02B). We wish to thank Andrej Tarkowski, Esbjörn Telemo and Samuel Lundin for critical reading of the manuscript and helpful suggestions.
REFERENCES
- 1.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells. their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 2.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 3.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–33. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 5.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–40. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 6.Appelmelk BJ, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls CM. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296–9. doi: 10.1016/s0167-5699(98)01281-x. [DOI] [PubMed] [Google Scholar]
- 7.Sutton P, Wilson J, Lee A. Further development of the Helicobacter pylori mouse vaccination model. Vaccine. 2000;18:2677–85. doi: 10.1016/s0264-410x(00)00052-9. [DOI] [PubMed] [Google Scholar]
- 8.Raghavan S, Svennerholm AM, Holmgren J. Effects of Oral Vaccination and Immunomodulation by Cholera Toxin on Experimental Helicobacter pylori Infection, Reinfection, and Gastritis. Infect Immun. 2002;70:4621–7. doi: 10.1128/IAI.70.8.4621-4627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beech JT, Bainbridge T, Thompson SJ. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Meth. 1997;205:163–8. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 10.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J Autoimmun. 2001;16:115–23. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 11.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–44. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson EL, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–20. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 15.Raghavan S, Hjulström M, Svennerholm AM, Holmgren J. Protection against Experimental Helicobacter pylori Infection after Immunization with Inactivated H. pylori whole-cell vaccines. Infect Immun. 2002;70:6383–8. doi: 10.1128/IAI.70.11.6383-6388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–7. [PubMed] [Google Scholar]
- 17.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Eaton KA, Mefford ME. Cure of Helicobacter pylori infection and resolution of gastritis by adoptive transfer of splenocytes in mice. Infect Immun. 2001;69:1025–31. doi: 10.1128/IAI.69.2.1025-1031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–61. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 20.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 22.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect Immun. 1999;67:337–41. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermak TH, Giannasca PJ, Nichols R, et al. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–88. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taams SL, Vukmanovic-stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4+CD25+ Treg cells. highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–91. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 26.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 27.McGuirk P, McCann C, Mills KH. Pathogen-specific T Regulatory 1 Cells Induced in the Respiratory Tract by a Bacterial Molecule that Stimulates Interleukin 10 Production by Dendritic Cells: a Novel Strategy for Evasion of Protective T Helper Type 1 Responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 29.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–95. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren A, Enarsson K, Suri-Payer E, Svennerholm A-M, Lundin BS. Helicobacter pylori-specific CD4+CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–62. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]