Abstract
In Wegener's granulomatosis (WG), a form of autoimmune systemic vasculitis, chronic carriage of Staphylococcus aureus constitutes a risk factor for the development of exacerbations. Circulating T cells in this disease are persistently activated, suggesting the presence of a chronic stimulus. A causal link between chronic carriage of S. aureus and chronic T cell activation in WG is conceivable, because S. aureus produces superantigens (SAg), which are potent T cell stimulators. Superantigenic stimulation of T cells results in expansion of T cell subsets expressing SAg-binding T cell receptor V-beta (Vβ) chains. In the present study we hypothesized that in WG the presence of staphylococcal SAg is accompanied by expansion of SAg-reacting T cell subsets. We tested our hypothesis in a cross-sectional and a longitudinal study in which the association between seven staphylococcal SAg genes [typed by poplymerase chain reaction (PCR)], eight SAg-binding Vβ chains and four SAg-non-binding Vβ chains (assessed by flow-cytometry) was assessed. Both studies showed that T cell expansions were present at a significantly higher rate in WG patients than in healthy individuals, but were not associated with the presence of either S. aureus or its SAg. Moreover, T cell expansions were generally of small extent, and did not appear simultaneously in both CD4 and CD8 subsets. We conclude that in WG S. aureus effects its supposed pathogenic function by a mechanism other than superantigenic T cell activation.
Keywords: superantigens, T cells, Wegener's granulomatosis
INTRODUCTION
Wegener's granulomatosis (WG) is a form of autoimmune systemic vasculitis with an as yet incompletely understood pathophysiology. Autoantibodies against proteinase 3 and, to a lesser extent, against myeloperoxidase (antineutrophil cytoplasmic antibodies, ANCA), are sensitive and specific markers of the disease [1–3]. Although the aetiology of WG is probably multi-factorial [4,5], several environmental factors, such as bacterial infections, have been proposed as probable initiators of the disease [4]. Our group has shown that chronic carriage of Staphylococcus aureus is a risk factor for disease exacerbation in WG [6]. This finding was corroborated by the observation that treatment of WG patients with the antibiotic trimethoprim–sulphamethoxazole (cotrimoxazole) resulted in a lower frequency of disease relapses [7]. Thus, unravelling the mechanisms by which S. aureus exerts its noxious effect is important for understanding and treating this form of vasculitis. One of these mechanisms may involve staphylococcal superantigens (SAg).
S. aureus produces a number of SAg, all of which are strong T cell activators [8]. Activation is T cell receptor V-beta chain-specific (TCR Vβ) [8,9], resulting in proliferation of the bound T cells and thus the expansion of the respective T cell subset. In WG, we and others have shown that circulating T cells are persistently activated [10,11], suggesting the presence of a chronic stimulus. Because the prevalence of carriage of S. aureus is increased in WG [6] (60–70% of the patients compared to 15–20% of healthy controls), we speculated that this stimulus might be a staphylococcal SAg [4,10]. Moreover, we found a high prevalence of S. aureus strains carrying genes encoding the SAg toxic shock syndrome-toxin 1 (TSST-1), staphylococcal enterotoxin A and C (SEA, SEC) and exfoliative toxin A (ETA) in WG patients [12]. Interestingly, in this study WG patients with SAg-positive S. aureus strains had a higher relapse risk than patients with a SAg-negative S. aureus strain. These findings suggest a possible link between T cell activation, SAg and disease activation in WG.
In the present study we hypothesized that in WG, staphylococcal SAg may activate T cells expressing SAg-binding TCR Vβ chains, leading to the expansion of the respective T cell subsets. We investigated this hypothesis in both a cross-sectional and a longitudinal study, by assessing the concomitant presence of S. aureus, SAg and T cell expansions. Both studies revealed no association between the presence of SAg and the presence of T cell expansions. To our knowledge, these are the first extensive studies correlating the simultaneous presence of S. aureus, staphylococcal SAg and T cell expansions in WG in particular and systemic autoimmune diseases in general.
MATERIALS AND METHODS
Study design
In a first, cross-sectional study we assessed whether T cell expansions were present in WG and whether they were associated with the presence of S. aureus and SAg. In that study we focused on the T cell subsets BV2S1, BV5S3 and BV17S1, as these bind TSST-1 [13,14], ETA [13], SEA [13–15] and SEC [13–15], respectively, which we found previously most frequently in WG patients [12].
In a second, longitudinal prospective study we assessed the relationship between T cell expansions, S. aureus carriage and staphylococcal SAg in time. Here, SAg and T cell expansions were assessed at three time-points (time intervals 0·5–15 months). Because different Vβ chains can recognize the same SAg, we extended the panel of T cell subsets to eight SAg-binding subsets (BV2S1, BV5S1 [15,16], BV5S3, BV8S1/2 [15], BV9S1 [15], BV12S2 [13–15], BV16S1 [15] and BV17S1) and four SAg-non-binding subsets (BV11S1, BV13S1, BV21S3, BV23S1), in order to avoid missing associations between SAg and T cell expansions.
Each study had a separate group of healthy controls.
Patients and controls
In the cross-sectional study, 36 consecutive patients (19 males, 17 females, mean age 57·8 years, range 25–86 years) with biopsy-proven WG and fulfilling the criteria of the American College of Rheumatology for the diagnosis of WG [17] were included. Patients received no or low dose immunosuppressive treatment (cyclophosphamide <50 mg/day, azathioprine <100 mg/day and/or prednisolone <7·5 mg/day) and had anti-PR3 ANCA at diagnosis [18]. The duration of disease ranged from 0 to 286 months (median 100 months). Active disease and complete remission were defined according to previously described criteria [7]. Nine patients had active WG and 28 patients were in complete remission at analysis. Four patients with active disease received antibiotics. None of the active patients was receiving immunosuppressive treatment at the time-point of analysis. Sixteen patients received antibiotics (cotrimoxazole tablets or mupirocin nasal ointment). Twenty-one healthy volunteers (13 males, eight females; mean age 43·5 years, range 25–86 years) served as controls. Control individuals had been free from infections for at least 3 weeks prior to analysis.
In the longitudinal study, 10 consecutive WG patients (five male, five female, mean age 59·3 years, range 29–75 years) were included. Three of these patients had participated previously in the cross-sectional study. Patients were in complete remission during the study. Three patients received low dose immunosuppressive treatment (see above) at one or more time-points of the study. Two patients received maintenance treatment with cotrimoxazole. A group of eight healthy individuals served as controls for this study (four male, four female, mean age 36·2 years, range 27–45 years). Control individuals had been free from infections for at least 3 weeks prior to analysis.
Patients 32–38 (Table 1) participated exclusively in the longitudinal study. Patients 1, 21 and 28 participated in both studies. All other patients participated exclusively in the cross-sectional study. The study was approved by the Institutional Review Board of the Medical Ethical Committee.
Table 1.
Clinical data of patients with WG
| pat | Age | Sex | Duration of disease (months) | Disease activity | i.s. treatment | Antibiotic treatment |
|---|---|---|---|---|---|---|
| 1 | 45 | m | 2 | active | Pr. | CT |
| 2 | 52 | f | 69 | active | – | – |
| 3 | 34 | m | 100 | active | Cy. | CT |
| 4 | 54 | m | 20 | active | Cy. | MC |
| 5 | 71 | f | 118 | active | Cy. | – |
| 6 | 51 | m | 87 | active | – | CT |
| 7 | 53 | f | n.p. | active | – | – |
| 8 | 56 | m | 250 | active | – | CT, MP |
| 9 | 31 | f | 106 | quiescent | – | CT |
| 10 | 59 | f | 27 | quiescent | – | – |
| 11 | 57 | m | 4 | quiescent | – | – |
| 12 | 25 | f | 16 | quiescent | Aza | CT |
| 13 | 63 | m | 149 | quiescent | – | – |
| 14 | 54 | m | 130 | quiescent | – | CT |
| 15 | 28 | f | 95 | quiescent | – | – |
| 16 | 78 | m | 118 | quiescent | – | – |
| 17 | 64 | f | 54 | quiescent | – | – |
| 18 | 69 | m | 72 | quiescent | Cy., Pr. | CT |
| 19 | 67 | m | 55 | quiescent | – | – |
| 20 | 68 | f | 119 | quiescent | – | – |
| 21 | 69 | m | 229 | quiescent | – | MP |
| 22 | 86 | m | 178 | quiescent | – | – |
| 23 | 67 | f | 286 | quiescent | – | CT |
| 24 | 86 | f | 107 | quiescent | – | – |
| 25 | 65 | m | 48 | quiescent | – | – |
| 26 | 45 | m | 154 | quiescent | Pr. | CT |
| 27 | 78 | m | 120 | quiescent | – | CT |
| 28 | 72 | f | 181 | quiescent | – | CT |
| 29 | 27 | f | 114 | quiescent | Pr. | – |
| 30 | 81 | f | 20 | quiescent | Aza. | – |
| 31 | 27 | f | 18 | quiescent | Aza. | CT |
| 32 | 68 | m | 36 | quiescent | – | – |
| 33 | 47 | f | 121 | quiescent | – | – |
| 34 | 64 | f | 156 | quiescent | – | – |
| 35 | 75 | m | 38 | quiescent | – | – |
| 36 | 56 | f | 160 | quiescent | – | – |
| 37 | 28 | f | 29 | quiescent | – | – |
| 38 | 20 | m | 118 | quiescent | – | – |
Abbreviations: pat; patient, Aza: azathioprine; CT: cotrimoxazole; Cy: cyclophosphamide; MC: minocyclin; MP: mupirocin; i.s. immunosuppressive; n.p. new patient. The dosage of treatment is described in the Methods section.
Monoclonal antibodies and conjugates
Monoclonal antibodies (moAbs) against CD4 and CD8 (both Cy-Q) were from IQP Diagnostics (Groningen, the Netherlands). MoAbs against the T cell receptor (TCR) chains BV2S1, BV5S1, BV5S3, BV8S1/2, BV9S1, BV11S1, BV13S1, BV12S2, BV16S1, BV17S1, BV21S3 and BV23S1 and goat-antimouse F(ab)2 (FITC) conjugate were from Immunotech (Marseille, France).
Detection of TCR Vβ T cell subsets
TCR Vβ chains on CD4+ or CD8+ T cells were detected by flow cytometry. One hundred µl of whole, heparinized blood was incubated with Vβ moAb and 5% goat serum, followed by goat-antimouse F(ab)2 (FITC) conjugate. Free binding sites on the conjugate were blocked with 10% mouse serum (CLB, Amsterdam, the Netherlands). Finally, cells were incubated in CD4 or CD8 moAb, respectively, without further washing. All incubations were carried out in PBS/0·5% BSA/0·01% sodium azide for 15 min at room temperature, in the dark, and were followed by appropriate washing steps. Erythrocytes were lysed with FACS-lysing solution. Cells were washed with PBS and analysed on an Epics Elite cytometer (Beckman Coulter, Mijdrecht, the Netherlands). CD4+ and CD8+ T cells were gated and 2000–5000 events were recorded within these gates, respectively. Population percentages were calculated using the WinList 3·2 software package (Verity Software House, Topsham, USA).
Detection of S. aureus
Nasal flora was sampled by rotating a sterile cotton swab in each anterior naris. Swabs were inoculated on 5% sheep-blood and salt mannitol agar. S. aureus was identified by coagulase and DNase positivity. Single S. aureus colonies were isolated and cultured on blood agar plates. Microbiological assays and preservation of bacterial strains were performed at the Department of Microbiology of the University Hospital.
Isolation of staphylococcal DNA
DNA was extracted from S. aureus strains by the method of Boom et al. [19], with several modifications. Briefly, one colony of S. aureus was prelysed in 250 µl Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, pH 8·0; Merck Darmstadt, Germany) containing 0·01 U/µl lysostaphin (Merck) for 5 min at 37°C. Bacteria were then lysed in lysis buffer (8·32 M guanidinium-isothiocyanate, Fluka BioChemika, Deisenhofen, Germany; 0·1 M Tris pH 6·4, 0·2 M EDTA, Merck; 2·6 g Triton X-100, Sigma, Zwijndrecht, the Netherlands) and DNA allowed to bind to Celite matrix (Sigma) (10 g in 32% w/v HCl) for 10 min at room temperature. The suspension was centrifuged briefly and the supernatants were discarded. The pellets were washed twice with 500 ml 70% ethanol, once with 500 µl acetone and dried at 37°C. The pellets were resuspended in 150 µl Tris-EDTA buffer and DNA was eluted from the matrix by incubating for 10 min at 56°C. The quality and quantity of the isolated DNA were assessed by gel electrophoresis on a 1% agarose gel (Eurogentec, Seraing, Belgium), using a serial dilution of lambda DNA (Amersham Pharmacia, Freiburg, Germany) as a reference. DNA imaging was perfomed on an Image Master digital camera (Pharmacia).
Detection of SAg genes by multiplex polymerase chain reaction (PCR)
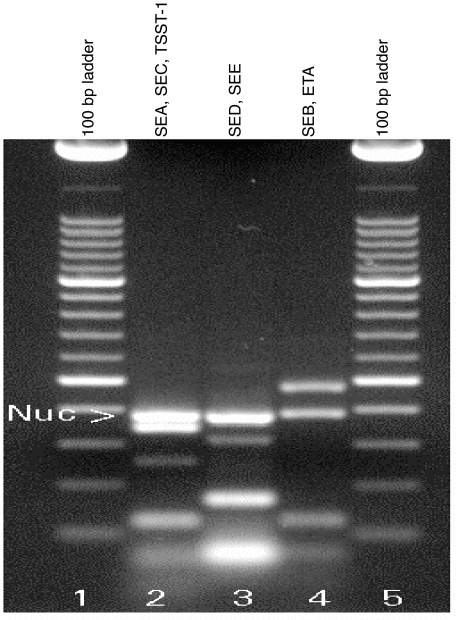
We established a multiplex PCR that allows the simultaneous detection of 2–3 SAg genes. Three separate PCR reactions were carried out for each strain. In these reactions, SEA, SEC and TSST-1, stapylococcal enterotoxin D (SED) and stapylococcal enterotoxin E (SEE) and, finally, staphylococcal enterotoxin B (SEB) and ETA can be detected simultaneously (Fig. 1). Oligonucleotide primer sequences for the staphylococcal SAg SEA, SEB, SEC, SED, SEE, TSST-1 and ETA have been described previously [20] and were from Life Technologies (Paisley, UK). PCR was carried out in a volume of 25 µl (0·58 mM dNTPs, Pharmacia; 21% Super-Taq reaction buffer; 2·5 U SuperTaq (Ambion, Austin, USA), 0·025 mM MgCl2, Merck; 25 pmol SEA primers, 12·5 pmol SEB primers, 75 pmol SED primers, 50 pmol SEC, SEE, TSST-1 and ETA primers, 10 pmol nuc-a primers, 50 ng DNA). The amplification protocol consisted of one preamplification cycle (5 min 94°C, 3 min 45°C, 3 min 72°C) followed by two cycles (10 s 94°C, 15 s 45°C, 30 s 72°C) and 37 cycles (10 s 94°C, 15 s 55°C, 30 s 72°C). PCR products were detected on an agarose gel as described above. The 100-bp ladder (Roche, Mannheim, Germany) was used as a reference for amplicon size.
Fig. 1.
Typing of staphylococcal SAg by multiplex PCR. Seven staphylococcal SAg genes (SEA-SEE, TSST-1 and ETA) can be typed in three separate reactions using this multiplex PCR protocol. Typing of the genes makes use of the fact that the SAg-specific primers yield different amplicon sizes. Thus, SEA, SEC and TSST-1 (lane 2), SED and SEE (lane 3) and SEB and ETA (lane 4) can be detected in the same reaction. Additionally, the S. aureus specific nuclease-a gene was co-amplified in order to confirm the strain species. To illustrate the method, total DNA from S. aureus strains carrying one of the genes encoding for the SAg SEA-SEE, TSST-1 or ETA, respectively, were mixed, thus creating an artificial positive control. The100 base pair ladder was used as a size marker.
Statistical analyses
Mann–Whitney's U-test, Fisher's R to Z transformation and exact test, Spearman's rank test and χ2 analyses were performed using GraphPad Prism software (GraphPad Software, CA, USA). Two-tailed P-values lower than 0·05 were considered significant. To determine T cell expansions we calculated the mean + 3 s.d. of the corresponding subset in healthy controls, after omitting values that were outside the normal distribution. In patients, T cell expansions were defined as a percentage of cells of the respective subset that exceeded the mean + 3 s.d. of the control group. As the control group of the cross-sectional study was not identical to that of the longitudinal study, normal (reference) values were calculated separately for the two studies.
RESULTS
Cross-sectional study
Our first aim was to determine whether WG patients enrolled in the cross-sectional study carried S. aureus at the time of inclusion. Of the 36 WG patients enrolled, 11 (30%) were S. aureus-positive. Eight of these patients were receiving maintenance antibiotic treatment with either cotrimoxazole or mupirocin. Three of 11 patients with S. aureus received low dose immunosuppressive treatment. Two patients with S. aureus also had active disease. All isolated S. aureus strains were coagulase-positive, DNase-positive (as confirmed by conventional microbiological tests) and nuclease-a positive (as confirmed by PCR, Fig. 1).
Five of the 11 S. aureus strains carried a SAg gene, as determined by multiplex PCR (Fig. 1). Two strains could not be typed due to failure to preserve the strains. Three S. aureus strains carried an SEA gene, two strains carried an ETA gene and four strains were negative for SAg genes. All patients who were SAg-positive were receiving maintenance antibiotic treatment. One of these patients (no. 30) was enrolled during active disease.
In the following step we assessed whether expansions of T cell subsets expressing SAg-binding TCR Vβ chains were present in this group of patients. Twelve of the 36 WG patients included had at least one T cell expansion, compared to 1/21 healthy controls (P = 0·01). Eight expansions were detected in CD4+ T cells and eight expansions in CD8+ T cells. Expansions were observed in all four Vβ subsets, except for CD8/BV2S1, and did not occur preferentially in one of the subsets. There was no correlation between the occurrence of T cell expansions and either disease activity, immunosuppressive or antibiotic medication, or age.
Finally, we investigated whether the presence of S. aureus and SAg in these patients was associated with the concomitant occurrence of T cell expansions. Two of 11 patients with S. aureus and 10/25 patients without S. aureus had a T cell expansion (P = 0·187).
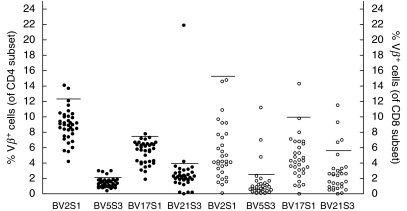
The SAg SEA and ETA, which were present in 5/11 patients carrying S. aureus, can induce expansions of BV5S3 and BV2S1-expressing T cells, respectively. However, none of the patients carrying these SAg had an expansion of one of these two or other T cell subsets. As mentioned, we did find T cell expansions in patients without S. aureus. Typical SAg-induced T cell expansions are substantial and present in both CD4 and CD8 T cells, as SAg-activation is independent of MHC type [8,9]. We occasionally observed twofold or higher expansions (Fig. 2); surprisingly, however, these were mainly present in the BV21S3 (control) subset. Expansions were never present simultaneously in both CD4+ and CD8+ T cells.
Fig. 2.
Expansions of T cell subsets expressing SAg-binding Vβ chains. Percentages of T cells expressing SAg-binding TCR Vβ chains were determined by flow cytometry. Closed symbols denote measurements in the CD4 T cell subset, open symbols denote measurements in the CD8 T cell subset. Horizontal lines represent the threshold for expansions, determined for the group of healthy controls.
Longitudinal study
Bacterial carriage is a dynamic process that depends on the immune status of the individual, exposure to environmental factors, medication, etc. Similarly, the composition of the T cell repertoire of an individual is subject to changes due to immunostimulatory or -inhibitory factors. Therefore, we conducted a second, longitudinal study, in which we followed S. aureus and SAg carriage in relation to T cell expansions in time.
Nine of 10 WG patients carried S. aureus at least at one time-point of assessment. In four of these nine patients (nos 3, 5, 9, 10) we found S. aureus at all three time-points, in four patients (nos 1, 2, 4, 7) S. aureus was present at two time-points and in one patient (no. 6) at only one time-point of assessment (Table 2a). One of the intermittent carriers (no. 2) was receiving maintenance cotrimoxazole treatment for the duration of the study. Three patients were receiving low dose immunosuppressive treatment at one time-point of assessment, which also coincided with S. aureus carriage.
SAg genes were detected in five of nine patients with S. aureus. The genes for SEA, SEB, SEC and TSST-1 were found, whereas the ETA gene was not present in any of the S. aureus strains analysed in the longitudinal study (Table 2a,b).
SAg genes were present in two of the four chronic S. aureus carriers (nos 9 and 10). Interestingly, whereas in patient no. 9 we found the same SAg (SEC) at all three time-points, patient no. 10 carried a SAg-positive strain only once (SEA, SEB), which suggests strongly that a switch of bacterial strains had taken place during chronic carriage (Table 2a,b). We observed the same phenomenon in one intermittent carrier of S. aureus (no. 2), who was also an intermittent SAg carrier. This patient was receiving maintenance treatment with cotrimoxazole. A group of eight healthy individuals were included in this study. Three of these were chronic S. aureus and SAg carriers. In contrast to the shifting pattern of S. aureus and SAg carriage in WG patients, the pattern of SAg carriage in control individuals was stable in time (not shown).
In order to determine whether WG patients had more T cell expansions than healthy individuals, we compared the frequency of expansions detected among all measurements in the two groups. In the CD4+ T cell subset there were 19 expansions among 357 measurements, compared to 4/285 expansions in healthy controls (P < 0·0001). In the CD8+ subset we found 19/350 expansions in WG patients and 4/284 expansions in healthy controls (P = 0·009). Expansions of BV2S1 and BV17S1 were not found (Table 2a,b). There was no preferential accumulation of expansions in one of the other T cell subsets.
There was no distinct pattern in the behaviour of T cell expansions in time. Whereas in some patients expansions persisted for several months (no. 1, CD4/BV12S2, no. 4, CD4/BV5S3 and BV12S2, no. 5, CD8/BV8S1), in other patients expansions were seen at only one or two time-points (Table 2a,b). There was no correlation between the presence of T cell expansions and immunosuppressive or antibiotic medication.
In order to investigate the relation between T cell expansions and S. aureus carriage we compared the sum of T cell expansions occurring in the presence of S. aureus with the sum of T cell expansions occurring in the absence of S. aureus. At time-points when S. aureus was present in WG patients, T cell expansions were detected in 35/436 measurements. In the absence of S. aureus, T cell expansions were detected in 14/222 measurements (P = 0·531). In healthy controls the presence of T cell expansions did not correlate with the presence of S. aureus (not shown).
As mentioned, in the longitudinal study we detected the SAg SEA (which can bind to BV5S3, BV9S1 and BV16S1), SEB (BV12S2, BV17S1), SEC (BV12S2, BV17S1) and TSST-1 (BV2S1). In only one patient was there a T cell expansion corresponding to the simultaneously present SAg gene (no. 4, SEC and CD4/BV12S2). However, in this patient there was no BV12S2 expansion present in the CD8 T cell subset, arguing against the induction of this expansion by the SAg SEC. Moreover, beside BV12S2, SEC is also able to bind and activate T cells expressing BV17S1. If T cell activation in this patient were truly induced by SEC, expansion of BV17S1 should also have been present [15]. This, however, was not the case (Table 2a,b). With the exception of patient no. 4, there were no further correlations between SAg genes and corresponding T cell expansions present at the same time-point.
DISCUSSION
In the present study we showed that in WG there is no association between the presence of staphylococcal SAg genes and expansions of peripheral blood T cell subsets expressing SAg-specific TCR Vβ chains.
Considerable effort has been invested in elucidating the mechanisms leading to vascular damage in WG. Autoantibodies with specificity for proteinase 3 or myeloperoxidase (ANCA) are key players in the development of the disease and their pathophysiological role has been studied extensively [21,22]. The production of autoantibodies is the result of an immune response, in which, classically (auto)antigen-specific B cells, T cells and the (auto)antigen are involved. An alternative scenario for the induction of autoimmunity has been put forward by Friedman and colleagues, who proposed that SAg-mediated B- and T cell activation could take place in a T cell antigen-independent manner [23]. In this scenario, SAg bridge MHC II molecules on autospecific B cells and TCR Vβ chains on T cells, thereby leading to B cell activation and autoantibody production. Since SAg are specific for conserved regions of the TCR Vβ chain shared by different members of a Vβ family, larger numbers of T cells expressing SAg-binding TCR Vβ chains can be recruited for the activation of autospecific B cells, independent of their autoantigen specificity. Indeed, He et al. have shown the ability of SED to drive production of rheumatoid factor by B cells [24]. Building on this scenario, we have previously postulated that in WG, in the presence of SAg-carrying S. aureus and the autoantigens proteinase 3 or myeloperoxidase, T cells expressing SAg-binding TCR Vβ chains may deliver co-stimulatory signals to autospecific B cells [10]. Our postulate was supported by the finding that a significantly increased number of activated T cells were present in WG patients, suggesting chronic activation by a persisting stimulus [10]. This stimulus could probably be staphylococcal SAg.
A consequence of SAg-mediated activation is the expansion of SAg-binding T cells. Among the vasculitides, Kawasaki disease is associated with expansions of BV2S1-expressing T cells, which can bind staphylococcal TSST-1 [25,26]. S. aureus carriage has been reported in this form of vasculitis [27] and in one study in two patients with Kawasaki disease the simultaneous presence of BV2S1 expansions and TSST-1 was demonstrated [28]. In WG, three reports documented T cell expansions of various Vβ families [29–31]. Except for the study in Kawasaki disease [28], however, none of the studies investigating SAg-mediated T cell activation in autoimmune diseases have associated the presence of expansions of T cell subsets expressing SAg-binding TCR Vβ chains with the simultaneous presence of the corresponding SAg. This was the goal of our present studies in WG patients.
Overall, we found SAg genes in 13/30 S. aureus strains. SEA and SEC were present most frequently (in 38·5% and 46% of all strains, respectively). In patients followed longitudinally, the pattern of SAg carriage was not always stable in time. There are two possible explanations for this finding. First, the location of SAg genes in the bacterial genome may determine whether this gene can be acquired or lost. The genes for the SAg SEA and SEE, for example, are situated on bacteriophages, which are not permanent components of the bacterial genome [32,33]. In patient 10 we observed a switch from an S. aureus strain harbouring no SAg gene to a strain carrying SEA and SEB genes. In this case, the acquisition of the SEA gene may have taken place via a bacteriophage. However, the gene encoding SEB, which was detected in the same strain, is located on the bacterial chromosome [34]. Therefore, a second possibility explaining strain switch could be considered. According to this possibility, the previous, SAg-negative may have been lost and replaced by a new, SAg-positive strain. This possibility is exemplified by patient no. 2, who carried a SEB-positive S. aureus at the first assessment and a SAg-negative S. aureus at the last assessment. Whether strain switch is enhanced by antibiotic or immunosuppressive treatment or is due to the acquisition of a new, dominant, S. aureus strain cannot be answered by this study. In the cohort of patients followed in the longitudinal study, neither acquisition of S. aureus, nor acquisition of or switch to a SAg-positive strain was associated with changes in disease activity. However, because the incidence of S. aureus- and SAg-carriage was low, it may be worthwhile to investigate the relation between SAg-carriage and disease (re)activation in a more extensive study. Interestingly, assessment of the diversity of S. aureus strains within one patient at a given time-point showed that by genetic fingerprinting of 10 random S. aureus colonies from the primary culture, all colonies were identical (E. Popa, unpublished observation). This suggests that a dominant S. aureus colony is established and that the chance of missing SAg by random picking of just one staphylococcal colony is low.
Our hypothesis that the presence of expansions of T cells expressing SAg-binding Vβ chains is associated with the presence of the corresponding SAg was not confirmed. While in both studies we found a significantly higher incidence of T cell expansions in WG patients than in healthy individuals, T cell expansions were associated with the presence of SAg in only one patient. Classically, T cell activation by SAg should be accompanied by expansion of activated T cells, occurring in both CD4 and CD8 subsets [35,36]. In our studies T cell expansions were modest and did not simultaneously appear in CD4 and CD8 T cells.
The question arises, why the presence of SAg genes is not associated with the expansion of the corresponding, SAg-responsive peripheral T cells. One possibility may be that the carriage of a SAg gene is not necessarily followed by the production of the SAg protein. In vitro it has been shown that carriage of SAg genes is associated with SAg production [20]. Whether this also occurs in vivo we cannot determine, as we did not investigate serum levels of SAg in our patients. Because SAg have been detected in the circulation only in severe cases of toxic shock [37], it may be difficult to detect these molecules in quiescent patients. A further possibility might be that, although produced, SAg could not reach the blood circulation or the lymphatics. Von Eiff et al. showed recently that in patients with bacteraemia S. aureus colonizing the nares could reach the bloodstream [38]. However, it could be speculated that in the absence of nasal lesions SAg cannot gain access to immunocompetent cells. Furthermore, it is possible that even though produced, SAg molecules would be scavenged by SAg-specific antibodies. In this line, antibody responses to various staphylococcal SAg have been detected in the serum of patients with toxic shock syndrome [39–41], rheumatoid arthritis [42], but also healthy nasal carriers of S. aureus[43]. According to a scenario proposed by Schiffenbauer et al. [44] it is also possible that, even though activated by SAg and having undergone expansion, T cells may have homed to sites containing the antigen for which these cells are specific. The nares, in which both the autoantigen proteinase 3 (secreted by neutrophils) and bacterial antigens are present, could be such a site. Finally, it may be argued that low dose of immunosuppressives, when present, may have affected T cell skewing. In our experience (E. R. Popa, unpublished results), however, dosages of <50 mg/day cyclophosphamide, <100 mg/day azathioprin or <7·5 mg/day prednisolone, did not affect T cell activation, as measured by the expression of activation marker CD25.
Because T cell expansions were not associated with the presence of SAg, the reason for these expansions remains obscure. Stimulation of T cells by staphylococcal SAg that were not investigated in this study, such as SEG, SEH, SEI, or by as yet undiscovered SAg, might be a possibility, as in this study we focused on SAg that were known previously to be present frequently in WG patients. Moreover, subclinical infections with viruses or bacteria as well as stimulation by (auto)antigens may explain changes in the T cell repertoire. Detailed functional and phenotypic investigation of these small populations of skewed T cells would be needed in order to determine their significance for the disease.
One of the questions of interest remains how S. aureus affects the course of disease in WG. As mentioned previously, S. aureus constitutes a risk factor for disease exacerbation in WG [7,12]. Our original hypothesis stated that staphylococcal SAg might indirectly induce ANCA production (see above). To test this hypothesis, information about the onset of SAg presence in a patient is essential, since (auto)antibody production occurs within a limited time interval after introduction of the SAg in the host. Unfortunately, in the present study the time-point of first presence of SAg in the included patients was unknown and thus correlation between SAg and ANCA titres was not possible. In the future, a prospective study aiming at identifying the onset of SAg-positive S. aureus carriage in WG patients, followed by prospective quantitative assessment of serum ANCA levels will be able to test this hypothesis. The present study suggests that the influence of this bacterium on the disease is not effected by the most plausible staphylococcal immune modulators, SAg. Other staphylococcal immune modulators have received little attention yet or are currently under investigation. Staphylococcal protein A, for example, which is known as a potent stimulator of a subset of B cells [44], may affect production of autoantibodies. Staphylococcal acid phosphatase has been described previously as a molecule with immunostimulatory capacity [46,47] and is currently under investigation in our group as a pathogen in WG. Obviously, multiple mechanisms that may lead to staphylococcal pathogenicity still need to be investigated. In WG, which is probably a multi-factorial disease, special emphasis will need to be placed on the interplay of S. aureus and other components of the immune system and its impact on disease activation.
Table 2a.
S. aureus and SAg carriage in WG patients in relation to Vβ expansion in a longitudinal study: CD4 subset
| Pat | Time-point | S.a. | SAg | BV2 | BV5/1 | BV5/3 | BV8 | BV9 | BV11 | BV12 | BV13 | BV16 | BV17 | BV21 | BV23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | + | – | 6·8 | 5·4 | 1·2 | 5·3 | 0·7 | 0·3 | 6·6 | 0·0 | 1·8 | 6·9 | 2·2 | 0·8 |
| 2 | + | n.d. | 5·8 | 4·6 | 0·5 | 4·5 | 0·0 | 0·6 | 4·8 | 0·9 | 0·7 | 6·0 | 1·5 | 0·5 | |
| 3 | – | 5·6 | 4·3 | 0·7 | 5·0 | 6·5 | 0·3 | 4·0 | 0·4 | 0·8 | 6·7 | 0·2 | 7·3 | ||
| 2 | 1 | + | SEB | 7·5 | 2·7 | 0·6 | 3·3 | 0·2 | 0·4 | 1·3 | 0·2 | 0·9 | 3·2 | 1·6 | 0·3 |
| 2 | – | 7·6 | 2·6 | 0·5 | 3·0 | n.d. | 0·5 | 1·0 | 2·8 | 0·8 | 2·9 | 1·5 | 0·3 | ||
| 3 | + | – | 6·2 | 2·3 | 0·4 | 2·9 | 2·5 | 0·7 | 0·8 | 4·6 | 1·0 | 2·7 | 1·3 | 0·4 | |
| 3 | 1 | + | – | 7·8 | 11·5 | 2·5 | 3·6 | 0·6 | 1·2 | 2·6 | 5·0 | 1·8 | 4·2 | 2·5 | 2·4 |
| 2 | + | – | 6·9 | 7·1 | 1·1 | 5·0 | 0·4 | n.d | 1·4 | 1·1 | 0·6 | 5·3 | 1·8 | 0·3 | |
| 3 | + | – | 8·0 | 6·3 | 0·9 | 5·4 | 0·8 | 0·4 | 2·4 | 0·3 | 1·2 | 3·1 | 0·3 | 0·4 | |
| 4 | 1 | + | SEC | 8·1 | 4·1 | 4·8 | 4·3 | 0·2 | 0·6 | 5·9 | 4·7 | 1·4 | 3·9 | 0·2 | 0·6 |
| 2 | + | SEC | 8·5 | 6·0 | 4·4 | 4·4 | 2·3 | 0·5 | 4·2 | 5·0 | 1·0 | 4·8 | 2·4 | 0·3 | |
| 3 | – | 8·5 | 5·5 | 4·3 | 4·0 | 0·4 | 0·0 | 4·6 | 0·3 | 1·3 | 5·3 | 2·0 | 0·5 | ||
| 5 | 1 | + | – | 11·3 | 5·2 | 0·5 | 3·4 | 2·2 | 0·4 | 1·9 | 0·1 | 1·0 | 6·0 | 0·0 | 12·2 |
| 2 | + | – | 12·5 | 5·3 | 0·9 | 4·0 | 1·9 | 0·8 | 2·1 | 2·1 | 1·0 | 3·7 | 0·0 | 0·5 | |
| 3 | + | – | 11·5 | 4·5 | 1·3 | 3·3 | 0·5 | 0·4 | 2·2 | 0·0 | 1·0 | 6·5 | 2·9 | 0·5 | |
| 6 | 1 | – | 3·9 | 5·2 | 1·3 | 4·0 | 1·4 | 0·9 | 2·1 | 1·0 | 1·1 | 5·4 | 3·2 | 1·8 | |
| 2 | – | nd | 0·7 | 0·1 | 3·3 | 0·1 | 0·0 | 0·2 | 0·1 | 0·4 | 5·0 | 1·0 | 0·0 | ||
| 3 | + | A,T | 7·8 | 7·8 | 1·0 | 4·4 | 2·3 | 0·9 | 1·7 | 0·7 | 1·1 | 5·9 | 1·6 | 1·0 | |
| 7 | 1 | + | – | 8·7 | 7·5 | 1·0 | 7·0 | 0·5 | 0·5 | 2·3 | 0·0 | 0·9 | 7·3 | 2·2 | 0·2 |
| 2 | + | n.d. | 9·4 | 6·4 | 0·8 | 6·6 | 1·1 | 0·9 | 2·7 | 0·7 | 1·9 | 6·0 | 3·5 | 0·3 | |
| 3 | – | 8·0 | 1·6 | 1·5 | 7·8 | 0·0 | 0·2 | 2·9 | 0·6 | 0·5 | 6·6 | 0·0 | 0·1 | ||
| 8 | 1 | – | 8·8 | 3·9 | 1·1 | 2·1 | 0·4 | 0·7 | 2·0 | 0·2 | 1·2 | 5·8 | 3·5 | 0·6 | |
| 2 | – | 7·8 | 7·3 | 0·7 | 3·6 | 0·0 | 0·2 | 1·9 | 0·2 | 0·3 | 0·1 | 0·1 | 0·5 | ||
| 3 | – | 8·7 | 6·1 | 1·2 | 3·9 | 2·4 | 0·7 | 1·7 | 1·3 | 1·3 | 5·9 | 0·0 | 0·4 | ||
| 9 | 1 | + | SEC | 10·0 | 7·3 | 1·5 | 5·4 | 0·9 | 1·0 | 2·2 | 2·6 | 1·5 | 3·7 | 2·3 | 0·5 |
| 2 | + | SEC | 10·3 | 6·0 | 1·3 | 5·6 | 0·1 | 0·7 | 0·1 | 0·2 | 1·6 | 4·8 | 2·5 | 0·0 | |
| 3 | + | SEC | 9·0 | 6·8 | 1·0 | 5·2 | 3·2 | 0·3 | 1·5 | 0·0 | 1·0 | 4·7 | 0·0 | 9·7 | |
| 10 | 1 | + | – | 8·8 | 9·8 | 1·2 | 4·2 | 4·2 | 0·4 | 1·9 | 2·6 | 0·7 | 5·2 | 2·3 | 0·2 |
| 2 | + | A, B | 4·4 | 4·8 | 1·4 | 3·2 | 2·0 | 0·0 | 1·1 | 0·2 | 1·4 | 3·5 | 0·9 | 0·3 | |
| 3 | + | – | 8·5 | 7·2 | 1·5 | 4·6 | 0·1 | 0·3 | 2·5 | 0·2 | 0·9 | 2·7 | 0·2 | 0·8 |
The three time-points of assessment are listed in chronological sequence. Bold type denotes T cell expansions. Underlined numbers indicate expansions that correspond to the SAg present at that moment. Abbreviations: S.a., S. aureus; n.d., not done, A, SEA; B, SEB; T, TSST-1. (a) CD4 subset. (b) CD8 subset.
Cotrimoxazole
Table 2b.
S. aureus and SAg carriage in WG patients in relation to Vβ expansion in a longitudinal study: CD8 subset
| Pat | Time-point | S.a. | SAg | BV2 | BV5/1 | BV5/3 | BV8 | BV9 | BV11 | BV12 | BV13 | BV16 | BV17 | BV21 | BV23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | + | – | 2·6 | 1·2 | 0·8 | 3·3 | 0·4 | 0·2 | 1·4 | 0·2 | 0·3 | 3·3 | 1·6 | 0·5 |
| 2 | + | n.d. | 1·6 | 2·3 | 0·1 | 3·4 | 0·1 | 0·1 | 0·8 | 0·1 | 0·3 | 3·4 | 1·7 | 0·5 | |
| 3 | – | 2·8 | 2·2 | 0·4 | 2·2 | 1·9 | 0·0 | 1·6 | 0·2 | 0·4 | 4·5 | 0·2 | 3·5 | ||
| 2 | 1 | + | SEB | 11·8 | 1·2 | 0·2 | 3·2 | 0·3 | 0·4 | 0·4 | 2·5 | 2·4 | 1·2 | 0·4 | 1·2 |
| 2 | – | 11·7 | 1·8 | 0·2 | 3·5 | n.d. | 0·1 | 0·7 | 5·3 | 1·1 | 1·1 | 0·8 | 1·0 | ||
| 3 | + | – | 11·7 | 1·6 | 0·1 | 1·9 | 2·9 | 0·8 | 0·2 | 4·7 | 1·9 | 0·9 | 1·00 | 1·0 | |
| 3 | 1 | + | – | 3·0 | 2·2 | 1·4 | 5·2 | 0·0 | 1·0 | 1·3 | 0·3 | 0·7 | 2·6 | 0·6 | 3·5 |
| 2 | + | – | 2·5 | 0·7 | 2·3 | 6·4 | 0·5 | 0·0 | 0·8 | n.d. | 0·3 | 1·5 | n.d. | n.d. | |
| 3 | + | – | 6·6 | 1·5 | 0·8 | 6·8 | 0·4 | 0·1 | 0·6 | 0·2 | 0·5 | 1·6 | 0·1 | 1·6 | |
| 4 | 1 | + | SEC | 5·1 | 3·2 | 1·2 | 1·7 | 0·1 | 0·1 | 0·7 | 2·7 | 0·6 | 2·9 | n.d. | n.d. |
| 2 | + | SEC | 5·6 | 2·8 | 0·8 | 1·8 | 1·0 | 0·3 | 0·8 | 2·7 | 0·6 | 2·6 | 9·5 | 1·4 | |
| 3 | – | 6·2 | 3·2 | 1·3 | 2·0 | 0·0 | 0·1 | 0·6 | 0·3 | 0·4 | 3·3 | 8·2 | 1·6 | ||
| 5 | 1 | + | – | 4·7 | 1·5 | 0·7 | 11·2 | 2·0 | 0·1 | 1·1 | 0·3 | 0·5 | 8·3 | 0·1 | 5·1 |
| 2 | + | – | 4·7 | 1·6 | 0·8 | 11·2 | 1·2 | 0·6 | 0·7 | 0·9 | 1·4 | 8·2 | 0·3 | 0·8 | |
| 3 | + | – | 5·7 | 1·9 | 0·7 | 12·9 | 0·1 | 0·3 | 1·0 | 0·4 | 0·3 | 7·9 | 9·3 | 1·5 | |
| 6 | 1 | – | 9·6 | 3·6 | 0·4 | 4·4 | 2·6 | 0·4 | 5·5 | 0·2 | 0·8 | 2·4 | 0·6 | 1·3 | |
| 2 | – | 5·0 | 2·3 | 1·8 | 5·7 | 1·4 | 1·0 | 1·5 | 1·6 | 1·4 | 4·3 | 2·8 | 2·3 | ||
| 3 | + | A,T | 3·7 | 2·1 | 0·4 | 1·1 | 1·3 | 0·2 | 1·6 | 0·3 | 0·7 | 4·0 | 1·5 | 2·7 | |
| 7 | 1 | + | – | 2·8 | 1·7 | 0·4 | 3·1 | 0·2 | 0·3 | 0·5 | 0·1 | 1·0 | 3·4 | 2·2 | 0·2 |
| 2 | + | n.d. | 3·5 | 0·8 | 0·1 | 3·3 | 0·1 | 0·0 | 0·2 | n.d. | 0·9 | 3·5 | n.d. | n.d. | |
| 3 | – | 2·3 | 0·9 | 1·1 | 1·8 | 0·3 | 0·1 | n.d | 0·2 | 0·5 | 2·3 | 0·0 | 0·1 | ||
| 8 | 1 | – | 3·2 | 0·1 | 0·6 | 3·2 | 0·2 | 0·0 | 1·0 | 2·4 | 0·6 | 1·8 | 1·7 | 0·1 | |
| 2 | – | 3·4 | 1·4 | 0·4 | 1·8 | 0·1 | 0·2 | 0·7 | 0·1 | 0·7 | 0·8 | 0·1 | 0·8 | ||
| 3 | – | 3·3 | 0·9 | 0·3 | 1·5 | 4·3 | 0·0 | 0·9 | 0·4 | 1·9 | 8·0 | 0·0 | 1·5 | ||
| 9 | 1 | + | SEC | 5·3 | 2·2 | 3·3 | 4·3 | 3·0 | 1·8 | 3·0 | 2·3 | 3·7 | 5·9 | 3·3 | 1·8 |
| 2 | + | SEC | 5·9 | 2·7 | 0·1 | 4·7 | 0·4 | 0·9 | 0·3 | 0·3 | 0·8 | 5·3 | 2·9 | 0·4 | |
| 3 | + | SEC | 5·3 | 3·1 | 0·1 | 4·4 | 1·3 | 0·2 | 3·0 | 0·0 | 0·9 | 5·1 | 0·0 | 5·1 | |
| 10 | 1 | + | – | 9·7 | 2·6 | 0·9 | 4·4 | 3·5 | 0·1 | 1·6 | 0·3 | 0·2 | 5·9 | 3·1 | 2·5 |
| 2 | + | A, B | 3·5 | 6·6 | 1·6 | 2·7 | 1·7 | 0·4 | 1·0 | 0·1 | 0·6 | 2·8 | 1·4 | 0·6 | |
| 3 | + | – | 5·2 | 2·9 | 1·1 | 4·2 | 0·0 | 0·3 | 1·8 | 0·1 | 0·8 | 2·7 | 0·1 | 2·1 |
The percentages of T cells expressing the SAg-binding TCR Vβ chains BV2S1 (binding TSST-1, ETA), BV5S3 (binding SEA), BV17S1 (binding SEB, SEC) and the control BV21S3 were assessed separately for the CD4 and CD8 T cell subsets. Bold type denotes expansions. Abbreviations: S. a., S. aureus; n.d., not done.
Acknowledgments
We thank the Department of Microbiology for performing the microbiological assays and N. van Dijk, B van der Meer and W. Postma for technical help. This study was supported by the Dutch Kidney Foundation, grant no. C-97–1627.
REFERENCES
- 1.Cohen Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Int Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 2.Nolle B, Specks U, Ludemann J, et al. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener granulomatosis. Ann Int Med. 1989;111:28–40. doi: 10.7326/0003-4819-111-1-28. [DOI] [PubMed] [Google Scholar]
- 3.Boomsma MM, Stegeman CA, van der Leij MJ, et al. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Cohen Tervaert JW, Popa ER, Bos NA. The role of superantigens in vasculitis. Curr Opin Rheumatol. 1999;11:24–33. doi: 10.1097/00002281-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Csernok E, Muller A, Gross WL. Immunopathology of ANCA-associated vasculitis. Intern Med. 1999;38:759–65. [PubMed] [Google Scholar]
- 6.Stegeman CA, Cohen Tervaert JW, Sluiter WJ, et al. Association of chronic nasal carriage of S. aureus and higher relapse rates in Wegener's granulomatosis. Ann Int Med. 1994;120:12–7. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Stegeman CA, Cohen Tervaert JW, de Jong PE, et al. Trimethoprim–sulfamethoxazole for the prevention of relapses of Wegener's granulomatosis. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 8.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–11. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 9.Dellabona P, Peccoud J, Kappler J, et al. Superantigens interact with MHC class II molecules outside the antigen groove. Cell. 1990;62:1115–21. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 10.Popa ER, Stegeman CA, Bos NA, et al. Differential B- and T-cell activation in Wegener's granulomatosis. J Allergy Clin Immunol. 1999;103:885–94. doi: 10.1016/s0091-6749(99)70434-3. [DOI] [PubMed] [Google Scholar]
- 11.Schlesier M, Kaspar T, Gutfleisch J, et al. Activated CD4+ and CD8+ T-cell subsets in Wegener's granulomatosis. Rheumatol Int. 1995;14:213–9. doi: 10.1007/BF00262300. [DOI] [PubMed] [Google Scholar]
- 12.Cohen Tervaert JW, Stegeman CA, Manson WL, et al. Staphylococcus aureus superantigens: a risk factor for disease reactivation in Wegener's granulomatosis. FASEB J. 1998;12:A488. [Google Scholar]
- 13.Choi Y, Kotzin B, Herron L, et al. Interaction of Staphylococcus aureus toxin ‘superantigens’ with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–5. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischer B, Necker A, Leget C, et al. Reactivity of mouse T-cell hybridomas expressing human Vβ gene segments with staphylococcal and streptococcal superantigens. Inf Immunity. 1996;64:987–99. doi: 10.1128/iai.64.3.987-994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappler J, Kotzin B, Herron L, et al. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–3. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 16.Hudson KR, Robinson H, Fraser JD. Two adjacent residues in staphylococcal enterotoxins A and E determine T cell receptor V beta specificity. J Exp Med. 1993;177:175–84. doi: 10.1084/jem.177.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 18.Cohen Tervaert JW, Goldschmeding R, Elema JD, et al. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 1990;37:799–806. doi: 10.1038/ki.1990.48. [DOI] [PubMed] [Google Scholar]
- 19.Boom R, Sol CJA, Salimans MMM, et al. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson WM, Tyler SD, Ewan EP, et al. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–30. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallenberg CGM, Brouwer E, Mulder AHL, et al. ANCA-pathophysiology revisited. Clin Exp Immunol. 1995;100:1–3. doi: 10.1111/j.1365-2249.1995.tb03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeringa P, Brouwer E, Cohen Tervaert JW, et al. Animal models of antineutrophil cytoplasmic antibody associated vasculitis. Kidney Int. 1998;53:253–6. doi: 10.1046/j.1523-1755.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 23.Friedman SM, Posnett DN, Tumang JR, et al. A potential role for microbial superantigens in the pathogenesis of systemic autoimmune disease. Arthritis Rheum. 1991;34:468–80. doi: 10.1002/art.1780340412. [DOI] [PubMed] [Google Scholar]
- 24.He X, Goronzy J, Weyand C. Selective induction of rheumatoid factors by superantigens and human helper T cells. J Clin Invest. 1991;89:673–80. doi: 10.1172/JCI115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe J, Kotzin BL, Jujo K, et al. Selective expansion of T-cell receptor variable regions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4966–070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashiro Y, Nagata S, Oguchi S, et al. Selective increase of Vβ2+ T cells in the small intestinal mucosal in Kawasaki disease. Pediatr Res. 1996;39:264–6. doi: 10.1203/00006450-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Leung DYM, Meissner CM, Fulton DR, et al. Toxic shock syndrome-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1993;342:1385–8. doi: 10.1016/0140-6736(93)92752-f. [DOI] [PubMed] [Google Scholar]
- 28.Leung DYM, Sullivan KE, Brown-Whitehorn TF, et al. Association of toxic shock syndrome toxin × secreting and exfoliative toxin-secreting Staphylococcus aureus with Kawasaki syndrome complicated by coronary artery disease. Pediatric Res. 1997;42:268–72. doi: 10.1203/00006450-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Simpson IJ, Skinner MA, Geursen A, et al. Peripheral blood T lymphocytes in systemic vasculitis: increased T cell receptor Vβ2 gene usage in microscopic polyarteritis. Clin Exp Immunol. 1995;101:220–6. doi: 10.1111/j.1365-2249.1995.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giscombe R, Grunewald J, Nityanand S, et al. T cell receptor (TCR) V gene usage in patients with systemic necrotizing vasculitis. Clin Exp Immunol. 1995;101:213–9. doi: 10.1111/j.1365-2249.1995.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grunewald J, Halapi E, Wahlstrom J, et al. T-cell expansions with conserved T-cell receptor beta chain motifs in the peripheral blood of HLA-DRB1*0401 positive patients with necrotizing vasculitis. Blood. 1998;92:3737–44. [PubMed] [Google Scholar]
- 32.Betley MJ, Mekalanos JJ. Staphylococcal enterotoxin A is encoded by phage. Science. 1985;229:185–7. doi: 10.1126/science.3160112. [DOI] [PubMed] [Google Scholar]
- 33.Couch JL, Soltis MT, Betley MJ. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988;170:3954–60. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafer WM, Iandolo JJ. Chromosomal locus for staphylococcus enterotoxin B. Infect Immun. 1978;20:273–8. doi: 10.1128/iai.20.1.273-278.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann T, Baschieri S, Lees RK, et al. In vivo responses of CD4+ and CD8+ cells to bacterial superantigens. Eur J Immunol. 1992;22:1935–8. doi: 10.1002/eji.1830220739. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann T, MacDonald HR. The CD8 T cell response to staphylococcal enterotoxins. Semin Immunol. 1993;5:33–9. doi: 10.1006/smim.1993.1005. [DOI] [PubMed] [Google Scholar]
- 37.Miwa K, Fukuyama M, Kunimoto T, et al. Rapid assay for detection of toxic shock syndrome toxin 1 from human sera. J Clin Microbiol. 1994;32:539–42. doi: 10.1128/jcm.32.2.539-542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study group. N Engl J Med. 2001;344:11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 39.Bonventre PF, Linnemann C, Weckbach LS, et al. Antibody responses to toxic-shock-syndrome (TSS) toxin by patients with TSS and by healthy staphylococcal carriers. J Infect Dis. 1984;150:662–6. doi: 10.1093/infdis/150.5.662. [DOI] [PubMed] [Google Scholar]
- 40.Whiting JL, Rosten PM, Chow AW. Determination by western blot (immunoblot) of seroconversions to toxic shock syndrome (TSS) toxin 1 and enterotoxin A, B, or C during infection with TSS- and non-TSS-associated Staphylococcus aureus. Infect Immun. 1989;57:231–4. doi: 10.1128/iai.57.1.231-234.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanclerski K, Soderquist B, Kjellgren M, et al. Serum antibody response to Staphylococcus aureus enterotoxins and TSST-1 in patients with septicemia. J Med Microbiol. 1996;44:171–7. doi: 10.1099/00222615-44-3-171. [DOI] [PubMed] [Google Scholar]
- 42.Origuchi T, Eguchi K, Kawabe Y, et al. Increased levels of serum IgM antibody to staphylococcal enterotoxins in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:713–20. doi: 10.1136/ard.54.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritz HL, Kirkland JJ, Bond GG, et al. Association of high levels of serum antibody to staphylococcal toxic shock antigen with nasal carriage of toxic shock antigen-producing strains of Staphylococcus aureus. Infect Immun. 1984;43:954–8. doi: 10.1128/iai.43.3.954-958.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiffenabuer J, Hohnson H, Soos J. Superantigens in autoimmunity. Their role as etiologic and therapeutic agents. In: Leung DYM, Huber BT, Schlievert PM, editors. Superantigens: molecular biology, immunology and relevance to human disease. New York: Marcel Dekker; 1997. pp. 525–49. [Google Scholar]
- 45.Silverman GJ. B-cell superantigens. Immunol Today. 1997;18:379–86. doi: 10.1016/s0167-5699(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 46.Yousif Y, Schiltz E, Okada K, et al. Staphylococcal neutral phosphatase. APMIS. 1994;102:891–900. [PubMed] [Google Scholar]
- 47.Jahreis A, Yousif Y, Rump JA, et al. Two novel cationic staphylococcal proteins induce IL-2 secretion, proliferation and immunoglobulins synthesis in peripheral blood mononuclear cells (PBMC) of both healthy controls and patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1995;100:406–11. doi: 10.1111/j.1365-2249.1995.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]