Abstract
It has been reported that bacterial superantigens induce interleukin (IL)-12 dependent expression of the cutaneous lymphocyte associated antigen (CLA) and that this may be relevant to the association between certain skin diseases and infections including psoriasis and streptococcal tonsillitis. We have confirmed that the streptococcal pyrogenic superantigen C (SpeC) increases CLA expression by both CD4+ and CD8+ T cells when PBMCs are incubated in medium enriched with fetal calf serum (FCS). However, such an increase could not be induced in medium enriched with human serum (HS) even when recombinant IL-12 was added to the PBMCs cultures. Strikingly, CD4+ T cells incubated with SpeC in HS showed a marked reduction in CLA expression, which was not due to apoptosis. In contrast, SpeC did induce T cell proliferation and expression of CD25, CD54 and CD103 in the presence of HS indicating that the absence of SpeC induced CLA expression in HS was not due to SpeC inhibitors. Although addition of low amounts of lipopolysaccharide endotoxin (LPS) caused a highly significant increase in CLA expression in the absence of SpeC in cultures enriched with HS, a combination of LPS and SpeC did not increase CLA expression beyond that induced by LPS alone. The superantigen-induced CLA expression in FCS was partially inhibited by anti-IL-12 but not by anti-IL-18 or antibodies to transforming growth factor (TGF)-β. It is concluded that IL-12 alone can not increase CLA expression but requires the help of other factor(s) present in FCS but not in HS. Although LPS can induce CLA expression it does not seem to be the factor that interacts with IL-12 to induce superantigen-mediated CLA expression in cultures enriched with FCS.
Keywords: superantigen, CLA, induction, interleukin-12
INTRODUCTION
Streptococcal pyrogenic exotoxins have been shown to act as superantigens that activate T lymphocytes expressing certain T cell receptor Vβ regions [1]. These may include potentially autoreactive T cells and superantigens have therefore been implicated in the aetiology of autoimmune diseases [2]. Cutaneous lymphocyte associated antigen (CLA) is a surface glycoprotein epitope that binds to E-selectin on vascular endothelium in skin [3] and is expressed by a subpopulation of memory-type T cells. Over 80% of T cells that infiltrate the skin express CLA while it is normally detected on less than 20% of peripheral blood T lymphocytes suggesting that CLA may play a major role in the homing of T cells to the skin [4–6]. Bacterial superantigens have been reported to induce IL-12 mediated expression of a CLA by lymphocytes and it has been argued that this may contribute to the development of T cell mediated skin diseases including psoriasis [7]. In contrast, superantigen-mediated induction of the mucosal integrin CD103 (αEβ7) has to our knowledge not been reported.
IL-12 is a potent inflammatory cytokine mainly produced by accessory cells and has been shown to be a powerful inducer of IFN-γ production and Th1 responses [8]. It is also the only known physiological inducer of CLA expression [7,9]. The intercellular adhesion molecule I (ICAM-1, CD54) is a ligand for the leucocyte function associated (LFA) integrin and its importance for binding of leucocytes to vascular endothelium and other cells is well recognized [10]. However, ICAM-1 can also be expressed by B- and T lymphocytes [6,11].
Using medium supplemented with human serum (HS) we have not been able to induce CLA expression by human T cells with streptococcal superantigens. As previously published studies of such expression have all been performed in medium enriched with fetal calf serum (FCS) [7,9,12] we decided to compare the effects of superantigens on the induction of CLA by T cells incubated in medium supplemented either with FCS or HS. Our findings indicate that IL-12 requires at least one cofactor for inducing CLA expression by human T cells and that this factor is unlikely to be endotoxin.
MATERIALS AND METHODS
Study population
Healthy individuals (n = 33) with no history of skin disorders or immunological diseases were included in the study. The study was approved by the Landspitali Bioethics Committee.
Reagents
Monoclonal antibodies to the following surface antigens that were fluorescein isothiocyanate (FITC), phycoerythrin (PE) and peridinin chlorophyll protein (PerCP) conjugated were used: CD4-PerCP (SK3), CD8-PerCP (SK1), CD25-PE (2A3), CD54-PE (LB-2), CD103-PE (Ber-ACT8), and CLA-FITC (HECA-452). Controls used were FITC or PE-conjugated mouse IgG1/IgG2 or rat IgM (Becton Dickinson, Heidelberg, Germany). Annexin V-FITC and propidium iodide were used for determining apoptosis (R & D Systems Europe Ltd, Oxon, UK).
Recombinant IL-12 and neutralizing anti-IL-12 (MAB219), anti-IL-18 (MAB318) and anti-TGF-β1,2,3 (MAB1845) antibodies were purchased from R & D. LPS was purchased from Sigma (Sigma-Aldrich, Vallensbæk Strand, Denmark).
Isolation and stimulation of peripheral mononuclear blood lymphocytes
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by density gradient. The cells were washed twice and resuspended in RPMI 1640 (Gibco BRL, Life Technologies, Paisley, UK) supplemented with 2 mM glutamine (Gibco), 100 U/ml penicillin/100 µg streptomycin (Gibco) with either 10% heat-inactivated human AB serum (HS) or 10% heat-inactivated fetal calf serum (FCS/Gibco). The FCS contained less than 10 units/ml of endotoxin. Streptococcus pyrogenic exotoxin C (SpeC) was purchased from Toxin Technologies (Saratoga, California, USA). PBMCs (2 × 105/well) were cultured in u-bottomed 96-well microtitre plates (Nunc, Life Technologies) with superantigen SpeC (100 ng/ml) or LPS (5, 10, 20 ng/ml) for 4 days. This concentration of the SpeC and the incubation time had previously been shown to give an optimal induction of CLA while a marked apoptosis is observed when the incubation time is extended beyond 5 days (unpublished results). In some experiments the PBMCs were incubated with different concentrations (0·2–5 ng/ml) of recombinant IL-12 or with different concentrations of neutralizing anti-IL-12, anti-IL-18 or anti-TGF-β1,2,3 antibodies. Proliferation was determined by pulsing SpeC stimulated cultures with tritiated thymidine (1 µCi/well; Amersham International, Aylesbury, UK) for 16 h before the cells were harvested after 4 days. The thymidine incorporation was measured by liquid scintillation spectroscopy,
Triple immunofluorescence staining and flow cytometry
On day 0 and after 2 and 4 days of incubation the lymphocytes were triple-stained with the FITC-, PE- and PerCP conjugated monoclonal antibodies for 30 min on ice. The cells were then washed twice in PBS before fixation in 0·5% formalin. Stained lymphocytes were immediately submitted to flow cytometric analysis using a FACScan (Becton Dickinson). The analysis on day 0 (d0) was performed using a light scatter including only small lymphocytes, whereas activated cultured populations were analysed with the light scatter set on both small lymphocytes and large T cell population. Nonspecific binding was never more than 1%. Cells expressing low amounts of CD4+ and CD8+ T cells were excluded to avoid small monocytes. Apoptosis was determined by flow cytometry analysis after staining with Annexin V and propidium iodide.
ELISA for detection of SpeC antibodies
To determine whether HS contained a significant amount of antibodies against SpeC, microwell plates were coated with different concentrations of SpeC (1–5 µg/ml), incubated for 2 h with serial dilutions of the HS or FCS, followed by 2 h with alkaline-phosphatase conjugated anti-human IgG (Sigma) and finally developed with p-Nitrophenyl Phosphate Disodium (Sigma) 1 mg/ml in diethanolamine-HCl buffer (pH = 9·8). Optical density was read at 405 nm.
Statistical analysis
Statistical significance of differences was determined by a paired t-test, as all the data had a normal distribution. P-values below 0·05 were considered to be statistically significant
RESULTS
Increased CLA expression by T cells was observed after superantigen stimulation in FCS but not in HS
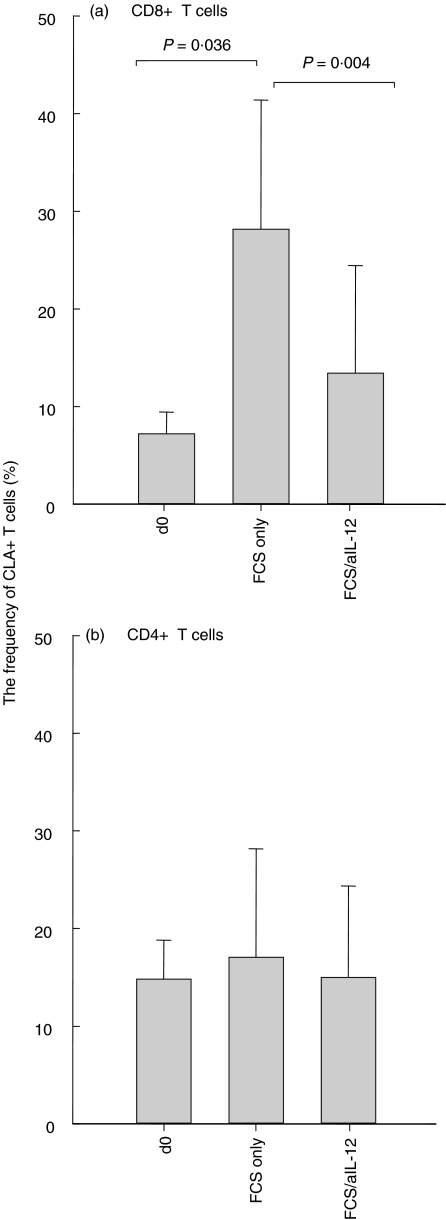
When PBMCs from 10 individuals were incubated in 10% FCS in the absence of superantigen, a significant increase in the frequency of CLA + CD8+ T cells was observed within 2 days and this increase could largely be inhibited by IL-12 antibodies (Fig. 1a). However, CLA expression by CD4+ T cells did not increase (Fig. 1b) and incubation in HS for up to 4 days did not significantly influence the CLA expression (Fig. 2).
Fig. 1.
PBMCs from 10 individuals were incubated for 2 days in medium containing 10% FCS but no superantigen. (a) A significant increase was observed in CLA expression by the CD8+ T cells and this increase was largely inhibited with antibodies against IL-12. (b) FCS did not influence CLA expression of CD4+ T cells.
Fig. 2.
(a) PBMCs from 33 individuals were cultured for 4 days in HS or FCS with or without SpeC. An increase in CLA expression was only observed in cultures enriched with FCS. Note that CD8+ T cells showed a significant increase in CLA expression after 4 days incubation in FCS without superantigen. SpeC stimulation in HS caused a marked decrease in CLA expression by CD4+ T cells while no change was observed in CD8+ T cells. (b) FACS histograms showing changes in CLA expression by T cells from 8 representative individuals, incubated with and without SpeC in the presence of HS or FCS. The X-axis shows the intensity of CLA expression and Y-axis the T cell counts.
When PBMCs from 33 healthy individuals were incubated with SpeC for 4 days in medium containing 10% FCS, a highly significant increase was observed in CLA expression by both CD4+ and CD8+ T cells (Fig. 2a). However, no such increases were observed for T cells that were incubated in HS. On the contrary there was a striking reduction in the frequency of CLA + CD4+ T cells but the frequency of CLA + CD8+ T cells remained unchanged (Fig. 2a). The difference in CLA expression by T cells cultured in FCS or HS was not due to higher rate of apoptosis in the HS nor to SpeC antibodies, as determined by a sensitive ELISA system (data not shown).
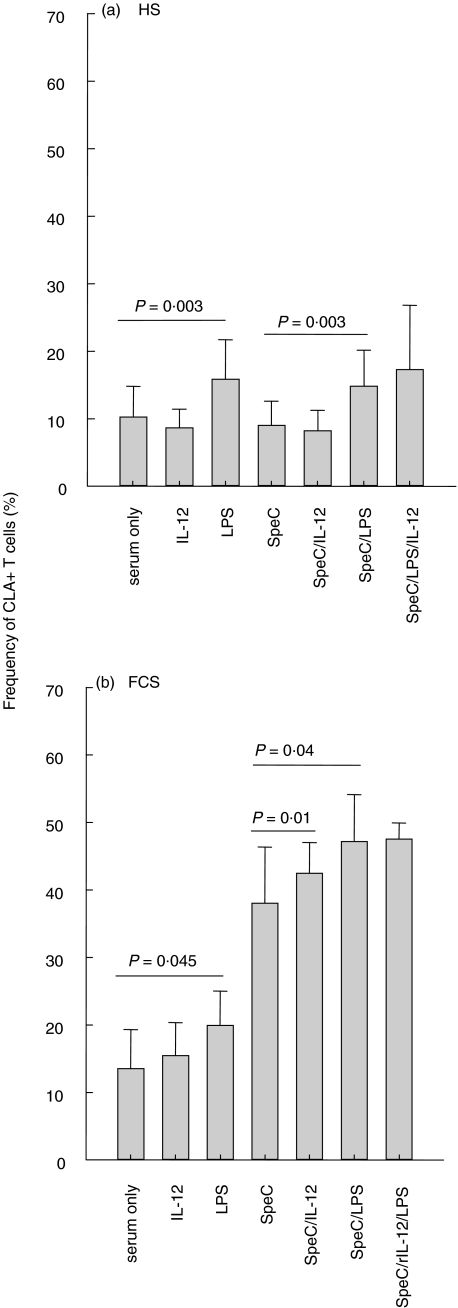
Addition of LPS but not IL-12 increased CLA expression by the T cells in the absence of SpeC
Addition of IL-12 (0·2, 2·0 or 5·0 ng/ml) to PBMCs from 12 individuals, cultured for 4 days in medium enriched with HS, did not increase CLA expression by the T cells regardless of the IL-12 concentration (Fig. 3a) and this applied both to CD4+ and CD8+ T cells (data not shown). However, addition of LPS (10 ng/ml) caused about 50% increase in CLA expression (P = 0·003) but no further increase was observed when PBMCs in HS were exposed to a combination of SpeC and IL-12, SpeC and LPS or to SpeC, IL-12 and LPS (Fig. 3a). Furthermore, a combination of IL-12 and LPS did not induce more CLA expression than LPS alone neither in HS nor FCS (data not shown).
Fig. 3.
PBMSc from 12 individuals were incubated for 4 days with various components. (a) No increase in CLA expression was detected when up to 5 ng/ml of IL-12 was added to PBMC cultured in HS while addition of LPS caused about 50% increase in CLA expression by the T cells. No further increase was observed when the T cells were exposed to a combination of LPS and SpeC, nor to a combination of IL-12, SpeC and LPS. (b) LPS caused only a borderline increase in CLA expression by T cells cultured in the presence of FCS with or without SpeC. Addition of IL-12 did not increase CLA expression above that induced by FCS alone while the addition of LPS or a combination of SpeC and LPS or SpeC and IL-12 induced a moderate increase in CLA expression.
Addition of IL-12 did not augment the spontaneous increase in CLA expression that was observed in cultures containing FCS without superantigen and LPS induced only a borderline increase in the absence of superantigen (Fig. 3b). However, addition of LPS or IL-12 to SpeC stimulated cultures induced a modest increase in CLA expression compared to cultures that were only incubated with SpeC but no further increase in CLA expression was observed in cultures incubated with a combination of LPS, SpeC and IL-12 (Fig. 3b).
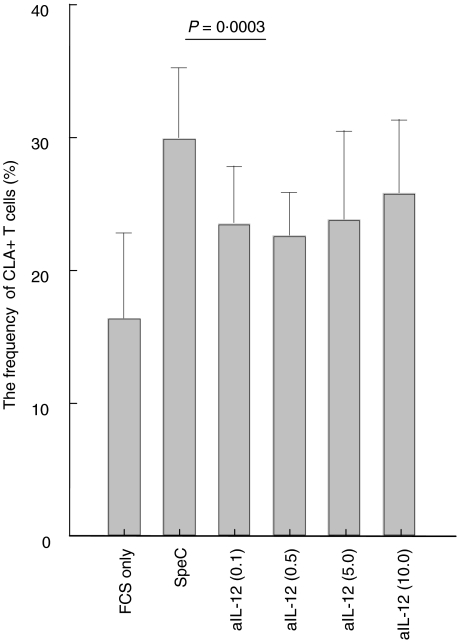
The SpeC induced increase in CLA expression by T cells exposed to FCS was only partially inhibited by anti-IL-12 even at the highest concentration (Fig. 4) while neutralizing monoclonal antibodies to IL-18 or TGF-β did not have any effect (data not shown).
Fig. 4.
SpeC induced CLA expression was only partially inhibited by neutralizing IL-12 antibodies. FCS exposed PBMCs were stimulated with SpeC and incubated with different concentrations of anti-IL-12 antibodies (µg/ml in brackets).
SpeC-induced lymphocyte proliferation and expression of CD25, CD54 and CD103 by T cells cultured in HS-enriched medium
Preliminary experiments showed that SpeC could, in addition to lymphocyte proliferation, also induce the expression of CD25, CD54 and CD103 by T cells cultured in medium enriched with FCS. In order to assess the possibility that human serum might contain inhibitors to SpeC, including antibodies, PBMCs from 15 individuals were incubated with and without SpeC in medium containing HS or FCS. As shown in Fig. 5, SpeC-induced lymphocyte proliferation and expression of CD54 was very similar in cultures enriched with HS or FCS. A highly significant SpeC-induced increase was also observed in the expression of CD25 and CD103 by T cells cultured in medium containing HS (P < 0·001), although these responses were much stronger in cultures containing FCS (Fig. 5). The induction of CD103 could be inhibited by anti-TGF-β antibodies (data not shown).
Fig. 5.
There was no difference in SpeC-induced proliferation or expression of CD54 by T cells after 4 day incubation in HS or FCS. The differences between the expression of CD25 and CD103 in unstimulated cultures versus SpeC-stimulated HS cultures were highly significant (P < 0·001), although the SpeC-induced stimulation was much stronger in FCS for both these parameters. The columns represent counts per minute (cpm) or percentages of T cells expressing each marker after subtraction of the values observed for unstimulated cultures.
DISCUSSION
Previous reports have shown that bacterial superantigens can be potent inducers of CLA expression by human T cells cultured in medium enriched by FCS, and it was concluded that this was mainly due to superantigen mediated production of IL-12 by macrophages [7]. Increased expression of CLA has also been observed when T cells are stimulated with antibodies including anti-CD3, in the presence of FCS and exogenous IL-12 [9,13,14].
Sofar IL-12 is the only physiological component known to increase CLA expression, but the mechanism of this activity remains to be elucidated. Our findings indicate that superantigen-induced up-regulation of CLA does not only involve the induction of IL-12 production as no increase in CLA expression is observed when PBMCs are cultured with superantigen in medium containing HS, even when large amounts of IL-12 are added. Furthermore, SpeC was equally active in stimulating T cell proliferation and the expression of CD54 regardless of whether the PBMCs are incubated in medium enriched with HS or FCS. The failure of superantigen to induce CLA in the presence of HS is therefore unlikely to be due to insufficient IL-12 production or SpeC inhibitors, including antibodies. Our findings therefore indicate that IL-12 alone can not induce CLA expression but requires some other factor(s) that are present in FCS but not HS. However, such cofactor(s) are not essential for superantigen induction of T cell proliferation nor expression of CD25, CD54 or CD103, although the expression of CD25 and CD103 may to some extend require cofactor(s) present in FCS (Fig. 5).
IL-12 is a universal and nontissue specific cytokine that is not only produced by macrophages but also by a variety of other cells [15–17]. Therefore, if IL-12 alone could increase CLA expression by leucocytes, inflammatory processes in any tissue of the body should give rise to increased numbers of leucocytes expressing this adhesion molecule that is selective for the skin, a scenario which conflicts with some recent observations indicating that inflammatory processes tend to induce tissue-specific homing receptors on memory lymphocytes [5,18–18].
Addition of low concentrations of LPS did induce a significant increase in the expression of CLA by T cells cultured in medium enriched by HS, and this has not been reported before. It is unlikely however, that LPS contamination of FCS explains the striking difference between the HS and FCS in promoting superantigen induction of CLA as the addition of both IL-12 and LPS to HS did not enhance CLA expression beyond that induced by LPS alone. Furthermore, IL-12-dependent CLA expression was only marginally increased by LPS, the increased CLA expression by CD8+ T cells cultured in FCS without superantigen could largely be inhibited by anti-IL-12 antibodies, and LPS had a very modest effect on the superantigen-mediated induction of CLA that takes place in FCS.
It is reasonable to assume that FCS may contain a variety of growth factors and cytokines that are present in low concentrations or not at all in serum from adult humans. However, we are not aware of any comparative studies in this respect. The failure to induce CLA expression with superantigens in the absence of FCS does in our view not exclude the possibility that such an induction can take place when skin or tonsils are infected by bacterial pathogens that produce superantigens. Inflammatory processes in these tissues might give rise to factors that interact with IL-12 to induce CLA expression and we are currently studying this possibility and the factors that may be involved.
In conclusion this study demonstrated that IL-12 requires some as yet unidentified cofactor(s) for enhancing the expression of the skin-specific adhesion epitope CLA.
Acknowledgments
This work was supported by the Icelandic Research Council and the Icelandic Graduate Training Fund.
REFERENCES
- 1.Fleischer B, Gerlach D, Fuhrmann A, Schmidt KH. Superantigens and pseudosuperantigens of gram-positive cocci. Med Microbiol Immunol. 1995;184:1–8. doi: 10.1007/BF00216783. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Walsh P, Giorno R, Norris DA. A potential role for superantigens in the pathogenesis of psoriasis. J Invest Dermatol. 1993;100:225–8. doi: 10.1111/1523-1747.ep12468941. [DOI] [PubMed] [Google Scholar]
- 3.Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–6. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–68. [PMC free article] [PubMed] [Google Scholar]
- 5.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–36. [PubMed] [Google Scholar]
- 6.de Boer OJ, Wakelkamp IM, Pals ST, Claessen N, Bos JD, Das PK. Increased expression of adhesion receptors in both lesional and non- lesional psoriatic skin. Arch Dermatol Res. 1994;286:304–11. doi: 10.1007/BF00402220. [DOI] [PubMed] [Google Scholar]
- 7.Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–53. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 9.Akdis M, Klunker S, Schliz M, Blaser K, Akdis CA. Expression of cutaneous lymphocyte-associated antigen on human CD4 (+) and CD8 (+) Th2 cells. Eur J Immunol. 2000;30:3533–41. doi: 10.1002/1521-4141(2000012)30:12<3533::AID-IMMU3533>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria BL, Moser R, Perez SM, Picker LJ, Blaser K, Hauser C. Migration of skin-homing T cells across cytokine-activated human endothelial cell layers involves interaction of the cutaneous lymphocyte-associated antigen (CLA), the very late antigen-4 (VLA-4), and the lymphocyte function-associated antigen-1 (LFA-1) J Immunol. 1995;154:1543–50. [PubMed] [Google Scholar]
- 11.Hjalmar V, Hast R, Kimby E. Cell surface expression of CD25, CD54, and CD95 on B- and T-cells in chronic lymphocytic leukaemia in relation to trisomy 12, atypical morphology and clinical course. Eur J Haematol. 2002;68:127–34. doi: 10.1034/j.1600-0609.2002.01515.x. [DOI] [PubMed] [Google Scholar]
- 12.Davison SC, Allen MH, Mallon E, Barker JN. Contrasting patterns of streptococcal superantigen-induced T-cell proliferation in guttate vs. chronic plaque psoriasis. Br J Dermatol. 2001;145:245–51. doi: 10.1046/j.1365-2133.2001.04341.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama F, Teraki Y, Kudo T, et al. Expression of cutaneous lymphocyte-associated antigen regulated by a set of glycosyltransferases in human T cells: involvement of alpha1, 3-fucosyltransferase VII and beta1,4-galactosyltransferase I. J Invest Dermatol. 2000;115:299–306. doi: 10.1046/j.1523-1747.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagers AJ, Kansas GS. Potent induction of alpha (1,3)–fucosyltransferase VII in activated CD4+ T cells by TGF–beta 1 through a p38 mitogen–activated protein kinase–dependent pathway. J Immunol. 2000;165:5011–5016. doi: 10.4049/jimmunol.165.9.5011. [DOI] [PubMed] [Google Scholar]
- 15.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 16.Perussia B, Chan SH, D’Andrea A, et al. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992;149:3495–502. [PubMed] [Google Scholar]
- 17.Cassatella MA, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 18.Quiding-Jarbrink M, Ahlstedt I, Lindholm C, Johansson EL, Lonroth H. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut. 2001;49:519–25. doi: 10.1136/gut.49.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantele JM, Arvilommi H, Kontiainen S, et al. Mucosally activated circulating human B cells in diarrhea express homing receptors directing them back to the gut. Gastroenterol. 1996;110:1061–7. doi: 10.1053/gast.1996.v110.pm8612994. [DOI] [PubMed] [Google Scholar]
- 21.Pitzalis C, Cauli A, Pipitone N, et al. Cutaneous lymphocyte antigen-positive T lymphocytes preferentially migrate to the skin but not to the joint in psoriatic arthritis. Arthritis Rheum. 1996;39:137–45. doi: 10.1002/art.1780390118. [DOI] [PubMed] [Google Scholar]