Abstract
Thyroglobulin (Tg) is a target autoantigen in autoimmune thyroid diseases, such as Graves’ disease (GD) and Hashimoto's thyroiditis. In a previous study we identified three 20mer Tg peptides bearing epitopes of autoantibodies associated with GD (TgP15, TgP26 and TgP41: sequences 2339–2358, 2471–2490 and 2651–2670 of human Tg, respectively). In the present study, we investigated the antigenicity of the above peptides in experimental immunization with Tg, the immunogenicity of antigenic peptides and the possibility of intramolecular B-cell epitope spreading during peptide immunization. For this purpose, two rabbits were injected with human Tg in CFA six times, every three weeks. Two control animals were injected only with CFA. Testing of antisera and of affinity-purified antibodies, by ELISA against the three peptides, revealed reactivity only to TgP41. This synthetic peptide was subsequently administered to two rabbits, in its free form (100µg in CFA six times, every two weeks). A strong serological response was developed not only against TgP41, but also to intact human and rabbit Tg. Immunization with TgP41 induced intramolecular B-cell epitope spreading, i.e. production of antibodies to sites on Tg other than that corresponding to TgP41, as revealed by immunoadsorption and competitive ELISA. Histopathological studies did not reveal any infiltration in thyroid glands. We conclude that peptide TgP41 encompasses not only an epitope of disease-associated autoantibodies, but also a dominant immunogenic epitope of experimentally induced Tg-specific antibodies, able to drive B-cell epitope spreading.
Keywords: thyroglobulin, B cell epitopes, epitope spreading, peptides, experimental immunization
INTRODUCTION
Thyroglobulin (Tg) is a large homodimeric iodinated glycoprotein (2 × 330 kD) that serves as a precursor for the thyroid hormones [1]. Autoantibodies to Tg are frequently detected in autoimmune thyroid diseases, Graves’ disease (GD) and Hashimoto's thyroiditis [2] and have been used as serological markers [3]. However, the pathogenic role of anti-Tg antibodies is still debatable [4,5]. Several researchers have characterized epitopes recognized by anti-Tg autoantibodies or by induced anti-Tg antibodies in animals, throughout the Tg sequence [6–10]. Mapping of serological epitopes on Tg has been usually performed using either large recombinant peptides [6–9] or proteolytic fragments [10]. In a previous study [11], we used small synthetic peptides to finely map autoepitopes on the C-terminal region of human Tg (hTg). This portion of Tg is highly immunogenic; encompasses four of the five pathogenic T-cell epitopes known in animal studies [12]. Moreover, this portion of Tg shares significant sequence homology (28%) [13] with acetylcholinesterase (AChE), an enzyme of the cholinergic synapses. Based on this structural homology, autoantibodies cross-reacting with Tg and orbital muscle AChE have been attributed a pathogenic role in thyroid eye disease [14,15]. Three 20mer peptides from this region were identified, preferentially recognized by autoantibodies in GD. These peptides (TgP15, TgP26 and TgP41) are located at the segments 2339–2358, 2471–2490 and 2651–2670 of human Tg (numbering of mature protein, not including signal peptide) [16]. These were the smallest Tg peptides known to bear B-cell epitopes, recognized by either patient sera or induced anti-Tg antibodies. In particular, one of these peptides, namely TgP26, was associated with thyroid eye disease in GD patients [11].
Immunization of rabbits with peptides has been reported to cause intramolecular B-cell epitope spreading in the case of autoantigens involved in systemic autoimmune diseases, such as Ro60 [17], Sm B/B′[18] and La (SSB) [19]. In the above cases, administration of a self-peptide to experimental animals resulted in the production of antibodies directed not only to the priming peptide sequence on the protein autoantigen, but also to sites on the protein not included in the peptide sequence. Intramolecular epitope spreading is considered to be a mechanism of expansion of the autoimmune response to numerous sites of a protein autoantigen; the initial response to certain dominant epitopes is followed by the production of antibodies directed to a multitude of secondary or cryptic epitopes through intramolecular epitope spreading [20,21]. Intramolecular epitope spreading has not been reported so far for autoantigens, such as Tg, related to organ-specific autoimmune diseases.
The aims of the present study were firstly, to check whether the three autoepitope-bearing peptides, TgP15, TgP26 and TgP41, are also targets of experimentally induced anti-Tg antibodies, secondly, to examine the immunogenicity and pathogenicity of antigenic peptides and finally, to investigate the possibility of intramolecular epitope spreading in Tg, using immunogenic Tg peptides.
MATERIALS AND METHODS
Reagents
Human Tg was purified from thyroid glands, obtained at operations, applying a well-established methodology [22]. Rabbit Tg (rTg) was purified in the same way from rabbit thyroid glands. The purity of the preparation was checked by SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis), under reducing and nonreducing conditions [23]. Anti-rabbit immunoglobulin antibody coupled to alkaline phosphatase was purchased from Sigma (St. Louis, Missouri, USA).
Synthetic peptides
The peptides TgP15, TgP26 and TgP41 (segments 2339–2358, 2471–2490 and 2651–2670 of hTg [16]; amino acid sequences: QVAALTWVQTHIRGFGGDPR, PPARALKRSLWVEVDL LIGS and PYEFSRKVPTFATPWPDFVP) were synthesized with free N- and C-terminals, at>80% purity, according to standard solid-phase methods [24] by Genosys Biotechnologies (Cambridge, UK). Peptide purity was assessed by analytical HPLC and mass spectral analyses.
Immunizations
Experimentation on living animals was performed in accordance with local ethical guidelines.
Two adult New Zealand White (NZW) rabbits (L46 and L47) were injected subcutaneously with 2·5 mg of hTg, in Complete Freund's Adjuvant (CFA) (Sigma) six times, every three weeks. Two control rabbits were injected only with CFA following the same protocol. Two more adult NZW rabbits (L48 and L49) were injected subcutaneously with 100 µg of TgP41, in CFA six times, every two weeks. Animals were bled before immunization (stage 0) and 8–10 days after each administration (stages 1–6). Antisera were stored at −70°C until use.
For histological examination of thyroid glands after administration of TgP41 a different protocol was followed. Three adult NZW rabbits were injected subcutaneously with 100 µg of TgP41, in CFA. Three weeks later, animals were boosted with the same peptide quantity and by the same route, in Incomplete Freund's Adjuvant (IFA). Two weeks later, boosting was repeated in the same way. Two weeks after the last administration, the animals were sacrificed and their thyroid glands were stored in 10% formaldehyde solution until histological examination.
Isolation of Tg-specific antibodies
Anti-Tg antibodies were isolated from total antiserum (L46, L47) on a Tg immunoadsorbent (4 mg of Tg per ml of beads); purified hTg was coupled to glutaraldehyde-activated polyacrylamide-agarose beads Ultrogel AcA22 (IBF biotechnics, Villeneuve-la-Garenne, France), as described previously [25]. Bound anti-Tg antibodies were subsequently eluted from the immunoadsorbent with 0·2 N HCl-glycine pH 2·2, at 4°C. After neutralization, concentration and dialysis against phosphate-buffered saline 10 mM pH 7·4, containing 150 mM NaCl (PBS), the anti-Tg fraction was stored in 50% glycerol, at − 20°C. Antibody concentration was determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories GmbH, München, Germany).
Isolation of TgP41-specific antibodies
Anti-TgP41 antibodies were depleted from total antiserum on a TgP41 immunoadsorbent (5 mg of TgP41 per ml of beads). TgP41 immunoadsorbent, was prepared by coupling the peptide TgP41 on CNBr-activated Sepharose 4B (Pharmacia, Uppsala, Sweden) according to standard protocol provided by the manufacturer. Anti-TgP41 antibodies were subsequently isolated and stored as described for Tg-specific antibodies.
ELISA
Non-competitive ELISA
Reactivity of (anti)sera and affinity-purified antibodies was tested by ELISA as described previously [26], with some modifications. Polystyrene plates Maxisorb (Nunc, Roskilde, Denmark) were coated overnight at 4°C with Tg or each of the peptides (10 µg/ml, 100 µl per well), in 0·1 M carbonate-bicarbonate buffer pH 9·6. Free binding sites were blocked with PBS containing 0·5% gelatin (PBS-G) for 45 min at 37°C. Sera (diluted 1:100–1:12 500), or purified antibodies (0·08–5 µg/ml) in PBS-G containing 0·1% Tween-20 (PBS-G-T), were added to the wells in duplicates and incubated with immobilized antigen for 90 min at 37°C. The plates were then incubated with anti-rabbit immunoglobulin-alkaline phosphatase conjugate (0·5 µg/ml in PBS-G-T) for 1 h at 37°C. After each step, the plates were extensively washed with PBS containing 0·1% Tween-20. The assay was completed by the addition of the enzyme substrate p-nitrophenyl phosphate. The absorbance (optical density) of the produced colour at 405 nm (OD405nm) was measured using a Dynatech MR5000 microplate reader (Dynatech, Chantilly, VA, USA).
Competitive ELISA
Binding of antisera or purified anti-Tg antibodies to immobilized hTg was checked for inhibition [27] by potential soluble inhibitors (hTg, control BSA, or peptides TgP41 and TgP26). The same procedure was followed as for non-competitive ELISA with the difference that the serum or purified antibodies (at dilution corresponding to 50% of maximum binding), had been preincubated with soluble inhibitor (Tg or BSA, up to 1 nmol/ml and TgP41 or TgP26, up to 25 nmol/ml) for 90 min at 37°C, before being placed into Tg-coated wells. The results were expressed as inhibition percent (%) plotted versus pmol/ml of inhibitor.
RESULTS
Reactivity to Tg peptides in experimental immunization with Tg
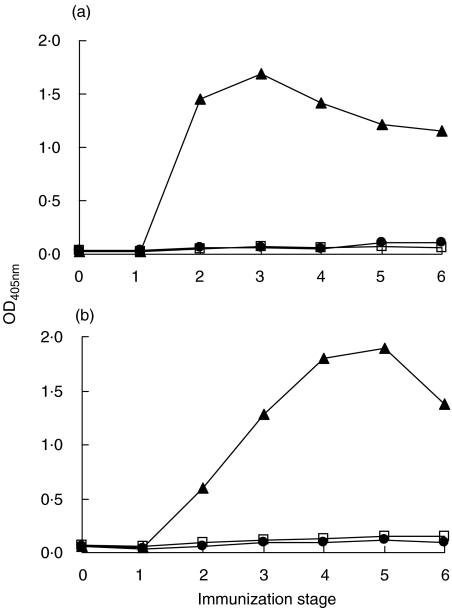
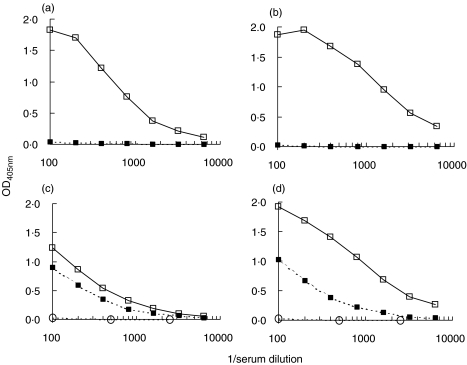
Rabbits L46 and L47, immunized with Tg, developed high serological response to Tg (data not shown). Antisera were also tested for reactivity against the Tg peptides TgP15, TgP26 and TgP41 (segments 2339–2358, 2471–2490 and 2651–2670 of hTg [16]). Figure 1 shows antipeptide reactivity of L46 and L47 antisera from successive immunization stages. After testing serial serum dilutions (data not shown), 1:100 dilution was selected, because at this dilution, differences of antibody reactivity between preimmune and serial immune sera were maximized. From stage 2 onward, high reactivity was developed to TgP41, but not to the other two peptides. Sera from control animals, injected only with CFA, did not react with the above peptides or with Tg. Apparently, TgP41 contains at least one epitope of Tg-specific antibodies induced in experimental immunization with Tg. In rabbit L46, reactivity to TgP41 peaks at stage 3 and then is gradually reduced, whereas, maximum reactivity for L47 is observed at stage 5 and is reduced at stage 6 (Fig. 1).
Fig. 1.
Reactivity (OD405nm) of antisera from rabbits L46 (a) and L47 (b), immunized with Tg, in successive immunization stages at serum dilution 1:100 against TgP15 (•), TgP26 (□) and TgP41 (▴).
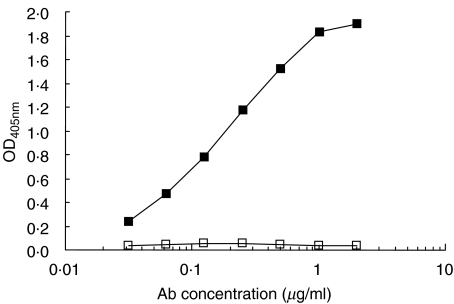
In order to definitely conclude that TgP41 bears Tg epitope(s) recognized by induced antibodies, we tested whether anti-TgP41 antibodies recognize intact Tg. For this purpose, TgP41-specific antibodies were affinity-purified from L46 antiserum at stage 6 on TgP41 immunoadsorbent. Serum concentration of isolated TgP41-specific antibodies was found to be 2·4 µg/ml (this quantity represents approximately 0·5% of total Tg-specific antibodies). Figure 2 shows that TgP41-specific antibodies were found to specifically react with intact Tg. Consequently, TgP41 comprise at least one epitope of Tg-specific antibodies induced in rabbits immunized with Tg.
Fig. 2.
Reactivity of purified TgP41-specific antibodies from Tg-immunized rabbits, against hTg (▪) and BSA (□) used as a control. OD405nm is plotted versus antibody concentration (µg/ml).
Serological response to TgP41 and to Tg in experimental immunization with TgP41
The immunogenicity of TgP41 was further tested, i.e. whether administration of this peptide induces the production of antibodies that recognize both the peptide and intact Tg.
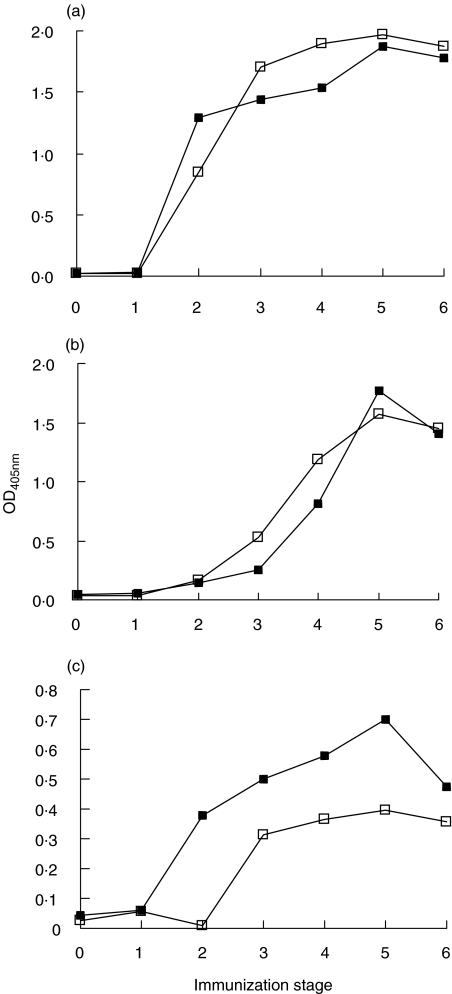
The serological response to TgP41 of rabbits L48 and L49, immunized with TgP41, was tested by ELISA. Figure 3a shows serum reactivity from successive immunization stages, at dilution 1:100 (this dilution was selected, because differences of antibody reactivity between immunization stages (0–6) were maximized). A strong response to TgP41 was exhibited after the second immunization (stage 2).
Fig. 3.
Antisera from TgP41-immunized rabbits, L48 (▪) and L49 (□) (serum dilution 1:100), were tested for reactivity to (a) TgP41, (b) hTg and (c) rTg, from all immunization stages.
The serological response of rabbits immunized with TgP41 was tested by ELISA against intact Tg. Antisera reactivity to hTg is more significant than reactivity to rTg (Fig. 3b,c). Antibody response to both Tgs reached maximum reactivity at stage 5 for both rabbits.
Reactivity of affinity-purified TgP41-specific antibodies to Tg
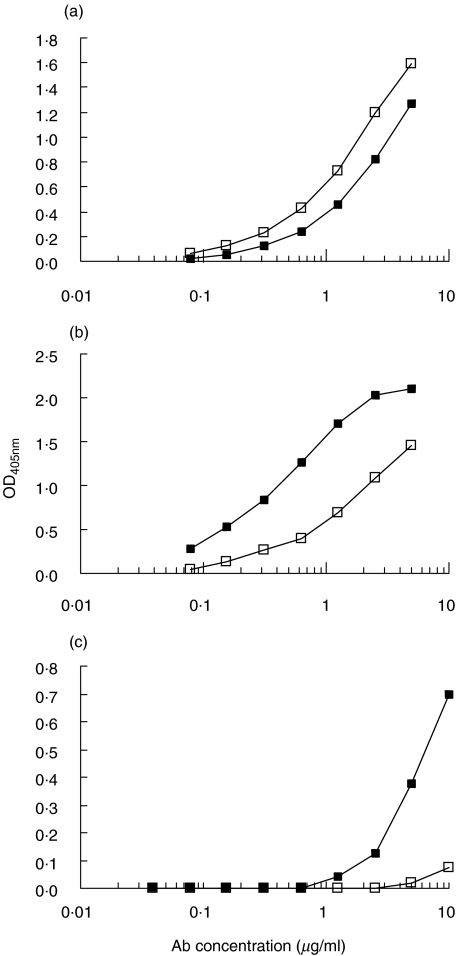
TgP41-specific antibodies were affinity-purified from antisera of stages 3 and 6 (where high reactivity is detected) from the rabbit L49. Antiserum antibody concentrations were 24·5 µg/ml from stage 3 and 29·7 µg/ml from stage 6. Reactivity of TgP41-specific antibodies was further tested against TgP41 (Fig. 4a), intact hTg (Fig. 4b) and intact rTg (Fig. 4c). Reactivity of TgP41-specific antibodies from stage 6 to TgP41 is slightly higher than that of stage 3. On the contrary, reactivity to hTg of antibodies from stage 3 is higher than that from stage 6. Presumably, in the course of immune stimulation, more antibodies to TgP41 are produced that do not bind (or have lower affinity) to the corresponding sequence on native hTg. TgP41-specific antibodies are also reactive with rTg (mainly those from stage 3). This reactivity is lower than that to hTg.
Fig. 4.
Affinity-purified TgP41-specific antibodies from antisera of stages 3 (▪) and 6 (□) from the rabbit L49, were tested for reactivity to (a) TgP41, (b) hTg and (c) rTg. OD405nm is plotted versus antibody concentration.
Evidence for intramolecular epitope spreading after immunization with TgP41
L49 antisera from stages 3 and 6, before and after depletion of TgP41-specific antibodies by immunoadsorption, were compared regarding their reactivity to TgP41 (Fig. 5a,b) and to hTg (Fig. 5c,d). After depletion of TgP41-specific antibodies, reactivity to the peptide was found to be abolished. On the contrary, after depletion of TgP41-specific antibodies, serum reactivity to Tg, although reduced, remained significant. Similar results were obtained by depletion of TgP41-specific antibodies from the stages 3 and 6 of rabbit L48 (data not shown). This result demonstrates that after depletion of TgP41-specific antibodies, antibodies directed to other sites of Tg do exist in antisera (this is known as intramolecular epitope spreading). This residual reactivity to Tg was inhibited by soluble Tg (approx. 90% inhibition at 1 nmol/ml soluble Tg), while no inhibition was detected by soluble peptide TgP41 up to 25 nmol/ml (data not shown). This suggests that reactivity to Tg, remaining after TgP41-specific antibody depletion, is specific for Tg and is not an effect of nonspecific binding.
Fig. 5.
Comparison of antisera from stages 3 and 6 of rabbit L49 before (□) and after (▪) depletion of TgP41-specific antibodies with immunoadsorption, regarding their reactivity to TgP41 [(a) stage 3 and (b) stage 6] and to hTg [(c) stage 3 and (d) stage 6]. OD405nm is plotted versus serum dilution. Serum reactivity of stage 0 (○) is plotted for comparison.
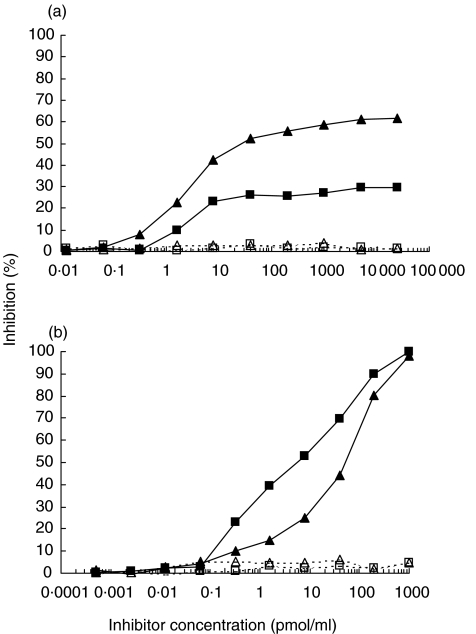
To definitely demonstrate the existence of antibodies directed to sites on Tg not included in TgP41, an additional experiment was performed. Tg-specific antibodies were purified by affinity chromatography from sera of stages 3 and 6 of rabbit L49 (immunized with TgP41). Subsequently, it was checked whether reactivity of these antibodies to immobilized Tg was inhibited by soluble TgP41. For stage 3, inhibition reaches a plateau not exceeding 30%, at 25 nmol/ml of inhibitor (Fig. 6a), whereas inhibition by soluble Tg reaches 100% at 1 nmol/ml (Fig. 6b). Inhibition by TgP41 at stage 6, although higher than that at stage 3, it reaches a plateau of approximately 60% at inhibitor concentration of 25 nmol/ml (Fig. 6a). Inhibition by soluble Tg at stage 6 approaches 100% at concentration 1 nmol/ml (Fig. 6b). Since the peptide TgP41 is not capable of completely inhibiting antibody reactivity to Tg, we conclude that antisera contain antibodies directed to sites on Tg not included in TgP41 sequence.
Fig. 6.
Inhibition of binding to hTg of purified Tg-specific antibodies from stages 3 (▪) and 6 (▴) of rabbit L49 (immunized with TgP41) by soluble (a) TgP41 and (b) hTg. Peptide TgP26 and BSA were used as control (open symbols/broken lines).
Histological examination of thyroid glands after administration of TgP41
The ability of TgP41 to cause experimental autoimmune thyroiditis (EAT) in rabbits was investigated. The peptide was administered to three rabbits following a protocol different from that used for antibody production, in order to optimize thyroid lymphocyte infiltration. The animals were sacrificed and the thyroid glands were histologically examined. No lymphocyte infiltration was observed in these thyroid glands.
DISCUSSION
In a previous study [11] we identified three 20mer synthetic peptides (TgP15, TgP26 and TgP41) from the C-terminal portion of hTg that contained autoepitopes associated with GD. Those peptides were the smallest peptides bearing Tg epitopes identified so far, owing to the method used; epitope mapping with small synthetic pin-bound peptides. Due to the large size of Tg, the identification of antigenic sequences on Tg, recognized by anti-Tg disease-associated or induced after immunization, was accomplished so far using either large recombinant peptides [6–9] or proteolytic fragments [10].
In the present study, TgP41 (a.a. 2651–2670 of hTg), was found to also contain epitope(s) recognized by anti-Tg antibodies induced in rabbits by Tg. Peptides TgP15 and TgP26 are not recognized by antisera, suggesting that they contain only epitopes recognized by autoantibodies.
Tg epitopes defined by TgP15 and TgP26 do not overlap with previously characterized Tg epitopes [6–10]. TgP41 sequence comprises an overlap of 14 residues with the sequence 2657–2748 of hTg, found previously to be recognized by a few Hashimoto's thyroiditis sera [10]. Moreover, it is encompassed in the segment 2644–2730 of hTg, found to bear an epitope of induced antibodies [7]. We cannot exclude the possibility that the epitope included in that 87 amino acids long peptide coincides with that defined by the 20mer peptide TgP41. TgP41 is the smallest peptide known to bear a target of induced anti-Tg antibodies.
TgP41 was also found here to contain a strongly immunogenic region of Tg; this peptide, when administered in rabbits, is capable of inducing antibodies recognizing the peptide, as well as native Tg. Of course, a portion of the antibodies recognizing the peptide do not bind to native Tg. It is possible that antibodies are produced directed to different sites on the 20mer peptide. Some of these epitopes are possibly not recognized on native Tg, presumably due to steric hindrance. It is remarkable that a small peptide like TgP41 was capable of inducing antibody production administered in rabbits free, not conjugated to a carrier molecule. Small molecules, in most cases are administered in rabbits conjugated to proteins or other types of carriers, such as, lysine tree [28] or sequential oligopeptide carrier (SOC) [29]. This way, additional epitopes are generated that can be recognized by helper T-cells, which subsequently stimulate antibody producing B-cells. Moreover, conjugation of a peptide to a carrier prevents its fast degradation within the recipient [30]. Consequently, TgP41 is strongly immunogenic, and possibly also includes a T-cell epitope.
In the present study, administration of TgP41 to rabbits, at least with our experimental procedure, did not result in the development of EAT. It is known that EAT is not always induced in rabbits, even if whole Tg is administered [31–33]. To ensure that TgP41 contains a pathogenic epitope, studies in susceptible strains of mouse are necessary.
In our study we concluded that experimental immunization with TgP41 induces B-cell epitope spreading. Reactivity to intact Tg, remaining after TgP41-specific antibody depletion, is specific for Tg and is not an effect of nonspecific binding. This residual reactivity to Tg is due to epitopes not encompassed in, and not homologous to TgP41, since, it is not inhibited by TgP41. During the course of successive immunizations with TgP41, more antibodies accumulate that recognize TgP41 sequence in native Tg, in parallel with antibodies directed to the ‘spread’ epitopes. This is indicated by the result showing that reactivity to Tg of purified anti-Tg antibodies from TgP41-immunized animals was inhibited by soluble TgP41 more significantly in stage 6 than in stage 3. Reactivity to ‘spread’ epitopes in Tg seems to remain unaltered between stages 3 and 6, since residual reactivity to Tg after depletion of TgP41-specific antibodies is comparable between stages 3 and 6, although anti-Tg reactivity is higher in stage 6. In the present study, B-cell epitope spreading after peptide-immunization, was demonstrated for the first time, for an autoantigen related to an organ-specific autoimmune disease. However, epitope spreading has often been reported for autoantigens associated with systemic autoimmune diseases [17–19]. It has also been reported for another thyroid antigen, thyrotropin receptor [21], but in that case, animals were primed with the extracellular domain of the protein and not with a small peptide. Intramolecular epitope spreading is believed to result from the activation of antigen-specific clones of helper T-cells capable of stimulating a series of B-cells specific for several epitopes on the protein [20,34]. In the case of immunization with a Tg peptide, intramolecular epitope spreading, can be explained via involvement of normally circulating Tg [35] or Tg fragments that may be recognized by anti-TgP41 antibodies and thus, create immune complexes [36]. These complexes can be further processed and give rise to antibodies directed to epitopes on Tg other than TgP41. However, we cannot exclude the possibility that transient lesions, although not observed, appear in the thyroid glands of TgP41-immunized rabbits, which could lead to release of Tg. B-cells specific for the immunizing peptide TgP41 could uptake Tg via surface immunoglobulins and, after processing, present new determinants to T-cells. Stimulated T-cell clones could, in turn, provide help to various B-cell clones specific for other epitopes on Tg. These mechanisms presume that epitope spreading is mediated by autologous Tg and that TgP41 encompasses an epitope located on the surface of Tg; indeed, calculation of Tg hydrophobicity profile confirmed the latter (data not shown). Moreover, the experiments confirmed antisera reactivity to autologous Tg (rTg).
Our model suggests involvement of normally circulating Tg in epitope spreading without tissue damage, in contrast to hypotheses proposed by other researchers, where tissue damage is a prerequisite for epitope spreading, either in T-cell-mediated or antibody-mediated autoimmune diseases [20]. Although rabbit Tg sequence is unknown, TgP41 (human sequence) shares 75% identity with other known Tgs from several species (bovine, porcine, mouse, rat). Presumably, the rabbit sequence homologous to TgP41 shares epitope(s) with the human sequence. Reactivity of TgP41-specific antibodies from stage 3 to rTg, confirmed cross-reactivity between TgP41 and the rabbit Tg sequence, responsible for epitope spreading. The very low reactivity of TgP41-specific antibodies from stage 6 to rTg can be attributed to the accumulation of TgP41-specific antibodies, during successive immunizations, that do not cross-react with rTg.
To conclude, region 2651–2670 of hTg (peptide TgP41), containing target(s) of autoantibodies and of antibodies produced after experimental immunization with Tg, is capable of inducing antibodies that bind to intact Tg and causing spreading of the reactivity to other sites of Tg.
Acknowledgments
The authors would like to thank the Academy of Athens for the fellowship of A. Thrasyvoulides.
REFERENCES
- 1.Malthiery Y, Marriq C, Berge-Lefranc JL, Franc JL, Henry M, Lejeune PJ, Ruf J, Lissitzky S. Thyroglobulin structure and function: recent advances. Biochimie. 1989;71:195–209. doi: 10.1016/0300-9084(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 2.Ericsson UB, Christensen SB, Thorell JI. A high prevalence of thyroglobulin autoantibodies in adults with and without thyroid disease as measured with a sensitive solid-phase immunosorbent radioassay. Clin Immunol Immunopathol. 1985;37:154–62. doi: 10.1016/0090-1229(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 3.Weetman AP, McGregor AM. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984;5:309–55. doi: 10.1210/edrv-5-2-309. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Niesen N, Milgrom F, Albini B. Transfer of experimental autoimmune thyroiditis by in situ perfusion of thyroids with immune sera. Clin Immunol Immunopathol. 1993;66:11–7. doi: 10.1006/clin.1993.1002. [DOI] [PubMed] [Google Scholar]
- 5.Tomer Y. Anti-thyroglobulin autoantibodies in autoimmune thyroid diseases: cross-reactive or pathogenic? Clin Immunol Immunopathol. 1997;82:3–11. doi: 10.1006/clin.1996.4243. [DOI] [PubMed] [Google Scholar]
- 6.Ludgate M, Dong Q, Dreyfus PA, Zakut H, Taylor P, Vassart G, Soreq H. Definition, at the molecular level, of a thyroglobulin-acetylcholinesterase shared epitope: study of its pathophysiological significance in patients with Graves’ ophthalmopathy. Autoimmunity. 1989;3:167–76. doi: 10.3109/08916938909099014. [DOI] [PubMed] [Google Scholar]
- 7.Dong Q, Ludgate M, Vassart G. Towards an antigenic map of human thyroglobulin: identification of ten epitope-bearing sequences within the primary structure of thyroglobulin. J Endocrinol. 1989;122:169–76. doi: 10.1677/joe.0.1220169. [DOI] [PubMed] [Google Scholar]
- 8.Henry M, Malthiery Y, Zanelli E, Charvet B. Epitope mapping of human thyroglobulin. J Immunol. 1990;145:3692–8. [PubMed] [Google Scholar]
- 9.Henry M, Zanelli E, Piechaczyk M, Pau B, Malthiery Y. A major human thyroglobulin epitope defined with monoclonal antibodies is mainly recognized by human autoantibodies. Eur J Immunol. 1992;22:315–9. doi: 10.1002/eji.1830220205. [DOI] [PubMed] [Google Scholar]
- 10.Saboori AM, Rose NR, Burek CL. Amino acid sequence of a tryptic peptide of human thyroglobulin reactive with sera of patients with thyroid diseases. Autoimmunity. 1995;22:87–94. doi: 10.3109/08916939508995304. [DOI] [PubMed] [Google Scholar]
- 11.Thrasyvoulides A, Sakarellos-Daitsiotis M, Philippou G, Souvatzoglou A, Sakarellos C, Lymberi P. B-cell autoepitopes on the acetylcholinesterase-homologous region of human thyroglobulin: association with Graves’ disease and thyroid eye disease. Eur J Endocrinol. 2001;145:119–27. doi: 10.1530/eje.0.1450119. [DOI] [PubMed] [Google Scholar]
- 12.Carayanniotis G, Rao VP. Searching for pathogenic epitopes in thyroglobulin. parameters and caveats. Immunol Today. 1997;18:83–8. doi: 10.1016/s0167-5699(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher M, Camp S, Maulet Y, Newton M, MacPhee-Quigley K, Taylor SS, Friedmann T, Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. Nature. 1986;319:407–9. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- 14.Ludgate M, Swillens S, Mercken L, Vassart G. Homology between thyroglobulin and acetylcholinesterase: an explanation for pathogenesis of Graves’ ophthalmopathy? Lancet. 1986;2:219–20. doi: 10.1016/s0140-6736(86)92515-8. [DOI] [PubMed] [Google Scholar]
- 15.Mappouras DG, Philippou G, Haralambous S, Tzartos SJ, Balafas A, Souvatzoglou A, Lymberi P. Antibodies to acetylcholinesterase cross-reacting with thyroglobulin in myasthenia gravis and Graves’ disease. Clin Exp Immunol. 1995;100:336–43. doi: 10.1111/j.1365-2249.1995.tb03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malthiery Y, Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987;165:491–8. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh US, Lewis JE, Gaskin F, Dhakephalkar PK, Kannapell CC, Waters ST, Fu SM. Ro60 peptides induce antibodies to similar epitopes shared among lupus-related autoantigens. J Immunol. 2000;164:6655–61. doi: 10.4049/jimmunol.164.12.6655. [DOI] [PubMed] [Google Scholar]
- 18.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yiannaki E, Vlachoyiannopoulos PG, Manoussakis MN, Sakarellos C, Sakarellos-Daitsiotis M, Moutsopoulos HM, Tzioufas AG. Study of antibody and T cell responses in rabbits immunized with synthetic human B cell epitope analogues of La (SSB) autoantigen. Clin Exp Immunol. 2000;121:551–6. doi: 10.1046/j.1365-2249.2000.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831–6. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlase H, Nakashima M, Graves PN, Tomer Y, Morris JC, Davies TF. Defining the major antibody epitopes on the human thyrotropin receptor in immunized mice: evidence for intramolecular epitope spreading. Endocrinology. 1995;136:4415–23. doi: 10.1210/endo.136.10.7664661. [DOI] [PubMed] [Google Scholar]
- 22.Schardt CW, McLachlan SM, Matheson J, Smith BR. An enzyme-linked immunoassay for thyroid microsomal antibodies. J Immunol Meth. 1982;55:155–68. doi: 10.1016/0022-1759(82)90028-x. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Merrifield RB. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. JACS. 1963;85:2149–54. [Google Scholar]
- 25.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–87. [PubMed] [Google Scholar]
- 26.Haralambous S, Blackwell C, Mappouras DG, Weir DM, Kemmett D, Lymberi P. Increased natural autoantibody activity to cytoskeleton proteins in sera from patients with necrobiosis lipoidica, with or without insulin-dependent diabetes mellitus. Autoimmunity. 1995;20:267–75. doi: 10.3109/08916939508995704. [DOI] [PubMed] [Google Scholar]
- 27.Dighiero G, Guilbert B, Fermand JP, Lymberi P, Danon F, Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin, and native DNA. immunologic studies and clinical correlations. Blood. 1983;62:264–70. [PubMed] [Google Scholar]
- 28.Tam JP. Synthetic peptide vaccine design. synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–13. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsikaris V, Sakarellos C, Cung MT, Marraud M, Sakarellos-Daitsiotis M. Concept and design of a new class of sequential oligopeptide carriers (SOC) for covalent attachment of multiple antigenic peptides. Biopolymers. 1996;38:291–3. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C291::AID-BIP1%3E3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Tam JP. Immunization with peptide-carrier complexes. traditional and multiple-antigen peptide systems. In: Wisdom GB, editor. Peptide AntigensA Practical Approach. New York: Oxford University Press; 1994. pp. 83–115. [Google Scholar]
- 31.Weigle WO. The induction of autoimmunity in rabbits following injection of heterologous or altered homologous thyroglobulin. J Exp Med. 1965;121:289–307. doi: 10.1084/jem.121.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velicky J, Titlbach M, Dvorak R, Lustig R, Raska I, Lhotova H, Mara M, Likovsky Z. Experimental thyroiditis in guinea pigs and rabbits. Immunization with thyroglobulin and bovine thyroid gland suspension. Funct Dev Morph. 1993;3:259–67. [PubMed] [Google Scholar]
- 33.Blumenthal HT, Premachandra BN. Immunopathological studies on thyroid immunity. IX. Thyroid and renal amyloidosis in thyroglobulin immunized rabbits. J Pathol. 1987;151:305–14. doi: 10.1002/path.1711510411. [DOI] [PubMed] [Google Scholar]
- 34.Mamula MJ, Janeway CA., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993;14:151–2. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 35.Van Herle AJ, Uller RP, Matthews NI, Brown J. Radioimmunoassay for measurement of thyroglobulin in human serum. J Clin Invest. 1973;52:1320–7. doi: 10.1172/JCI107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Y, Carayanniotis KA, Eliades P, Lymberi P, Shepherd P, Kong Y, Carayanniotis G. Enhancing or suppressive effects of antibodies on processing of a pathogenic T cell epitope in thyroglobulin. J Immunol. 1999;162:6987–92. [PubMed] [Google Scholar]