Abstract
The objective of this study was to investigate the effect of the oral administration of type II collagen (CII) on pro-inflammatory mediator production by synoviocytes in rats with adjuvant arthritis (AA). Sprague-Dawley rats were fed with bovine CII either before immunization with Complete Freund's adjuvant (CFA) or after initiation of arthritis. Hind paw secondary swelling was measured and synoviocytes were harvested. Sera from portal vein of oral tolerized rats were collected and in vitro synoviocytes culture or synoviocytes-Peyer's Patches (PP) cells coculture system were developed. Interleukin (IL)-1 activity was measured by a mouse thymocyte activation assayed by MTT dye reduction and tumour necrosis factor (TNF) activity was measured by an L929 cytotoxicity bioassay. Nitric oxide (NO) and malondialdehyde (MDA) levels were measured by biochemical methods. We found that feeding with CII (5, 50 and 500 µg/kg) for 7 days before immunization significantly suppressed hind paw secondary swelling measured at day 16, 20, 24 and 28 (all P < 0·01) and pro-inflammatory mediator (IL-1, TNF, NO and MDA) production by synoviocytes (all P < 0·01) in rats with AA. Feeding with CII (5, 50 and 500 µg/kg) for 7 days after initiation of arthritis had a similar effect. CII (1, 10, 100 µg/ml) had no effect on IL-1 and TNF production by synoviocytes in vitro, but CII 10 µg/ml suppressed IL-1 and TNF production by synoviocytes-PP cells coculture system (P < 0·01), which was antagonized by anti-TGF-β antibody (10 µg/ml) (P < 0·01). Portal serum (1 : 10) from oral tolerized rats suppressed IL-1 and TNF production by synoviocytes (P < 0·01), which was also antagonized by anti-TGF-β antibody (10 µg/ml) (P < 0·01). We conclude that oral administration of CII had prophylactic and therapeutic effects on AA and over-production of IL-1, TNF, NO and MDA by synoviocytes was suppressed. Bystander active suppression may be the main mechanism of oral CII in the suppression of synoviocyte function.
Keywords: type II collagen, oral tolerance, adjuvant arthritis, synoviocyte, TGF-β
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease characterized by hyperplasia of the synovium with resultant cartilage destruction. Proliferation of the synovium is associated with increased production of pro-inflammatory cytokines, mainly interleukin (IL)-1 and tumour necrosis factor (TNF), which play crucial roles in cartilage degradation [1,2]. Nitric oxide (NO) and oxygen radials also play an important role in chondrocyte catabolic activity [3]. Therefore, suppression of production of these pro-inflammatory mediators by synovial cells may be an important target of therapy for RA.
Oral tolerance, a state of immunological unresponsiveness induced by oral administration of antigen, has posed intriguing possibilities for the treatment of autoimmune diseases including RA, multiple sclerosis and insulin dependent diabetes [4–8]. Oral tolerance has been applied to prevent and treat autoimmune disease in several animal models including arthritis. Oral administration of type II collagen (CII) has been shown to suppress collagen [9–11], adjuvant [12], antigen [13] and pristane [14] induced arthritis in mice and rats. However, the precise mechanisms of oral tolerance are not fully known. Studies of experimental autoimmune encephalomyelitis (EAE) have shown that oral tolerance of myelin basic protein (MBP) was associated with down-regulation of pro-inflammatory cytokines such as IL-1 and TNF expressions and up-regulation of ssuppressive cytokines such as transforming growth factor (TGF)-βand IL-4 expressions in brain tissue [15]. It is generally agreed that the regulation of mucosally induced tolerance involves several mechanisms, including active suppression induced by low doses of antigen and clonal anergy or deletion induced by high doses of antigen [4–8,16]. Cells from Peyer's patches (PP) in the gut-associated lymphoid tissue (GALT) and TGF-β are reported to mediate the induction of active suppression [17,18]. But there are few reports about the mechanisms of mucosal tolerance to CII in animal models of arthritis [11–13,19–22], especially its effect on synoviocytes.
Adjuvant arthritis (AA) is a commonly used model of human RA with an incidence of around 90%, which makes it ideal to investigate anti-inflammatory effects. In the present study therefore we tested the effect of oral administration of CII on pro-inflammatory mediator production by synoviocytes in rats with AA.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley (SD) rats weighing 150–190 g were obtained from Animal Centre, Anhui Medical University (China). They were housed five per cage and fed a standard laboratory chow and water ad libitum. Inbred C57BL/6 J mice weighing 16–20 g were also obtained from this Animal Centre.
Induction and evaluation of AA
Complete Freund's adjuvant (CFA) was prepared by suspending heat-killed Mycobacterium tuberculosis (MT) in liquid paraffin at 10 mg/ml. AA was induced by a single intradermal injection of 100 µl of CFA into the left hind paw. At day 0, 16, 20, 24 and 28 after immunization, the right hind paw volume was measured with a water replacement plethsmometer (Mukomachi Kiai CD, Japan). Paw swelling (ml) was calculated by subtracting paw volume at day 0 from that at day 16, 20, 24 and 28 respectively.
Oral administration of CII
In the pretreatment protocol, rats were fed daily doses of 5, 50 or 500 µg/kg of bovine CII (provided by Qilu Pharmaceutical Company, China) dissolved in 0·01 M acetic acid using an 18-gauge stainless steel animal-feeding needle for 7 days prior to immunization. Control rats were fed the same volume of vehicle (0·01 M acetic acid) only. In the treatment protocol, rats were treated orally with above doses of CII or vehicle for 7 days from day 13 after immunization, since paw swelling appeared on days 10–13.
Synovial cell isolation and cultures
Synovial cells (synoviocytes) were isolated as described [23,24]. After measurement of paw swelling, rats were killed at day 30 after immunization, and the synovial tissue from right ankle was excised. The synovial membranes were minced aseptically, then dissociated enzymatically using collagenase (4·0 mg/ml, Sigma, St Louis, MO, USA) in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Grand Island, NY, USA) for 4 h at 37°C. After centrifugation for 10 min at 500 g, cells were resuspended in DMEM with 2 mM L-glutamine, 100 U/ml penicillin, 50 µg/ml gentamicin, 20 nM HEPES buffer, and 10% fetal calf serum (FCS). Cells were cultured in 50 ml culture dishes in humidified 5% CO2 atmosphere at 37°C. After adherence for 18 h, cells were washed thoroughly with phosphate-buffered saline (PBS) solution. Adherent synovial cells were removed by adding trypsin-EDTA followed by washing with PBS containing 2% FCS. Synovial cells collected from different groups were used in 4–5 passages for subsequent experiments, when they showed fibroblastoid morphology. They (5 × 105/well) were cultured for 48 h with 5 µg/ml lipopolysaccharide (LPS, Sigma) and the supernatants were collected for measurement.
Serum collection from oral tolerized rats
Rats were treated orally with CII 5, 50 or 500 µg/kg or vehicle daily for 7 days. Two days later, blood samples were obtained from portal vein while rats were under light ether anaesthesia. After centrifugation, pooled serum was added to culture medium of synovial cells, and the dilution of 1 : 10 was found to be most effective on synoviocyte function.
In vitro experiments
Synovial cells obtained from AA rats were used in 4–5 passages for subsequent in vitro experiments. Cells (5 × 105/well) were cultured for 48 h in 24-well plates containing DMEM with 10% FCS and 5 µg/ml LPS in absence or presence of CII (1, 10, 100 µg/ml), 1 : 10 serum (from untreated or tolerized rats) or anti-TGF-β antibody (R & D Systems, Minneapolis, MN, USA; 10 µg/ml) in humidified 5% CO2 atmosphere at 37°C. Supernatants were collected for measurement.
Peyer's patches (PP) were harvested from normal rats and pooled, minced into single cell suspensions though nylon mesh in DMEM, and washed 3 times. Then PP cells (106/well) were cocultured with synovial cells(5 × 105/well) for 48 h in 24-well plates containing DMEM with 10% FCS and 5 µg/ml LPS in absence or presence of CII (10 µg/ml) or anti-TGF-β antibody (10 µg/ml) in humidified 5% CO2 atmosphere at 37°C. Supernatants were collected for measurement.
IL-1 activity bioassay
IL-1 activity was measured by a mouse thymocyte activation assayed by MTT dye reduction [25,26]. The tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma) was dissolved in sterile PBS to a concentration of 5 mg/ml and stored in the dark at 4°C for up to 1 week. Immediately before use, stock MTT was filtered (0·22 µm) to remove any formazan precipitate. Thymocytes (2 × 106/well) from mice were cultured for 48 h in 96-well plates containing RPMI 1640 medium (Gibco) supplemented with 5 µg/ml concanvalin A (ConA, Sigma) and 0·1 ml collected supernatants in triplicate. Three hours before the termination of culture, cells were pulsed with MTT stock (20 µl/well), returned to 37°C and incubated for an additional 3 h. The plates were centrifuged for 10 min at 1000 g to pellet cells and MTT formazan product. The supernatant was carefully aspirated without disturbing the pellet, and the formazan was solubilized by the addition of isopropanol (100 µl/200 µl supernatant). Insoluble material was then removed by centrifugation for 10 min at 1000 g. The solubilized formazan in isopropanol was collected and distributed into 12-well, flat-bottom ELASA plates at a final volume of 100 µl/well. Plates were read at 570 nm in EL 301 Strip Reader (Bio-Tek, Instruments Inc, Winooski, VT, USA) within 1 h of addition of isopropanol. Values were expressed as mean absorbance (A) of triplicate wells.
TNF bioassay
TNF activity in the supernatants was measured by an L929 cytotoxicity bioassay [27,28]. Briefly, L929 cells (obtained from Department of Immunology, Beijing University) were cultured in RPMI 1640 medium with 5% FCS at 105 cells/well for 24 h in 96-well, flat-bottom plates at 37°C in a 5% CO2 atmosphere. The medium above the nonconfluent cell layer was replaced with fresh medium containing actinomycin D (4 µg/ml, Sigma) and serial dilutions of supernatants to be tested for TNF activity. All samples were tested in triplicate. After a further 24 h incubation the medium was removed and a 0·5% in 20% methanol solution of crystal violet was added to the wells. The absorbance in each well was assessed with a plate reader at 570 nm. Cell lysis was calculated as follows:
A is the absorbance of L929 cells cultured only with complete medium and B is the absorbance of L929 cells cultured with a test samples.
Nitrite estimation
Nitrite concentration represented NO level in the supernatant was measured as described before [29]. Briefly, 100 µl aliquots were removed from collected supernatants and incubated with an equal volume of Griess reagent (1% sulphanilamide/0·1% naphthylethylene diamine dihydrochloride/2·5% H3PO4) at room temperature for 10 min The absorbance at 550 nm was determined in a plate reader. A standard curve was established with a set of serial dilutions of sodium nitrite. All samples were assayed in triplicate. Results were expressed as µmol/l.
MDA estimation
Malondialdehyde (MDA) levels represented lipid peroxidation in supernatants were determined using the method described before [30]. Briefly, MDA was reacted with thibarbituric acid (TBA) by incubating for 1 h at 95–100°C. Following the reaction, absorbance was measured with a spectrophotometry at 535 nm. Results were expressed as µmol/l.
Statistical analysis
Results were expressed as mean ± SD. Student's t-test was used to make comparisons between the groups. P-values < 0·05 were considered statistically significant.
RESULTS
CII oral pretreatment suppresses AA and synoviocyte function
To determine if oral administration of CII has prophylactic effect on AA, AA rats were prefed with vehicle or three doses of CII for 7 days before immunization. As shown in Table 1, AA rats prefed with vehicle had significant hind paw secondary swelling measured at day 16, 20, 24 and 28 after immunization with CFA. Daily pre-feeding with CII 5, 50 and 500 µg/kg significantly suppressed AA at day 16, 20, 24 and 28 after immunization (all P < 0·01) with the most pronounced effects seen in the group fed with 50 µg/kg of CII. The suppression rates ranged between 33·9% and 58·4%.
Table 1.
Inhibitory effects of oral CII pretreatment on hind paw swelling in AA rats
| Paw swelling (ml) | ||||
|---|---|---|---|---|
| group | day16 | day 20 | day 24 | day 28 |
| Normal | 0·10 ± 0·02 | 0·15 ± 0·02 | 0·20 ± 0·02 | 0·23 ± 0·02 |
| AA | 0·46 ± 0·09** | 0·67 ± 0·12** | 0·87 ± 0·14** | 1·05 ± 0·12** |
| CII | ||||
| 5 (µg/kg) | 0·30 ± 0·07‡ (33·9) | 0·37 ± 0·07‡ (49·9) | 0·42 ± 0·08‡ (51·2) | 0·48 ± 0·08‡ (54·1) |
| 50 (µg/kg) | 0·20 ± 0·02‡ (56·1) | 0·30 ± 0·04‡ (55·9) | 0·36 ± 0·04‡ (58·4) | 0·45 ± 0·03‡ (57·5) |
| 500 (µg/kg) | 0·27 ± 0·04‡ (44·3) | 0·38 ± 0·03‡ (44.0) | 0·45 ± 0·02 (47·6) | 0·54 ± 0·02‡ (49.0) |
SD rats were fed with vehicle (0·01 M acetic acid) or different daily doses of bovine CII for 7 days prior to immunization. Adjuvant arthritis (AA) was induced by a single intradermal injection of 100 µl of Complete Freund's Adjuvant into the left hind paw. The right hind paw volume was measured with a water replacement plethsmometer at day 0, 16, 20, 24 and 28 after immunization. Paw swelling (ml) was calculated by taking away the paw volume at day 0 from the relevant one at day 16, 20, 24 and 28. Results are representative of two similar experiments and expressed as mean ± SD, n= 5.
P < 0·01 versus Normal group,
P < 0·01 versus AA group. Numbers in parenthesis represent the percentage inhibition.
To investigate the effects of pre-feeding with CII on synoviocyte function in AA rats, the rats were decapitated at day 30 after immunization and IL-1, TNF, NO and MDA levels produced from synoviocytes were measured. As shown in Table 2, IL-1, TNF, NO and MDA produced from synoviocytes increased significantly in AA rats as compared with those in normal rats (all P < 0·01). Daily pre-feeding of 3 doses of CII had significant inhibitory effects on IL-1, TNF, NO and MDA production from synoviocytes as compared with daily pre-feeding with vehicle (all P < 0·01) in AA rats. The most pronounced effect was seen in the group fed with 50 µg/kg of CII.
Table 2.
Effects of oral CII pretreatment on synoviocyte function in AA rats
| Group | IL-1 (A) | TNF (%) | NO (µmol/l) | MDA (µmol/l) |
|---|---|---|---|---|
| Normal | 0·44 ± 0·05 | 28·92 ± 9·81 | 5·84 ± 1·29 | 0·63 ± 0·18 |
| AA | 0·67 ± 0·07** | 65·54 ± 6·49** | 12·14 ± 1·09** | 1·86 ± 0·27** |
| CII | ||||
| 5 (µg/kg) | 0·48 ± 0·05‡ | 38·46 ± 9·36‡ | 6·89 ± 1·07‡ | 0·96 ± 0·34‡ |
| 50 (µg/kg) | 0·49 ± 0·06‡ | 32·61 ± 8·86‡ | 6·46 ± 0·64‡ | 0·61 ± 0·17‡ |
| 500 (µg/kg) | 0·53 ± 0·05‡ | 39·08 ± 10·12‡ | 6·91 ± 1·39‡ | 0·72 ± 0·22‡ |
SD rats were fed with vehicle (0·01 M acetic acid) or different daily doses of bovine CII for 7 days prior to immunization. Adjuvant arthritis (AA) was induced by a single intradermal injection of 100 µl of Complete Freund's Adjuvant into the left hind paw. At day 30 after immunization, rats were killed and synoviocytes were isolated and cultured. IL-1, TNF, NO and MDA secreted from synoviocytes were measured as described in materials and methods. Results are representative of two similar experiments and expressed as mean ± SD, n= 5.
P < 0·01 versus Normal group,
P < 0·01 versus AA group.
CII oral treatment suppresses AA and synoviocyte function
To test if oral administration of CII has therapeutical effect on AA, AA rats were fed with vehicle or three doses of CII for 7 days from day 13 after immunization. As shown in Table 3, daily oral treatment with CII 5, 50 and 500 µg/kg also significantly suppressed hind paw secondary swelling measured at day 20, 24 and 28 after immunization (all P < 0·01). The suppression rates ranged between 20·2% and 35·8%.
Table 3.
Inhibitory effects of oral CII treatment on hind paw swelling in AA rats
| Paw swelling (ml) | |||
|---|---|---|---|
| group 20 | day 20 | day 24 | day 28 |
| Normal | 0·15 ± 0·04 | 0·23 ± 0·06 | 0·32 ± 0·07 |
| AA | 0·53 ± 0·07** | 0·77 ± 0·03** | 0·94 ± 0·07** |
| CII | |||
| 5 (µg/kg) | 0·43 ± 0·09‡ (20·2) | 0·56 ± 0·04‡ (27·6) | 0·65 ± 0·06‡ (30·5) |
| 50 (µg/kg) | 0·37 ± 0·06‡ (30·9) | 0·57 ± 0·06‡ (20·6) | 0·41 ± 0·08‡ (34·5) |
| 500 (µg/kg) | 0·37 ± 0·05‡ (31·1) | 0·49 ± 0·09‡ (35·8) | 0·62 ± 0·06‡ (34·2) |
Adjuvant arthritis (AA) was induced by a single intradermal injection of 100 µl of Complete Freund's Adjuvant into the left hind paw. Vehicle (0·01 M acetic acid) or different daily doses of bovine CII were fed for 7 days from day 13 after immunization. The right hind paw volume was measured with a water replacement plethsmometer at day 0, 20, 24 and 28 after immunization. Paw swelling (ml) was calculated by taking away the paw volume at day 0 from the relevant one at day 20, 24 and 28. Results are representative of two similar experiments and expressed as mean ± SD, n= 5.
P < 0·01 versus Normal group,
P < 0·01 versus AA group. Numbers in parenthesis represent the percentage inhibition.
To examine the effects of oral treatment with CII on synoviocyte function in AA rats, the rats were decapitated at day 30 after immunization and IL-1, TNF, NO and MDA produced from synoviocytes were measured. As shown in Table 4, daily feeding of 3 doses of CII had significant inhibitory effects on IL-1, TNF, NO and MDA production from synoviocytes as compared with daily feeding with vehicle (all P < 0·01) in AA rats. The most pronounced effect was also seen in the group fed with 50 µg/kg of CII.
Table 4.
Inhibitory effects of oral CII treatment on synoviocyte functions in AA rats
| Group | IL-1 (A) | TNF (%) | NO (µmol/l) | MDA (µmol/l) |
|---|---|---|---|---|
| Normal | 0·46 ± 0·06 | 28·31 ± 9·40 | 7·25 ± 1·38 | 0·80 ± 0·24 |
| AA | 0·69 ± 0·04** | 64·00 ± 11·39** | 13·61 ± 1·56** | 2·19 ± 0·32** |
| CII | ||||
| 5 (µg/kg) | 0·50 ± 0·06‡ | 47·07 ± 5·92† | 8·81 ± 1·24‡ | 1·02 ± 0·28‡ |
| 50 (µg/kg) | 0·47 ± 0·05‡ | 40·31 ± 9·81‡ | 6·74 ± 0·73‡ | 0·81 ± 0·17‡ |
| 500 (µg/kg) | 0·51 ± 0·04‡ | 49·85 ± 5·17‡ | 7·48 ± 0·68‡ | 0·93 ± 0·25‡ |
Adjuvant arthritis (AA) was induced by a single intradermal injection of 100 µl of Complete Freund's Adjuvant into the left hind paw. Vehicle (0·01 M acetic acid) or different daily doses of bovine CII were fed for 7 days from day 13 after immunization. At day 30 after immunization, rats were killed and synoviocytes were isolated and cultured. IL-1, TNF, NO and MDA secreted from synoviocytes were measured as described in materials and methods. Results are representative of two similar experiments and expressed as mean ± SD, n= 5.
P < 0·01 versus Normal group,
P < 0·05,‡P < 0·01 versus AA group.
CII suppresses synoviocyte function via PP cells and TGF-β in vitro
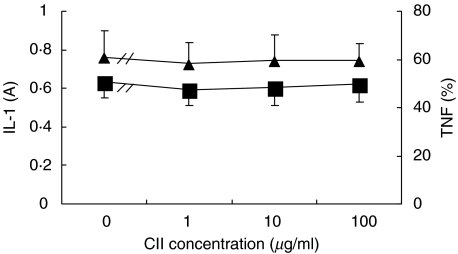
Experiments were performed to test if CII has a direct effect on synoviocytes, in vitro. As shown in Fig. 1, CII 1, 10, 100 µg/ml had no effects on IL-1 and TNF production by synoviocytes of AA rats in vitro.
Fig. 1.
Effects of CII on IL-1 (▪) and TNF (▴) production by synoviocytes of AA rats in vitro. Synovial cells (5 × 105/well) obtained from AA rats on day 30 after immunization were cultured for 48 h in 24-well plates containing DMEM with 10% FCS and 5 µg/ml LPS in absence or presence of CII (1, 10, 100 µg/ml). Supernatants were collected for IL-1 and TNF measurement. Results are expressed as mean ± SD of 5 independent experiments.
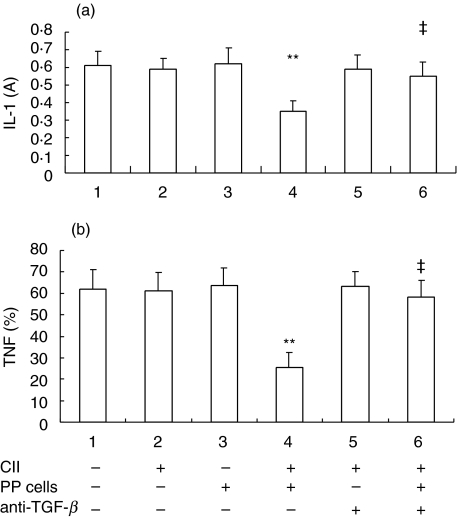
As CII has been shown to stimulate lymphocyte proliferation in vitro[31, Ding CH et al. unpublished data], we investigated whether CII acts on synoviocytes via lymphocytes and TGF-β secreted by Th3 cells. As shown in Fig. 2, CII 10 µg/ml had no effect on IL-1 and TNF production from synoviocytes of AA rats in vitro (group 2 versus group 1, P > 0·05). PP cells (106/well) from normal rats also had no effect on IL-1 and TNF production when cocultured with synoviocytes (5 × 105/well) from AA rats (group 3 versus group 1, P > 0·05). However, CII 10 µg/ml suppressed IL-1 and TNF production significantly in the coculture system (group 4 versus group 2 and 3, P < 0·01). Meanwhile, anti-TGF-β antibody (10 µg/ml) was added and it was demonstrated that anti-TGF-β, which had no effect on IL-1 and TNF production by synoviocytes (group 5 versus group 2, P > 0·05), significantly antagonized the suppressive effects of CII on IL-1 and TNF production in the coculture system (group 6 versus group 4, P < 0·01).
Fig. 2.
Effects of CII on (a) IL-1 and (b) TNF production by synoviocytes cocultured with PP cells in vitro and antagonism of anti-TGF-β antibody. Peyer's patches (PP) were harvested from normal rats and synovial cells were obtained from AA rats on day 30 after immunization. PP cells (106/well) were cocultured with synovial cells (5 × 105/well) for 48 h in 24-well plates containing DMEM with 10% FCS and 5 µg/ml LPS in absence or presence of CII (10 µg/ml) or anti-TGF-β antibody (10 µg/ml). Supernatants were collected for IL-1 and TNF measurement. ‘+’ represents ‘added’, ‘–’ represents ‘not added’. Results are expressed as mean ± SD of 5 independent experiments. **P < 0·01 versus group 2, ‡P < 0·01 versus group 4.
Serum from oral tolerized rats suppresses synoviocyte function in vitro
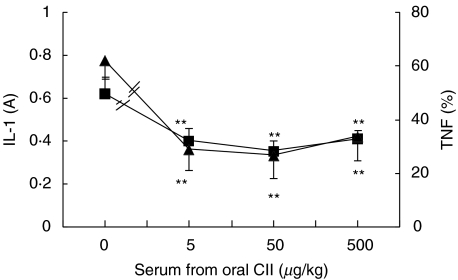
To determine if the suppressive effects of oral administration of CII on synoviocyte function are through blood mediators, we collected the portal vein sera from the rats fed with CII 5, 50 or 500 µg/kg or vehicle (as a control) daily for 7 days and tested the effects of these sera on synoviocyte function in vitro. As shown in Fig. 3, as compared with serum (1 : 10) from rats fed with vehicle, serum (1 : 10) from rats fed with CII 5, 50 or 500 µg/kg significantly inhibited IL-1 and TNF production from synoviocytes of AA rats (all P < 0·01).
Fig. 3.
Effects of serum from oral tolerized rats on IL-1 (▪) and TNF (▴) production by synoviocytes of AA rats in vitro. Sera in portal vein were obtained from rats treated orally with CII 5, 50 or 500 µg/kg daily or vehicle for 7 days. Synovial cells (5 × 105/well) obtained from AA rats were cultured for 48 h in 24-well plates containing DMEM with 10% FCS and 5 µg/ml LPS in absence or presence of the different serum (1 : 10). Supernatants were collected for IL-1 and TNF measurement. Results are expressed as mean ± SD of 5 independent experiments. **P < 0·01 versus cells treated with serum from vehicle-fed rats.
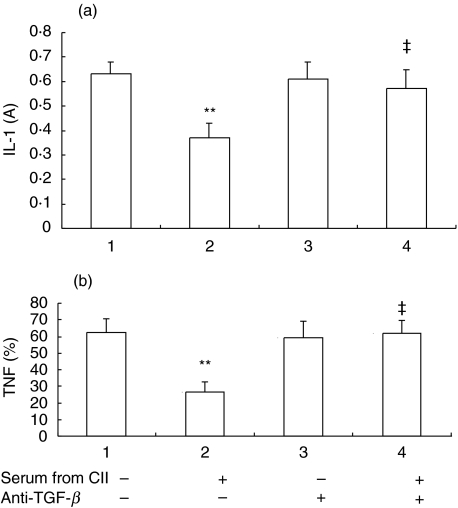
Furthermore, to test if the inhibitory effects of these sera operate via TGF-β in blood, we added anti-TGF-β antibody (10 µg/ml) together with serum from rats fed with CII 50 µg/kg or vehicle into the synoviocytes culture medium. As shown in Fig. 4, serum (1 : 10) from rats fed with CII 50 µg/kg had inhibitory effects on IL-1 and TNF production from synoviocytes of AA rats (group 2 versus group 1, P < 0·01), which antagonized by anti-TGF-β (group 4 versus group 2, P < 0·01).
Fig. 4.
Antagonism of anti-TGF-β antibody against the effects of serum from oral tolerized rats on IL-1 and TNF production by synoviocytes of AA rats in vitro. Sera in portal vein were obtained from rats treated orally with CII 50 µg/kg daily or vehicle for 7 days. Synovial cells (5 × 105/well) obtained from AA rats were cultured for 48 h in 24-well plates containing DMEM with 10% FCS and 5 µg/ml LPS in absence or presence of the different serum (1 : 10) or anti-TGF-β antibody (10 µg/ml). Supernatants were collected for IL-1 and TNF measurement. ‘+’ represents ‘added’, ‘–’ represents ‘not added’. Results are expressed as mean ± SD of 5 independent experiments. **P < 0·01 versus group 1, ‡P < 0·01 versus group 2.
DISCUSSION
Our study demonstrated that oral CII pre-feeding or feeding suppressed secondary hind paw swelling and pre-inflammatory mediators production in AA. CII had no direct effect on synoviocyte function in vitro, but it could suppress IL-1 and TNF production by synoviocytes via TGF-β secrected by PP cells. Furthermore, portal serum from oral tolerized rats suppressed IL-1 and TNF production by synoviocytes via TGF-β in vitro.
Beginning with collagen-induced arthritis (CIA) in rats [10] and mice [9], oral administration of CII has been found to suppress virtually all experimentally inducible animal models that exist for RA [11–14,32]. Histopathological studies showed that oral administration of CII resulted in reduction of synovial hyperplasia, mononuclear infiltration, pannus formation, and cartilage erosions [14]. In AA models, using Lewis rats weighing between 125 and 150 g, daily doses of 3 or 30 µg of native chicken CII abrogated the disease to a statistically significant degree, whereas higher doses or a dose of 0·3 µg/day were ineffective. Both pre-feeding and feeding suppressed the disease of AA [12]. In our present study, using 150–190 g SD rats, we found that daily oral administration of bovine CII 5, 50 and 500 µg/kg for 7 days either before or after immunization suppressed secondary hind paw swelling of AA. Our results and doses are consistent with the previous findings [12]. Moreover, we demonstrated, for the first time, that oral administration of CII either before or after immunization inhibited the production of pro-inflammatory mediators, i.e. IL-1, TNF, NO and MDA, from synoviocytes in AA. These results suggest that suppression of paw swelling by oral CII may be through suppression of pro-inflammatory mediator production by synoviocytes. This implies that oral administration of CII could modify the disease activity of arthritis, for these pro-inflammatory mediators play crucial roles in the degradation of cartilage in inflammatory arthritis [1–3]. Although the effects of oral administration of CII in the treatment of RA from clinical trials are inconsistent, showing no effect, non significant, or significant improvement in the disease [8,33–36], our present study in AA supports the concept that oral tolerance induction by CII has a potential prospect in modifying disease activity of RA.
So far there has not been a convincing explanation why oral administration of CII suppresses synovial inflammation. In other animal systems a number of studies have pinpointed the ways by which oral tolerance is likely to be achieved [4–8,37–39]. It has been observed in mice and rats that multiple feedings of low doses of a soluble antigen leads to development of active suppression and regulatory T cells (CD4+ Th2 and Th3 cells) in GALT containing PP. Th2 cells secrete IL-4 and IL-10, whereas Th3 cells secrete TGF-β. These regulatory T cells migrate to lymphoid organs and target organs expressing the same antigen. Antigen exposure stimulates cells from these organs to secrete anti-inflammatory cytokines (TGF-β, IL-4, and IL-10). Multiple feedings of low doses of a soluble antigen also induced splenic CD4+ CD25+ regulatory cells to produce the anti-inflammatory cytokines [40,41]. These cytokines down-regulate other T-cells responses in the organ in which the autoimmune attack occurs. The ability to suppress immune response in the microenvironment in an antigen nonspecific manner is known as bystander suppression [4–8,37–39]. In the course of bystander suppression induction, PP cells and TGF-β secreted by Th3 or CD4+ CD25+ cells play important roles. PP cells have been used to transfer tolerance to soluble antigen [42]. CD4+ and CD8+ regulatory cells were detected in PP as early as 6 h following oral antigen in TCR transgenic mice [43]. That PP-null mice lack ovalbumin-specific oral tolerance suggests that PP cells are required in oral tolerance [17]. Similarly, TGF-β is an important mediator of the active component of oral tolerance. Increased production of this cytokine has been reported in a number of models of oral tolerance and TGF-β -secreting Th3 or CD4+ CD25+ T cells can be produced from animals tolerized in this way [4–8,40]. Administration of anti-TGF-β antibody in vivo negates the ability of oral MBP to protect against induction of EAE [18].
The roles of PP cells and TGF-β in oral tolerance were verified by our present in vitro study. We found that CII (1, 10 and 100 µg/ml) had no direct effect on IL-1 and TNF production by synoviocytes. However, when PP cells were added to the culture system, CII 10 µg/ml significantly inhibited IL-1 and TNF production by synoviocytes. This effect of CII was antagonized by anti-TGF-β antibody (10 µg/ml). Our findings strongly suggest that bystander active suppression is the main mechanism of oral tolerance to CII, which is consistent with previous reports [11–13,19–22]. It has been reported that spleen cells, and nylon wool-filtered splenic T cells from CII-induced oral tolerance rats adoptively transferred hyporesponsiveness to normal recipients that were less susceptible to AA or CIA [12,19], suggesting regulatory T cells mediate the bystander active suppression. T cell lines established from CII fed or nasally treated mice showed increased production of IL-2, IL-10 and TGF-β, and decreased production of IFN-γ. Meanwhile, TNF-α and IL-6 mRNA expressions in the joint were diminished by nasal treatment of CII in mice with CIA [11], showing CII induced mucosal tolerance could suppress local inflammation of joint in CIA. Neutralization of IL-4 by an anti-IL-4 antibody prevented the suppression of CIA in mice by oral administration of CII, implying that IL-4 may play a role in its suppression [22]. TGF-β 1 increased the degree of oral tolerance induced by feeding of low-dose CII in mice and augmented the induction of immunoregulatory CD8 T cells, which transferred the resistance to CIA induction to normal recipients [18]. Single administration of PLGA (a carrier for drug delivery systems) entrapping CII showed a higher level of TGF-β mRNA expression in PP, but a lower level of TNF-α mRNA expression in draining lymph nodes in CIA mice [44], suggesting TGF-β plays an important role in OT induction by CII.
Surgical approaches using portacaval shunts showed that the development of oral tolerance is dependent on the hepatic portal circulation [45,46], so we studied the effect of the serum in portal vein from oral tolerized rats. We found that the serum suppressed IL-1 and TNF production by synoviocytes in vitro, which was antagonized by anti-TGF-β antibody. This result suggests that TGF-β level in the hepatic portal circulation may also play a role in suppression of synovial inflammation by oral administration of CII.
In conclusion, oral administration of CII had prophylactic and therapeutic effects on AA and over-production of IL-1, TNF, NO and MDA by synoviocytes was suppressed. CII had no direct effect on synoviocytes, but it could suppress IL-1 and TNF production by synoviocytes via TGF-β secreted by PP cells in vitro. Portal vein serum from oral tolerized rats also suppressed IL-1 and TNF production by synoviocytes via TGF-β in vitro. Our study suggests that bystander active suppression may be the main mechanism of oral tolerance to CII in the suppression of synovial inflammation.
Acknowledgments
This work was awarded Servier Young Investigator Prize in Pharmacology by the Chinese Pharmacological Society and the Institute de Recherches Internationales Servier (France). A special thanks to Ms Lili Zhang who donated CII to us. This work was supported by the Education Committee Foundation of Anhui Province, Research Foundation of Qilu Pharmaceutical Company in China.
REFERENCES
- 1.Arend WP. Cytokines and cellular interactions in inflammatory synovitis. J Clin Invest. 2001;107:1081–2. doi: 10.1172/JCI12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldmann M. Pathogenesis of arthritis: recent research progress. Nat Immunol. 2001;2:771–3. doi: 10.1038/ni0901-771. [DOI] [PubMed] [Google Scholar]
- 3.Mazzetti I, Grigolo B, Pulsatelli L, Dolzani P, Silvestri T, Roseti L, Meliconi R, Facchini A. Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin Sci. 2001;101:593–9. [PubMed] [Google Scholar]
- 4.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–51. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 5.Wardrop RM, III, Whitacre CC. Oral tolerance in the treatment of inflammatory autoimmune diseases. Inflamm Res. 1999;48:106–19. doi: 10.1007/s000110050433. [DOI] [PubMed] [Google Scholar]
- 6.Whitacre CC, Gienapp IE, Meyer A, Cox KL, Javed N. Treatment of autoimmune disease by oral tolerance to autoantigens. Clin Immunol Immunopathol. 1996;80:S31–9. doi: 10.1006/clin.1996.0139. [DOI] [PubMed] [Google Scholar]
- 7.Xiao BG, Link H. Mucosal tolerance. a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85:119–28. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 8.Trentham DE. Oral tolerization as a treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24:525–36. doi: 10.1016/s0889-857x(05)70024-7. [DOI] [PubMed] [Google Scholar]
- 9.Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci USA. 1986;83:7443–6. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson HS, Staines NA. Gastric administration of type II collagen delays the onset and severity of collagen-induced arthritis in rats. Clin Exp Immunol. 1986;64:581–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL. Suppression of collagen-induced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999;13:315–24. doi: 10.1006/jaut.1999.0320. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZY, Lee CS, Lider O, Weiner HL. Suppression of adjuvant arthritis in Lewis rats by oral administration of type II collagen. J Immunol. 1990;145:2489–93. [PubMed] [Google Scholar]
- 13.Yoshino S, Quattrocchi E, Weiner HL. Suppression of antigen-induced arthritis in Lewis rats by oral administration of type II collagen. Arthritis Rheum. 1995;38:1092–6. doi: 10.1002/art.1780380811. [DOI] [PubMed] [Google Scholar]
- 14.Thompson SJ, Thompson HS, Harper N, Day MJ, Coad AJ, Elson CJ, Staines NA. Prevention of pristane-induced arthritis by the oral administration of type II collagen. Immunology. 1993;79:152–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–7. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR. Peyer's patches are required for oral tolerance to proteins. Proc Natl Acad Sci USA. 2001;98:3310–5. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–5. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson HS, Harper N, Bevan DJ, Staines NA. Suppression of collagen induced arthritis by oral administration of type II collagen: changes in immune and arthritic responses mediated by active peripheral suppression. Autoimmunity. 1993;16:189–99. doi: 10.3109/08916939308993327. [DOI] [PubMed] [Google Scholar]
- 20.Thorbecke GJ, Schwarcz R, Leu J, Huang C, Simmons WJ. Modulation by cytokines of induction of oral tolerance to type II collagen. Arthritis Rheum. 1999;42:110–8. doi: 10.1002/1529-0131(199901)42:1<110::AID-ANR14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Bayrak S, Mitchison NA. Bystander suppression of murine collagen-induced arthritis by long-term nasal administration of a self type II collagen peptide. Clin Exp Immunol. 1998;113:92–5. doi: 10.1046/j.1365-2249.1998.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino S. Treatment with an anti-IL-4 monoclonal antibody blocks suppression of collagen-induced arthritis in mice by oral administration of type II collagen. J Immunol. 1998;160:3067–71. [PubMed] [Google Scholar]
- 23.Dechanet J, Briolay J, Rissoan MC, Chomarat P, Galizzi JP, Banchereau J, Miossec P. IL-4 inhibits growth factor-stimulated rheumatoid synoviocyte proliferation by blocking the early phases of the cell cycle. J Immunol. 1993;151:4908–17. [PubMed] [Google Scholar]
- 24.Migita K, Tanaka F, Yamasaki S, et al. Regulation of rheumatoid synoviocyte proliferation by endogenous p53 induction. Clin Exp Immunol. 2001;126:334–8. doi: 10.1046/j.1365-2249.2001.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z, Tu JH, Small CB, Schnipper SM, Rosenstreich DL. Increased urine interleukin-1 levels in aging. Gerontology. 1993;39:19–27. doi: 10.1159/000213510. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Di Giovine FS, Nuki G, Duff GW. Tumour necrosis factor in synovial exudates. Ann Rheum Dis. 1988;47:768–72. doi: 10.1136/ard.47.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojeda Ojeda M, de Silva CVJ, Arana Rosainz M, Fernandez-Ortega C. TNFalpha production in whole blood cultures from healthy individuals. Biochem Biophys Res Commun. 2002;292:538–41. doi: 10.1006/bbrc.2002.6688. [DOI] [PubMed] [Google Scholar]
- 29.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 30.Gavino VC, Miller JS, Ikharebha SO, Milo GE, Cornwell DG. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res. 1981;22:763–9. [PubMed] [Google Scholar]
- 31.Catchpole B, Hamblin AS, Staines NA. T cell lines generated with type II collagen proliferate in an autologous mixed lymphocyte response. J Autoimmun. 2001;17:181–9. doi: 10.1006/jaut.2001.0537. [DOI] [PubMed] [Google Scholar]
- 32.Yoshino S. Downregulation of silicone-induced chronic arthritis by gastric administration of type II collagen. Immunopharmacology. 1995;31:103–8. doi: 10.1016/0162-3109(95)00038-5. [DOI] [PubMed] [Google Scholar]
- 33.Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–30. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 34.Sieper J, Kary S, Sorensen H, et al. Oral type II collagen treatment in early rheumatoid arthritis. A double-blind, placebo-controlled, randomized trial. Arthritis Rheum. 1996;39:41–51. doi: 10.1002/art.1780390106. [DOI] [PubMed] [Google Scholar]
- 35.Barnett ML, Kremer JM, St Clair EW, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290–7. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 36.Choy EH, Scott DL, Kingsley GH, Thomas S, Murphy AG, Staines N, Panayi GS. Control of rheumatoid arthritis by oral tolerance. Arthritis Rheum. 2001;44:1993–7. doi: 10.1002/1529-0131(200109)44:9<1993::AID-ART347>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–73. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 39.Inobe J, Slavin AJ, Komagata Y, Chen Y, Liu L, Weiner HL. IL-4 is a differentiation factor for transforming growth factor-beta secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomyelitis. Eur J Immunol. 1998;28:2780–90. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 40.Strobel S. Oral tolerance, systemic immunoregalation, and autoimmunity. Ann N Y Acad Sci. 2002;958:47–58. doi: 10.1111/j.1749-6632.2002.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 41.Cobelens PM, Kavelaars A, van der Zee R, van Eden W, Heijnen CJ. Dynamics of mycobacterial HSP65-induced T-cell cytokine expression during oral tolerance induction in adjuvant arthritis. Rheumatology. 2002;41:775–9. doi: 10.1093/rheumatology/41.7.775. [DOI] [PubMed] [Google Scholar]
- 42.Santos LM, al-Sabbagh A, Londono A, Weiner HL. Oral tolerance to myelin basic protein induces regulatory TGF-beta-secreting T cells in Peyer's patches of SJL mice. Cell Immunol. 1994;157:439–47. doi: 10.1006/cimm.1994.1240. [DOI] [PubMed] [Google Scholar]
- 43.Gonnella PA, Chen Y, Inobe J, Komagata Y, Quartulli M, Weiner HL. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J Immunol. 1998;160:4708–18. [PubMed] [Google Scholar]
- 44.Kim WU, Lee WK, Ryoo JW, et al. Suppression of collagen-induced arthritis by single administration of poly (lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46:1109–20. doi: 10.1002/art.10198. [DOI] [PubMed] [Google Scholar]
- 45.Callery MP, Kamei T, Flye MW. The effect of portacaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res. 1989;46:391–4. doi: 10.1016/0022-4804(89)90208-4. [DOI] [PubMed] [Google Scholar]
- 46.Yang R, Liu Q, Grosfeld JL, Pescovitz MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg. 1994;29:1145–8. doi: 10.1016/0022-3468(94)90297-6. [DOI] [PubMed] [Google Scholar]