Abstract
Release of soluble Granzymes (sGranzymes) is considered to reflect activation of cytotoxic T lymphocytes and NK cells. sGranzymes and a number of pro-inflammatory cytokines were measured in plasma of malaria patients with natural or experimentally induced Plasmodium falciparum infections. Concentrations of sGranzyme A and B, IL-10, IL-12p70 and CRP were significantly increased in African children presenting with clinical malaria; IL-10 and CRP concentrations were significantly correlated with disease severity. In nonimmune Dutch volunteers which were experimentally infected by P. falciparum-infected mosquitoes, sGranzyme A increment started 1–2 days prior to clinical symptoms and microscopically detectable parasitaemia. This coincided with increases in IFNγ, IL-12p40 and IL-8, while sGranzyme B and IL-10 levels increased 24–48 h later. The elevation of sGranzyme A and IFNγ in nonimmune volunteers suggests that NK cells are activated upon release of parasites by infected liver cells and subsequently during blood stage infection; thus, NK cells are likely involved innate immune human host resistance in the early phase of a malaria infection.
Keywords: CTL, NK cells, granzyme, infectious immunity-parasites, human-clinical studies
INTRODUCTION
Clinical malaria is well known to be accompanied by increased plasma concentrations of pro-inflammatory cytokines, which often correlate with disease severity [1]. Plasma samples for such analysis are usually obtained from patients with overt clinical malaria symptoms of varying severity. Little is known on the dynamics and kinetics of these mediators in the earlier phases of infection including hepatic phase when some cytokines are considered to play an important role in disease progression. Plasmodium falciparum malaria starts with the bite of an infected mosquito and injected parasites disappear from the circulation within 10–20 min to mature and multiply in liver cells. This incubation period, which usually lasts 5–7d, ends with release of parasites from ruptured liver cells into the circulation. Subsequently, cyclic proliferation of parasites starts in red blood cells which is soon followed by the occurrence of clinical symptoms.
IFNγ, which is produced in large quantities by activated natural killer (NK) cells, NKT [2, review] and CD8+ T-cells, is one of the pro-inflammatory cytokines that probably play a key role in the early phase of malaria, i.e. during the liver stages as has been shown in several murine models [3]. It has been proposed in murine malaria that NK cells, one of the mediators of innate immune response, limit the initial phase of asexual parasite replication [4]. In human malaria, IFNγ concentration is increased and correlates with NK cell activation during blood stage infection [5].
CTL activity depends on antigen presentation in association with class I major histocompatibility complex (MHC-I) molecules, whereas NK cells recognize their target in a MHC-unrestricted manner and in the absence of antibody. Both CTL and NK cells can destroy their target cells indirectly by production of IFNγ or directly by using a Fas-dependent mechanism, or by exocytosis of the cytotoxic granules [6]. These granules contain perforin and a group of serine proteinases which are called granzymes [7]. Granzymes are mainly, if not exclusively, produced by CTL and NK cells [8]. Granzymes can be released during cytotoxicity and their levels in biological fluids constitute a soluble marker for activation of CTL and NK cells and increased concentrations are considered to reflect the involvement of CTL and NK cells in various disease states. Soluble Granzymes levels in circulation are elevated in viral infection [9], rheumatoid arthritis [10] and melioidosis [11]. Although the biological functions of most granzymes remain to be resolved Granzyme A, which is a trypsine-like enzym, is less efficient in DNA fragmentation as compared to Granzyme B which has its activity towards procaspase substrates [12]. We investigated the levels of sGranzyme A, B and other cytokines during the hepatic and erythrocytic stages of P falciparum infection as possible indicators of the human immune response.
VOLUNTEERS, PATIENTS, MATERIALS AND METHODS
Study sites
The patient study was conducted in Yaoundé, Cameroon at the Department of Paediatrics of the Central Hospital and in the outpatient clinic of the Messa Dispensary. The patients studied were enrolled between February 1994 and October 1996.
The volunteer study was carried out at the Departments of Medical Microbiology and General Internal Medicine of the University Medical Center Nijmegen, the Netherlands.
Patients from Cameroon
Children of mean age 5·8 years (range 0·2–15·0 years) with symptoms of clinical malaria according to World Health Organization (WHO) standards [13], attending the outpatient department or admitted to the Central Hospital of Yaoundé were enrolled after informed consent. Children with clinical malaria were admitted to the Emergency Room of the Department of Paediatrics and examined by the clinical staff. All children underwent a complete physical examination and a thick smear was made. When the thick smear was positive for P. falciparum severity of malaria was assessed according to WHO standards as uncomplicated cases (n = 35) and severe malaria cases (n = 57; cerebral malaria n= 22, respiratory distress n= 4, repeated generalized convulsions n= 4, impaired consciousness but rousable n= 31, hyperpyrexia n= 6). Respiratory distress was defined according to Marsh et al. [14]. The level of consciousness was quantified using the Blantyre Coma Scale [15]. According to hospital guidelines, severe malaria patients were treated with quinine i.v. or artemether i.m. After admission the patients were prospectively followed until the day of discharge. Children in the outpatient department were treated with a standard dose of amodiaquine and asked to return after one week. To constitute a control group (n = 19), plasma was collected from siblings of the malaria patients.
Experimental malaria infection of human volunteers
Anopheles stephensi mosquitoes were maintained in the insectary at the animal house, UMC, Nijmegen. Three days old mosquitoes were fed on blood containing gametocytes [16] produced in a standardized semiautomated culture system seeded with the chloroquine-sensitive NF54 isolate of P. falciparum[17]. Mosquitoes were allowed to bloodfeed for 10 min and fed and unfed mosquitoes were separated 3 h later. Oocysts and sporozoite counts were performed 7 and 14 days after the feed, respectively, and batches with 100% infected mosquitoes were used for experimental infection of volunteers.
Five healthy volunteer males (age 18–34 years) with no prior exposure to malaria were selected after informed consent. Sets of 7 female mosquitoes that were deprived of sugar for 17 h, were exposed to bites on both forearms for 10 min. This procedure was repeated until at least 5 mosquitoes that contained salivary glands sporozoites upon subsequent dissection had fully engorged. From 5 days after infection onset of infection was assessed by daily blood films and the development of malaria symptoms. Parasites were counted against 500 WBCs in Giemsa-stained thick smears. Volunteers were immediately treated with a standard curative regimen of chloroquine upon microscopic detection of parasites. This study was approved by the Ethical Committee of the University Medical Centre Nijmegen (CWOM 9712–0232).
Inflammatory mediators
Plasma samples were obtained from all patients; they were encoded and initially stored at − 20°C. After transport, samples were stored at − 80°C until analysis. The ELISA to detect sGranzyme A and B has been described in detail [9]. Briefly, 100 µl of purified mouse Mab GA 29 (0·5 µg/ml; CLB, http://www.CLB.nl) or Mab GB 11 (1·0 µg/ml; CLB), for the detection of sGranzyme A and B, respectively, were coated onto microtitre plates (Nunc Maxisorp, VWR, Amsterdam, The Netherlands) overnight at 4°C. Diluted test samples and standards (purified sGranzyme A and B (CLB) at different concentrations) were incubated for 1 h. Subsequently, the plates were incubated with biotinylated GA28 (0·5 µg/ml; CLB) or GB10 MoAb (0·5 µg/ml; CLB) for the detection of sGranzyme A and B, respectively, for 1 h. Streptavidin-horseradish peroxidase (poly HRP; CLB) was added and the plates were developed using hydrogen peroxidase as substrate and tetramethylbenzidine (Merck, VWR, Amsterdam, The Netherlands). The absorbance at 450 nm was determined with a Titertek plate reader (Labsystems, VWR, Amsterdam, The Netherlands). The concentrations of sGranzymes were expressed in pg/ml. Minimum detection level for sGranzyme A was 3 pg/ml and for sGranzyme B 2 pg/ml.
IL-8 [18], IL-10 [19], IL-12p40, IL-12p70, IFNγ[20] and CRP [21] were also measured by ELISA, accordingly the publication procedure.
Statistical analyses
For statistical analyses the Mann–Whitney U-test was used for comparison of mean and the Pearson correlation coefficient (r) was calculated to detect significant relationship between different cytokines. P-values <0·05 were considered to be significant.
RESULTS
Concentrations of sGranzymes and cytokines in endemic malaria patients
Median plasma concentrations of sGranzyme A and B of Cameroonian children with malaria were significantly higher on enrolment than in the nonmalaria control group. The percentage of patients with elevated sGranzyme B increased with severity of disease (Table 1). A strong correlation was found between sGranzyme A and B concentrations in the Cameroonian children (r = 0·83, P < 0·001, n= 111). Table 2 shows that plasma IL-10, IFNγ (only severe malaria) and CRP concentrations were significantly increased while IL-12 p40 and IL-12 p70 showed no obvious changes compared to controls. CRP and IL-10 concentrations were associated with disease severity, while sGranzyme A and B show a positive trend (Tables 1 and 2). Plasma concentrations of sGranzyme A and B did not correlate with those of the cytokines IFNγ, IL-10 and IL-12 p70. In addition, none of these mediators correlated with parasite density on admission, or duration of parasitaemia (n = 111; data not shown).
Table 1.
sGranzyme concentrations from Cameroonian children with P falciparum malaria
| sGranzyme A pg/ml | P-value* | sGranzyme B pg/ml | P-value* | |
|---|---|---|---|---|
| Control | 37 (18–90) † | 9 (2–32) † | ||
| Uncomplicated malaria | 103 (33–1798) | < 0·001* 74%§ | 42 (2–4132) | < 0·001* 57% |
| Severe malaria | 130 (3–1290) | < 0·001*″72% | 69 (2–1980) | < 0·001* 72% |
| 0·70‡ | 0·17‡ |
median concentrations (range) on day of enrolment.
P-value as compared to control value.
P-value as compared to uncomplicated malaria.
percentage of patients with significant higher concentrations as compared to controls (cut off level is mean + 2SD).
Table 2.
IFNγ, IL-10, IL-12 and CRP concentrations from Cameroonian children with P falciparum malaria
| IFNγ | IL–10 | IL–12p40 | IL–12p70 | CRP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (pg/ml) | P-value | (pg/ml) | P-value | (pg/ml) | P-value | (pg/ml) | P-value | (mg/l) | P-value | |
| Control | 20‡ (10–117) | 20 (9–126) | 298 (117–581) | 0·9 (0·1–2·4) | 2 (0·1–60) | |||||
| Uncomplicatedmalaria | ND | 39 (20–3190) | 0·001* | 275 (150–2672) | 0·6* | ND | 63 (14–522) | <0·001* | ||
| Severe malaria | 10 (1–1132) | 0·04* | 2496 (18–21020) | <0·001* | 242 (55–2456) | 0·9* | 1·5 (0·1–11·4) | 0·05* | 217 (15–545) | <0·001* |
| <0·001§ | 0·4§ | <0·001§ | ||||||||
median concentrations on day of enrolment (range).
P-value as compared to control value.
P-value as compared to uncomplicated malaria. ND, Not Done.
Clinical course of volunteers experimentally infected with Plasmodium falciparum
The above result indicated that sGranzyme A and B are increased in plasma of children from a P. falciparum endemic area presenting with clinical malaria. This prompted us to investigate the earliest changes of sGranzymes and cytokines during experimental P. falciparum infection in adult human volunteers. After exposure to blood feeding infected mosquitoes, all five volunteers developed parasitaemia after a mean of 9 days (range 7·5 and 11) (Table 3). Maximum parasitaemia as determined by thick smear varied between 30 and 132 parasites/µl. Four of the five volunteers developed fever (Table 3) accompanied by mild to moderate malaria symptoms in the same period. Upon antimalaria drug treatment, the clinical course was uncomplicated with resolution of the symptoms within 2–3d and clearance of parasites from the blood within 1–2d.
Table 3.
Parameters of P. falciparum infection following bites by infected mosquitoes in 5 volunteers
| Volunteer | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Patency (days)* | 9 | 9 | 9 | 7·5 | 11 |
| Maximum parasite density/µl | 60 | 42 | 132 | 30 | 60 |
| Onset‡ of fever (> 37·5°C) | 8 | <§ | 9 | 7 | 11 |
| Fever peak values (°C) | 40·3 | – | 39·0 | 38·7 | 38·7 |
| Discharge‡ | 20 | 20 | 20 | 16 | 21 |
Patency is defined as the interval between infection through mosquitoes and the detection of P. falciparum parasites in thick smears.
Days after infection.
No fever.
Soluble granzymes in volunteers infected with P. falciparum infected mosquitoes
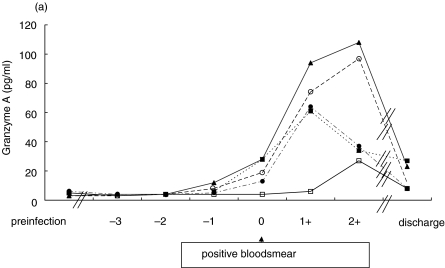
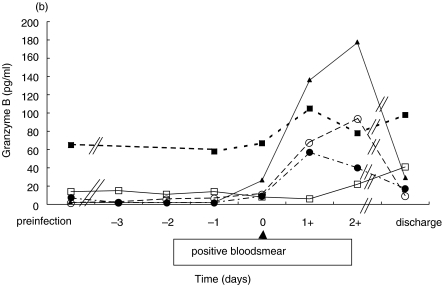
Pre-infection sGranzyme A concentrations were 4·4 ± 1·1 pg/ml (mean ± sd). In four volunteers the mean concentration of sGranzyme B was 6·3 ± 5·7 pg/ml, but volunteer 5 exhibited 69 pg/ml. The kinetics of sGranzyme A and B concentrations in all volunteers is shown in Fig. 1(a+b). Day 0 is defined as the first day of detection of parasites as determined by thick smear. SGranzyme A concentrations started to increase between day −2 and day −1 in volunteers (n = 4) that became febrile. SGranzyme A started to increase 1–2 days prior to the increase of sGranzyme B that more closely coincided with clinical symptoms and parasitaemia. Volunteer number 2 did not develop fever and both sGranzymes did not increase until day 2. On discharge, both sGranzyme A and B concentrations were still above base-line concentrations in all volunteers.
Fig 1.
Kinetics of plasma (a) sGranzyme A and (b) sGranzyme B during the experimental Plasmodium falciparum infection in volunteers. ▴ Volunteer 1; □ Volunteer 2; ○ Volunteer 3; ▪ Volunteer 4; • Volunteer 5.
There is a significant correlation between sGranzyme A and sGranzyme B concentrations in the volunteers (r = 0·84, P < 0·001, n= 43).
Concentrations of sGranzymes and cytokines in volunteers infected with P. falciparum infected mosquitoes
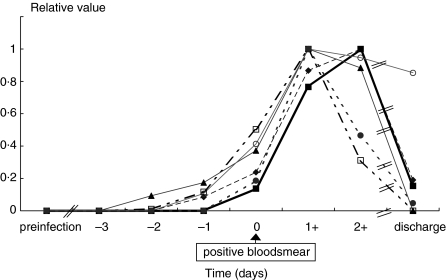
Figure 2 shows the kinetics of plasma concentrations of sGranzyme A, sGranzyme B, IFNγ, IL-8, IL-10 and IL-12 p40 in volunteer 1 being representative for all febrile volunteers. Kinetics of these mediators in the a-febrile volunteer showed a similar pattern but with a delay of 2 days. The increment of plasma concentrations of sGranzyme A, IFNγ, IL-8 and IL-12 p40 started between day −3 and day − 2, while increases of sGranzyme B and IL-10 started 48 h later. One day after the start of the antimalaria drug treatment IL-10 and IFNγ decreased sharply while the other mediators remained elevated for a further 1–2 days. At discharge, concentrations of most mediators had declined to background levels with the exception of sGranzyme A, sGranzyme B and IL-12 p40.
Fig 2.
Kinetics of inflammatory mediators in plasma of volunteer 1 during experimental P. falciparum infection. The maximum value of each mediator is defined as 1 and the concentration before infection as zero. The concentration of mediators before infection, respectively, at the maximum levels are the following: sGranzyme A 3 and 108 pg/ml, sGranzyme B 2 and 177 pg/ml, IFNγ 2 and 276 pg/ml, IL-8 3 and 24 pg/ml, IL-10 4 and 47 pg/ml, IL-12 p40 60 and 134 pg/ml, CRP 0·1 and 78 mg/l. ¥ Granzyme A; ▪ Granzyme B; □ IFN; ▴ IL-8; • IL-10; ○ IL-12p40.
The sGranzyme A, B and CRP and IL-10 concentrations in the uncomplicated malaria children from Cameroon and the experimentally infected volunteers were similar with no significant difference being detectable (Table 1, Fig. 2). Remarkably, the IL-12 p40 concentrations in the nonmalaria control group from Cameroon was 275 pg/ml (range 150–2672), which is relatively high compared to concentrations observed in the experimental study (range 60–134 pg/ml, Fig. 2).
DISCUSSION
This study shows that plasma concentrations of sGranzyme A, sGranzyme B, CRP, IL-10 and IL-12 are significantly increased in patients with clinical P. falciparum malaria in (semi)-immune Cameroonian children. In those children IL-10 and CRP concentrations correlate with disease severity in contrast to IFNγ and IL-12p40. Associations of IL-10 and CRP concentrations with severity of disease corresponds with data from others [22–24]; in contrast with our data, two of these studies describe a decrease of IL-12 (p70) in severe as compared to mild malaria [23,24]. SGranzymes represent a convenient marker for activation and degranulation of CTL and NK cells [8]. Thus, the significant increase of sGranzymes in the (semi)immune children indicates the activation of these cytotoxic cells and the possible involvement in the host defense mechanism against malaria.
In the experimentally infected volunteers sGranzyme A and B as well as the other mediators were not detectable during the first 5–6 days, but started to increase 1–2d prior to clinical symptoms and parasitaemia. No increment of IL-12 p70 could be detected (data not shown). Changes in some of the inflammatory mediators as described in this paper occur 3–4 d prior as compared to the results of Harpaz et al. [1], which may be due to sensitivity of the assays. Previously we showed that blood parasites are at first detectable by real-time quantitative PCR between 6·3 and 7 days after infection which is mean 2·7days (range 1·3–3·7 days) before parasites are microscopically detectable [25]. These data suggest that sGranzyme A, IFNγ, IL-12p40 and IL-8 increments, are related to the latest stages of hepatic infection including release of merozoites and early blood stage infection.
These data illustrate the strong potency of malaria parasites to activate the release of pro-inflammatory mediators and sGranzymes, as only a few million parasites in total [25] circulate at this early stage of subclinical infection.
Based on existing immunity in the African patients CTL, NK and NKT cells are probably the most likely cell source that contribute to production of IFNγ and sGranzymes. NK cells will be the more likely cell origin in the nonimmune Dutch volunteers as it is unexpected that specific CTL and NKT cells will have been generated at this early stage of infection. NK cell activity in humans has been shown to correlate with serum IFNγ concentrations [5]. The functional effect of IFNγ is illustrated in murine malaria by showing that elimination of infected hepatocytes is directly the result of IFNγ release [3]. While CD8+ T cells are clearly responsible in this effect, the role of NK and NKT cells is less obvious as conflicting data are obtained in different murine malaria models [26–28]. Next to IFNγ, rather than perforin, Granzyme B and FasL may be involved in pre-erythrocytic protection in murine gene k/o models [3,29].
In our human volunteers, we do not observe signs of activation in peripheral blood during the liver stage period but rather upon immediate release of parasites in the bloodstream. The elevations of sGranzyme A and B and concomitant decreases in numbers of circulating NK cells (data not shown), suggest that parasitaemia activates the NK cell population, although there is no formal proof for functional activity.
Our data show a marginal correlation (0·54 and 0·50, respectively) between IFNγ and sGranzymeA or sGranzyme B in the experimentally infected volunteers and no obvious correlation in the patients from Cameroon. No correlation was found between IL-12 p40 and sGranzyme A or B in the experimental and in the natural malaria infection. Possible explanations may be the differences in clearance rate of IFNγ, IL-12 p40 and sGranzyme A and sGranzyme B, and/or IFNγ can be produced by noncytotoxic cells such as CD4+ Th1 cells and dendritic cells [30] which do not secrete sGranzymes.
A high correlation was found between sGranzyme A and sGranzyme B in both experimental and natural P. falciparum infections (0·84 and 0·83, respectively). This suggests that both, sGranzyme A and B, are more or less secreted concomitantly and probably from the same cellular source. Our findings are in agreement with data from a human endotoxemia study in which sGranzyme A peaked 3–4 h earlier than sGranzyme B [11]. The concentrations of sGranzyme A and B described in our study are in the same range as in CMV [31], rheumatoid arthritis [10] and melioidosis [11] and peak concentrations of sGranzyme A and B are similar in uncomplicated naturally acquired malaria patients and in experimentally infected individuals. This shows that the kinetics and plasma concentrations of sGranzyme A and B are similar in these diseases despite the great differences in aetiology.
In conclusion, the increased sGranzyme A and sGranzyme B concentration, reflecting cytotoxic T and/or NK, NKT cells responses, in the (semi)-immune population and in the nonimmune population indicate that NK, NKT cells and CTL's are activated at a time when parasites are first being released into the circulation and subsequently activated during the erythrocytic phase reflecting a mechanism that contributes to the host defense mechanism against malaria.
Acknowledgments
This study has been financially supported by a grant of WOTRO (W93-230), the Netherlands. We thank M. Bolmer-van de Vegte, J. Hooghof and G.J. van Gemert for culturing the malaria parasites and preparing P. falciparum infected mosquitoes, T. Arens for his skilled work at malaria diagnostics, J. van der Ven-Jongekrijg for her support in measuring the cytokine ELISA’s, J. Verstraeten for the clinical follow-up of the volunteers, R. Ramasamy for reading the manuscript and T. Bousema for his statistical help. We are indebted to the medical students, nursing and medical staff of the Department of Paediatrics of the Central Hospital and the Messa Dispensary in Yaoundé, Cameroon.
REFERENCES
- 1.Harpaz R, Edelman R, Wasserman SS, Levine MM, Davis JR, Sztein M. Serum cytokine profiles in experimental malaria. J Clin Invest. 1992;90:515–23. doi: 10.1172/JCI115889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronenberg M, Gapin L. The unconventional lifestile of NKT cells. Nature Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 3.Doolan D, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–62. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 4.Fell AH, Smith NC. Immunity to asexual blood stages of Plasmodium: is resistance to acute malaria adaptive or innate? Parasitol Today. 1998;14:3649. doi: 10.1016/s0169-4758(98)01298-8. [DOI] [PubMed] [Google Scholar]
- 5.Ojo-Amaize EA, Salimonu LS, Williams AI, Akinwolere OA, Shabo R, Alm GA, Wigzell H. Positive correlation between degree of parasitaemia, interferon titers and natural killer cell activity in Plasmodium falciparum-infected children. J Immunol. 1981;127:2296–300. [PubMed] [Google Scholar]
- 6.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–2. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 7.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–9. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 8.Bleackley RC, Lobe CG, Duggan B, et al. The isolation and characterization of a family of serine protease genes expressed in activated cytotoxic T lymphocytes. Immunol Rev. 1988;103:5–19. doi: 10.1111/j.1600-065x.1988.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 9.Spaeny-Dekking EH, Hanna WL, Wolbink AM, et al. Extracellular granzymes A and B in man: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–6. [PubMed] [Google Scholar]
- 10.Tak PP, Spaeny-Dekking L, Kraan MC, Breedveld FC, Froelich CJ, Hack CE. The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;116:366–70. doi: 10.1046/j.1365-2249.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauw FN, Simpson AJH, Hack CE, et al. Soluble granzymes are released during human endotoxemia and in patients with severe infection due to gram-negative bacteria. J Infect Dis. 2000;182:206–13. doi: 10.1086/315642. [DOI] [PubMed] [Google Scholar]
- 12.Kam C, Hudig D, Powers JC. Granzymes (lymphocyte serine proteases). characterization with natural and synthetic substrates and inhibitors. Biochem Biophys Acta. 2000;1477:307–23. doi: 10.1016/s0167-4838(99)00282-4. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:51–74. [Google Scholar]
- 14.Marsh K, Foster D, Waruiru C, et al. Indicators of life threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59. [PubMed] [Google Scholar]
- 16.Lensen A, van Druten J, Bolmer M, van Gemert G, Eling W, Sauerwein R. Measurement by membrane feeding of reduction in Plasmodium falciparum transmission induced by endemic sera. Trans R Soc Trop Med Hyg. 1996;90:20–2. doi: 10.1016/s0035-9203(96)90464-2. [DOI] [PubMed] [Google Scholar]
- 17.Ponnudurai T, Lensen AHW, van Gemert GJA, Bensink MPE, Bolmer M, Meuwissen THETh. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology. 1989;98:165–73. doi: 10.1017/s0031182000062065. [DOI] [PubMed] [Google Scholar]
- 18.Hack CE, Hart M, Strack van Schijndel RJM, Eerenberg AJM, Nuijens JH, Thijs LG, Aarden LA. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992;60:2835. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Pouw Kraan TCTM, Boeije LCM, Smeenk RJT, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen PM, van der Pouw Kraan TCTM, de Jong IW. Release of interleukin-12 in experimental Escherichia coli septic shock in baboons: relation to plasma levels of interleukin-10 and interferon-g. Blood. 1996;12:5144–51. [PubMed] [Google Scholar]
- 21.Wolbink GJ, Bossink AW, Groeneveld AB, de Groot MC, Thijs LG, Hack CE. Complement activation in patients with sepsis is in part mediated by C-reactive protein. J Infect Dis. 1998;177:81–7. doi: 10.1086/513803. [DOI] [PubMed] [Google Scholar]
- 22.Kremsner PG, Winkler S, Wilding E, Prada J, Bienzle U, Graninger W, Nussler AK. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90:44–7. doi: 10.1016/s0035-9203(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 23.Luty A, Perkins D, Lell B, et al. Low interleukin 12 activity in severe Plasmodium falciparum malaria. Inf Imm. 2000;68:3909–15. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins D, Weinberg J, Kremsner P. Reduced interleukin-12 and transforming growth factor-β1 in severe malaria: relationship of cytokine balance with disease severity. J Inf Dis. 2000;182:988–92. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- 25.Hermsen C, Telgt D, Linders E, van de Locht L, Eling W, Mensink E, Sauerwein R. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol. 2001;118:247–51. doi: 10.1016/s0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 26.Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, Cazenave PA. Liver CD4-CD8-NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000;164:1463–9. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 27.Romero JF, Eberl G, MacDonald H, Corradin G. CD1d-restricted NK T cells are dispensable for specific antibody responses and protective immunity against liver stage malaria infection in mice. Par Immunol. 2001;23:267–9. doi: 10.1046/j.1365-3024.2001.00381.x. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. Alpha-galactosylceramide-activated Va14 natural killer cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is dependent of Fas (CD95/Apo-1) or perforin-mediated cytotoxicity. Parasite Immunol. 1997;19:145. doi: 10.1046/j.1365-3024.1997.d01-190.x. [DOI] [PubMed] [Google Scholar]
- 30.Asselin-Paturel C, Boonstra A, Dalod M. Mouse type I IFN-producing cells are immature APC's with plasmacytoid morphology. Nat Immunol. 2001;2:1144–50. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 31.ten Berge IJ, Wever PC, Wolbink AM, Surachno J, Wertheim PM, Spaeny LH, Hack CE. Increased systemic concentrations of soluble granzyme A and B during primary cytomegalovirus infection after renal transplantation. Transplant Proc. 1998;30:3972–4. doi: 10.1016/s0041-1345(98)01308-6. [DOI] [PubMed] [Google Scholar]