Abstract
The clearance of intracellular bacteria requires the appropriate induction of proinflammatory cytokines and chemokines to recruit macrophages and T cells to the site of infection. In this study, we investigated the production of tumour necrosis factor (TNF)-α, interleukin (IL)-8 and interferon (IFN)-γ by the peripheral blood mononuclear cells (PBMC) of patients with multidrug-resistant tuberculosis (MDR-TB) in response to in vitro stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. The results were compared with those from cases of newly diagnosed TB (N-TB) and TB with treatment failure (TF-TB), and healthy tuberculin reactors (HTR). The most significantly depressed TNF-α levels were found in MDR-TB patients. IFN-γ production was depressed significantly in all groups of TB patients compared with the HTR group. TNF-α secretion in response to the 30-kDa antigen was unchanged by coculturing with recombinant human interferon (rhIFN)-γ, and was increased dramatically following IL-10 neutralization with an anti-human IL-10 antibody. The IL-8 levels were depressed significantly in MDR-TB patients compared with N-TB patients, but were similar to the IL-8 levels in TF-TB patients. Furthermore, rhTNF-α directly increased IL-8 secretion, and neutralizing antibody to TNF-α inhibited IL-8 production by the PBMC of MDR-TB patients that were stimulated with the 30-kDa antigen. Taken together, these data suggest that the PBMC of MDR-TB patients typically show TNF-α depression in response to the 30-kDa antigen, and this effect is modulated by IL-10. In addition, we highlight the role of TNF-α in IL-8 secretion in MDR-TB patients.
Keywords: interferon-gamma, interleukin-8, multidrug-resistant tuberculosis, tumour necosis factor-alpha, 30-kDa antigen
INTRODUCTION
Tuberculosis (TB) worldwide has been accompanied by an increase in the incidence of multidrug-resistant tuberculosis (MDR-TB), which is a significant public health and therapeutic problem that may rapidly worsen as the human immunodeficiency virus epidemic spreads [1]. Research on host defence and the immunopathogenesis of TB is necessary, because there is an urgent need for a new vaccine and adjunctive immunotherapy, particularly in patients with drug-resistant TB. The cytokine and chemokine signals in mycobacterial infection play crucial roles in protective immunity, because this is dependent on the precise co-ordination of T-lymphocyte sensitization and monocyte recruitment. Previous studies [2] showed that MDR-TB patients with low CD4 T cell counts had impaired interferon (IFN)-γ and interleukin (IL)-2 responses. In addition, our previous study [3] showed that MDR-TB patients had significantly elevated IL-10 and IL-18 production, when compared with healthy tuberculin reactors (HTR). However, little is known about tumour necrosis factor (TNF)-α or chemokine expression in patients with MDR-TB.
The important proinflammatory cytokine TNF-α plays a key role in the defence against TB [4,5]. Detailed studies of murine models have indicated that TNF plays an essential role in protective immunity against TB [6,7]. TNF-α contributes to the prevention of reactivation of persistent TB, and limits the pathological response of the host [8]. In humans, the critical role of TNF-α was emphasized by the reactivation of TB in rheumatoid arthritis patients who were treated with anti-TNF antibodies [4,9,10]. However, TNF-α may also be responsible for the toxic syndrome and tissue necrosis that accompany TB, since it has important proinflammatory activities [11]. Thus, successful protective immunity to TB may require a balance between antimycobacterial cytokine responses and proinflammatory cytokine responses that may result in unwanted tissue damage.
Chemokines belong to a large family of structurally related secreted proteins that are important for leucocyte trafficking during host defence and inflammation [12,13]. IL-8, which is the best characterized of the CXC subfamily of chemokines, appears to be an important chemokine in the mycobacterial host–pathogen interaction, and is involved in cellular recruitment to the granuloma [14]. IL-8 attracts neutrophils and T cells, both directly and indirectly, to sites of infection, and has recently been implicated in monocyte recruitment [15]. Bronchoalveolar lavage fluid from patients with active pulmonary TB contains elevated levels of IL-8, compared with healthy controls [16,17], which suggests that IL-8 is involved in protective immune responses to TB through the recruitment of cells for granuloma formation.
Currently, there is great interest in the secreted protein antigen (Ag) of M. tuberculosis in relation to immune responses to infection. The 30-kDa Ag is a very effective cytokine inducer and has been reported to strongly induce IFN-γ[18–20], IL-12, IL-10 [20] and TNF-α[21] in human monocytes or peripheral blood mononuclear cells (PBMCs). In addition, the 30-kDa Ag is recognized differently by the immune systems of infected healthy and diseased subjects, and may constitute a potential marker for protection against TB. Ours [20,22] and other [18] previous studies emphasized the role of the 30-kDa Ag, a major secretory antigen from M. tuberculosis, in eliciting differential IFN-γ induction in HTR and active pulmonary TB patients. However, little is known about the 30-kDa-induced cytokine or chemokine responses in patients with MDR-TB.
Given this background, this study analysed the TNF-α, IFN-γ and IL-8 secretion profiles of PBMCs from MDR-TB patients after in vitro stimulation with the 30-kDa Ag. The data were compared with those from conventional TB [newly diagnosed TB (N-TB), treatment failure TB (TF-TB)] and HTR. This study showed that MDR-TB patients produce less TNF-α and IFN-γ in response to the 30-kDa Ag. However, both the TNF-α and IL-8 levels were elevated significantly in PBMCs from newly diagnosed drug-sensitive TB patients compared with those from MDR-TB patients. In addition, IL-10 neutralization significantly increased the 30-kDa Ag-induced TNF-α levels in PBMCs from HTR and MDR-TB patients. Furthermore, TNF-α may play a major role in IL-8 expression by the PBMCs of MDR-TB patients following in vitro stimulation with the 30-kDa Ag of M. tuberculosis.
MATERIALS AND METHODS
Subjects
Whole blood was obtained by venipuncture from ‘MDR-TB (n = 17)’ patients and ‘not MDR-TB (n = 40)’ patients at the National Mokpo Tuberculosis Hospital (Mokpo, Chonnam, Korea), the Catholic University Hospital (Daejeon, Korea) and Chungnam National University Hospital (Daejeon, Korea). This study was approved by the bioethics committee of Chungnam National University Hospital, and all 1the participants gave their written consent.
The ‘not MDR-TB’ patients were divided into patients with N-TB (n = 19) and TF-TB (n = 21). The N-TB patients participated in this study within 1 month of beginning first-line antituberculosis drug medication. Their diagnoses were bacteriologically confirmed active TB. The N-TB patients had minimal to moderate TB, except for two patients who exhibited advanced TB in chest X-rays.
The TF-TB patients were undergoing a second round of treatment for TB, because the primary treatment had failed. These patients had histories of incomplete or irregular prior treatments and/or inappropriate treatments with the available chemotherapeutic agents. The 17 patients had culture-proven MDR-TB, and all of them had bacteria that were resistant to rifampicin and isoniazid. All of the patients had parenchymal TB, but none had miliary or pleural TB. None of the patients had a previous history of diabetes mellitus or steroid therapy, and all were HIV negative. Both the TF-TB and MDR-TB patients had moderate to advanced radiographic abnormalities upon chest X-ray examination. The mean durations of anti-TB treatment between the TF-TB and MDR-TB groups were 18·0 ± 15·2 and 31·8 ± 13·3 months, respectively, and the difference between these two groups was statistically significant (P < 0·05). A complete history was taken and a physical examination was performed on each patient by one of the investigators.
The 20 HTR control subjects exhibited skin reactions of more than 15 mm after intradermal inoculation of 5 units of PPD-RT23 (Statens Seruminstitut, Copenhagen, Denmark) within 1–3 years before this study. The HTR individuals had no previous history of clinical TB. All the healthy controls had received Mycobacterium bovis Bacille Calmette–Guérin (BCG) vaccination as children.
Antigen and antibodies
The 30-kDa Ag was purified from M. tuberculosis strain H37Rv culture filtrate, as described previously [23]. The endotoxin content in the 30-kDa Ag preparations was < 0·02 ng/mg protein for 30-kDa Ag, determined by the Limulus amoebocyte assay. Neutralizing rat antihuman IL-10 antibodies and the appropriate IgG isotype control antibodies were purchased from Endogen (Boston, MA, USA). The recombinant human IFN-γ (rhIFN-γ) and rhTNF-α were purchased from R&D Systems (Minneapolis, MN, USA). Phytohaemagglutinin (PHA; Sigma) and lipopolysaccharide (LPS; Sigma) were used as positive controls for Ag stimulation in this study.
Preparation of PBMC
Whole heparinized venous blood was obtained from each subject. PBMCs were prepared by centrifugation of the blood over Ficoll-Paque gradients, as described previously [20], and were counted and resuspended in complete medium.
Enzyme-linked immunosorbent assays (ELISA)
The TNF-α, IFN-γ, IL-10 and IL-8 content of the supernatant from PBMCs stimulated with the 30-kDa Ag was assessed using commercially available ELISA kits (PharMingen, San Diego, CA, USA), according to the manufacturer's instructions. The lower limit of detection for all the cytokines is less than 4 pg/ml, except for IL-8, for which it is less than 3 pg/ml.
Statistical methods
The results are presented as the mean ± s.d. Statistical significance was calculated using ANOVA, Student's t-test or linear regression analysis.
RESULTS
TNF-α and IFN-γ production by PBMCs from TB patients and HTR controls after in vitro stimulation with the 30-kDa Ag
Production of TNF-α, IFN-γ and IL-10 in response to the 30-kDa Ag was determined using ELISA in PBMC cultures from each group of patients and HTR. The supernatants were collected 18 (TNF-α, IL-10) and 96 (IFN-γ) h after stimulation with the 30-kDa Ag (1·0 µg/ml), and the cytokine levels peaked at 18 h for TNF-α and IL-10, and at 96 h for IFN-γ (data not shown). As a positive control, supernatants were collected from cells stimulated with PHA (10 µg/ml) at 48 h, or LPS (1 µg/ml) at 18 h.
TNF-α
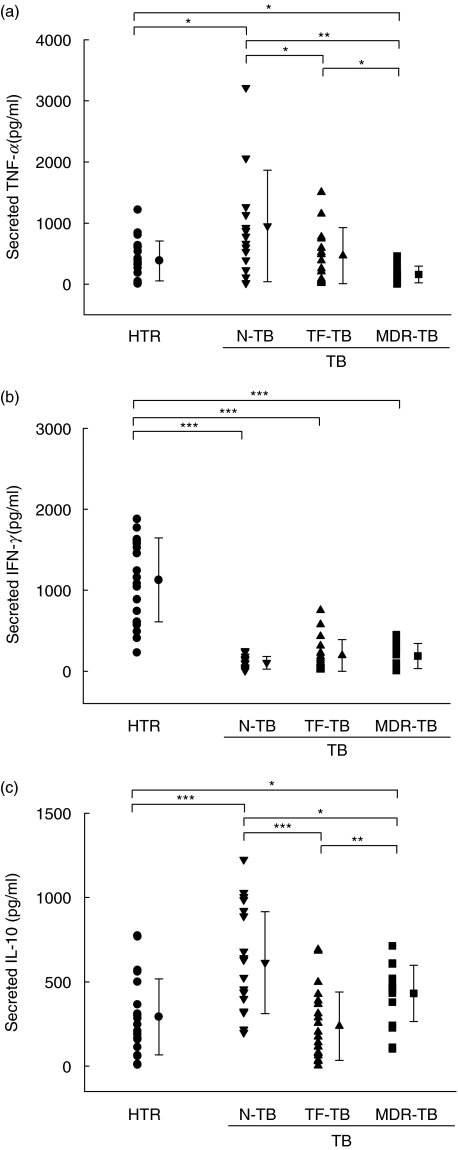
As shown in Fig. 1a, MDR-TB patients had significantly reduced TNF-α production after stimulation with the 30-kDa Ag for 18 h, as compared with the HTR controls or TF-TB patients (P < 0·05 in both cases), or the N-TB patients (P < 0·01). The TNF-α levels of PBMCs from MDR-TB patients were decreased more significantly than those of PBMCs from the other groups (P < 0·001) after a 96-h stimulation with the 30-kDa Ag (data not shown). The greatest increase in TNF-α production in response to the 30-kDa Ag was in the N-TB patients, compared with the other groups (HTR, P < 0·05; TF-TB, P < 0·05; MDR-TB, P < 0·01). LPS induced similar TNF-α titres in both healthy controls and MDR-TB patients (6510 ± 2420 versus 5800 ± 3190 pg/ml, P > 0·1; data not shown).
Fig. 1.
TNF-α and IFN-γ production by PBMCs from TB patients and HTR after in vitro stimulation with the 30-kDa Ag of M. tuberculosis. The PBMCs were stimulated with the 30-kDa Ag, and the supernatant harvested after 18 and 96 h, respectively. Secreted TNF-α and IFN-γ levels in the culture supernatants were measured by ELISA, as described in Materials and methods. The TNF-α (a), IFN-γ (b) and IL-10 (c) levels in PBMCs from each group of TB patients and HTR were determined after in vitro stimulation with the 30-kDa Ag. The values are shown as the mean ± s.d. of triplicate supernatant samples. The data are from one representative experiment. *P < 0·05; **P < 0·01; ***P < 0·001 (Student's t-test).
IFN-γ
The data for IFN-γ production by PBMCs were compared between groups at 96 h (Fig. 1b). The mean IFN-γ concentrations after stimulation with the 30-kDa Ag were significantly lower in each of the TB patient groups than in the HTR controls (N-TB, P < 0·001; TF-TB, P < 0·001; MDR-TB, P < 0·001; Fig. 1b). However, there were no significant differences between the groups of TB patients in IFN-γ production after stimulation with the 30-kDa Ag. PHA induced similar IFN-γ titres in both healthy controls and MDR-TB patients (9820 ± 4190 versus 8160 ± 5340 pg/ml, P > 0·1; data not shown).
IL-10
As shown in Fig. 1c, IL-10 production was significantly higher in the MDR-TB patients than in the HTR controls after an 18-h stimulation with the 30-kDa Ag (P < 0·05). PBMCs from N-TB patients produced significantly more IL-10 after stimulation with the 30-kDa Ag than did those from HTR controls (P < 0·01) or TF-TB patients (P < 0·05). LPS induced similar IL-10 titres in both healthy controls and MDR-TB patients (1700 ± 555 versus 1520 ± 580 pg/ml, P > 0·1; data not shown).
Effect of IFN-γ and endogenous IL-10 neutralization on 30-kDa Ag-induced TNF-α production
Because TNF-α secretion in response to the 30-kDa Ag was depressed significantly in MDR-TB patients, subsequent experiments were carried out to further evaluate the effects of IFN-γ or IL-10 on 30-kDa-induced TNF-α production by PBMCs. PBMCs from five HTR individuals were cultured in complete RPMI with or without the 30-kDa Ag (1 µg/ml) following co-culture with rhIFN-γ (10 ng/ml) or neutralizing antibodies to IL-10 (2 µg/ml). Culture supernatants were collected at 18 h and assayed for TNF-α production by ELISA. Endogenous TNF-α production by PBMCs from HTR or MDR-TB patients was unchanged after co-culture with IFN-γ(Fig. 2). However, TNF-α secretion as a result of LPS stimulation was increased significantly in both the HTR and MDR-TB patients (data not shown).
Fig. 2.
The effect of rhIFN-γ or endogenous IL-10 on TNF-α induction by the 30-kDa Ag of M. tuberculosis. PBMC from HTR (n = 5) and MDR-TB patients (n = 5) were cultured with or without rhIFN-γ, neutralizing antibody to IL-10 (2 µg/ml), or control antibody (2 µg/ml). The 30-kDa Ag (1 µg/ml) was added to each of the cultures. Immunoreactivity for TNF-α was assessed in the culture supernatants at 18 h. The data are the mean ± s.d. of a representative result of three independent experiments. The percentage increase in TNF-α immunoreactivity relative to the TNF-α level of a culture that was treated with the 30-kDa Ag alone (100%) is shown. □, 30-kDa only;  , 30-kDa + rhIFN-γ; ▪, 30-kDa + α-IL-10;
, 30-kDa + rhIFN-γ; ▪, 30-kDa + α-IL-10;  , 30-kDa + control IgG.
, 30-kDa + control IgG.
Interestingly, the TNF-α levels were elevated significantly in cultures from HTR (P < 0·001) and MDR-TB patients (P < 0·05) that contained the neutralizing antibody to IL-10, whereas an IgG1 isotype control antibody used at the same concentration did not affect TNF-α secretion (Fig. 2). The increases in TNF-α production were greater in the HTR group than in the MDR-TB group (1·6-fold increase for MDR-TB; 3·4-fold for HTR). Therefore, we found that endogenous IL-10 neutralization, but not the addition of rhIFN-γ, increases the 30-kDa-induced TNF-α secretion from the PBMCs of MDR-TB patients.
IL-8 production as a result of in vitro stimulation with the 30-kDa Ag of PBMCs from TB patients and HTR controls
We investigated whether chemokine levels were expressed differentially in the various clinical forms of TB. To compare the differences in IL-8 production between subject groups, the optimal IL-8 production was determined using ELISA in PBMC cultures from HTR subjects 6, 18, 48 and 96 h after stimulation with the 30-kDa Ag (1·0 µg/ml), and the peak IL-8 level was observed after 96 h of stimulation in different donors (data not shown). Therefore, we compared the individual IL-8 concentrations between the groups at 96 h poststimulation.
IL-8 production was significantly depressed in MDR-TB patients in response to the 30-kDa Ag, compared with the N-TB patients (MDR-TB, 316·2 ± 260·5 ng/ml; N-TB, 686·1 ± 346·8 ng/ml, P < 0·01; Fig. 3a). However, the IL-8 levels in PBMC from MDR-TB patients did not differ significantly when compared with those from either TF-TB patients or HTR (P > 0·1). N-TB patients showed significantly elevated IL-8 levels, when compared with HTR (P < 0·001) or MDR-TB patients. In addition, a significant correlation was observed between IL-8 and TNF-α production in the culture supernatants of PBMC from TB patients (n = 57, r = 0·58, P < 0·001; Fig. 3b), but not those from HTR controls (n = 20, r = 0·17, P > 0·1; Fig. 3b). LPS induced similar IL-8 titres in both healthy controls and MDR-TB patients (790 ± 340 versus 620 ± 390 ng/ml, P > 0·1; data not shown).
Fig. 3.
IL-8 production by PBMC from TB patients and HTR in response to the 30-kDa Ag of M. tuberculosis. (a) IL-8 production in TB patients and HTR. The supernatants were prepared after 96 h, and the cytokine concentrations were measured by ELISA. The values shown are the mean ± s.d. of triplicate supernatant samples. The data are from one representative experiment. (b) There was significant correlation between the IL-8 and TNF-α levels in 30-kDa Ag-stimulated PBMCs from TB patients (n = 57, r = 0·58, P < 0·001), but not those from HTR controls (n = 20, r = 0·17, P > 0·1). •, HTR; —, HTR reg; r, TB; ···, TB reg.
Effect of IFN-γ and TNF-α on 30-kDa Ag-induced secretion of IL-8
Next, we investigated whether IFN-γ or TNF-α interact with chemokine production, thereby contributing to the overall host defense and pathogenic responses in TB. Therefore, we assessed the effects of IFN-γ and TNF-α on the levels of IL-8 induced in human PBMCs by the 30-kDa Ag. PBMCs from HTR controls (n = 7) were cultured in complete RPMI with or without the 30-kDa Ag (1 µg/ml) following co-culture with rhIFN-γ (10 ng/ml), rhTNF-α (10 ng/ml), or both, or rhIFN-γ with anti-TNF-α moAb (2 µg/ml). Culture supernatants were collected at 96 h and assayed for IL-8 production by ELISA.
As shown in Fig. 4a, there was significant up-regulation of endogenous IL-8 in the Ag-stimulated PBMCs after co-culture with rhTNF-α (P < 0·01), but not rhIFN-γ (P > 0·05). In addition, IL-8 production was activated more significantly by stimulation with both rhTNF-α and rhIFN-γ (P < 0·001) than after co-culture with rhTNF-α only; however, this effect was abrogated by the addition of neutralizing anti-TNF-α MoAb (2 µg/ml). Co-culture with the same concentration of isotype control antibody had no effect on IL-8 formation (Fig. 4a).
Fig. 4.
Effect of IFN-γ and TNF-α on 30-kDa Ag-induced IL-8 production by PBMC of MDR-TB patients. (a) Effect of rhIFN-γ or rhTNF-α (each 10 ng/ml) on IL-8 production induced by the 30-kDa Ag. PBMC from HTR (n = 7) were cultured with or without rhIFN-γ, rhTNF-α, rhIFN-γ+ rhTNF-α, rhIFN-γ+ anti-TNF-α antibody or rhIFN-γ+ control antibody. Then 30-kDa Ag (1 µg/ml) was added to each of the cultures. The culture supernatants were collected at 96 h and assayed for IL-8 production by ELISA. The percentage increase in IL-8 immunoreactivity relative to the IL-8 level of a culture that was treated with 30-kDa Ag alone (100%) is shown. (b) Effect of endogenous TNF-α on IL-8 production that was induced by the 30-kDa Ag. PBMC from HTR (n = 7) were stimulated with the 30-kDa Ag and cultured with or without neutralizing antibody to TNF-α (2 µg/ml) or control antibody (2 µg/ml). The percentage increase in IL-8 immunoreactivity relative to the IL-8 level of a culture that was treated with 30-kDa Ag alone is shown. (c) Effects of rhTNF-α on 30-kDa Ag-induced IL-8 production by PBMC from MDR-TB patients (n = 7). The data are representative of three separate experiments. ▪, 30-kDa; □, 30-kDa + rhTNF-α.
We also assessed the effect of endogenous TNF-α neutralization on Ag-induced IL-8 secretion. PBMCs from HTR (n = 7) were stimulated with the 30-kDa Ag and cultured in complete RPMI with or without neutralizing antibody to TNF-α (2 µg/ml). The culture supernatants were collected at 96 h and assayed for IL-8 production by ELISA. Neutralization of the endogenous TNF-γ with an anti-TNF-α MoAb (2 µg/ml) decreased the IL-8 level approximately twofold in Ag-primed PBMCs from HTR, whereas the co-culture with the same concentration of isotype control antibody had no effect on IL-8 formation (Fig. 4b).
Furthermore, PBMCs from MDR-TB patients (n = 7) were cultured in complete RPMI with or without the 30-kDa Ag (1 µg/ml) following co-culture with rhIFN-γ (10 ng/ml) for 96 h. Although there was a substantial heterogeneity in the increase in IL-8 production in 30-kDa Ag-induced PBMCs from MDR-TB patients, PBMCs from MDR-TB patients produced more IL-8 after co-culture with rhTNF-α, compared with those by 30-kDa Ag alone (Fig. 4c). The mean immunoreactivity of PBMC from MDR-TB patients for IL-8 at 96 h was increased significantly after treatment with rhTNF-α (P < 0·01).
DISCUSSION
In this study, we found that depressed TNF-α levels in response to stimulation with the 30-kDa Ag were characteristic of MDR-TB patients. MDR-TB patients in this study had clinically advanced disease; the mean treatment duration was more than 2 years, despite antituberculosis drug therapy. Our data are in partial agreement with previous studies in which patients with fatal M. tuberculosis infection showed reduced secretion of proinflammatory cytokines, such as TNF-α, after ex vivo stimulation of whole blood leucocytes [24]. In addition, monocytes from patients with chronic refractory TB have been found to release significantly lower amounts of TNF-α than those from patients with N-TB [25]. In contrast, a recent study showed that TNF-α production was elevated in cells from pulmonary TB patients in response to the 30-kDa Ag, compared with cells from healthy controls [26]. This inconsistency may be due to differences in the clinical status of the TB patients studied. It seems that decreased TNF-α levels do not simply reflect the chronic conditions of these patients, since there were no significant correlations between treatment duration and TNF-α levels. In addition, there were no significant associations between various parameters (performance status, malnutrition examined by body mass index, chest X-ray findings or IFN-γ levels) and TNF-α levels in the patients with MDR-TB and TF-TB. Therefore, our results suggest that TNF-α depression induced by the 30-kDa antibody is a unique finding associated with the strains infecting MDR-TB patients.
The depressed levels of IFN-γ in these patients may not be responsible for the decrease in TNF-α secretion. Our data are partly in accordance with the previous finding that preincubation of monocytes with IFN-γ did not augment BCG-induced TNF-α production, whereas LPS induced the production of large amounts of TNF-α from cultured monocytes [25]. Although IFN-γ activates mononuclear phagocytes, which results in enhanced endotoxin-induced TNF-α release [27], both endotoxin and mycobacterial Ag may stimulate monocytes to produce TNF-α by different mechanisms.
The mechanism underlying the decreased TNF-α levels in MDR-TB patients may be associated with the presence of IL-10, based on the finding that IL-10 neutralization significantly increased the TNF-α levels in PBMC from HTR and MDR-TB patients. Our data are partially consistent with previous findings that neutralization of IL-10 prolonged TNF-α mRNA expression, and significantly increased net TNF-α production in human monocytes after LPS stimulation [28]. The same study demonstrated that potentiation of TNF-α production by IFN-γ in monocytes was coupled to the inhibition of endogenous IL-10 expression [28]. Our previous results [3] and the current data suggest that increased IL-10 production by PPD-stimulated PBMC from MDR-TB patients is associated with depressed TNF-α secretion in MDR-TB patients. However, the TNF-α increases were greater in HTR than in MDR-TB, although the IL-10 levels were significantly elevated in MDR-TB patients versus HTR patients. Therefore, our findings show that IL-10 neutralization plays an important, but not exclusive, role in the TNF-α secretion from PBMCs of MDR-TB patients following in vitro stimulation with the 30-kDa Ag.
We suggest that the rate of apoptosis in monocyte-derived cells from MDR-TB patients is higher than in HTR after exposure to mycobacterial Ags, as seen in a previous study [26]. The percentage of apoptotic monocytes tends to be higher in TB patients than in healthy controls, both in non-stimulated cell cultures and in those incubated in the presence of mycobacterial Ags [26]. Although we were not able to examine the rate of apoptosis in the Ag-stimulated monocytes from these patients, the number of monocytes, which is the main source of TNF-α, was not different in MDR-TB patients and HTR (data not shown). In addition, the PBMC of MDR-TB patients showed similar cytokine production patterns in response to LPS when compared with HTR.
Previous studies indicate that spontaneous TNF-α and IL-1β secretion by peripheral blood monocytes is significantly higher in TB patients [29]. Although we did not measure the spontaneous secretion of TNF-α in all the patients in this study, we suggest that the mononuclear cells from these patients are anergic due to repetitive, chronic antigenic stimulation in vivo. Further studies should clarify the mechanisms underlying the TNF-α depression after stimulation with the 30-kDa Ag.
Several reports have demonstrated that IL-8 is produced by monocytes and alveolar macrophages that are infected in vitro with M. tuberculosis or stimulated in vitro with PPD [17,30]. IL-8 is a member of the CXC chemokine subfamily, and the most prominent sources of IL-8 are monocytes and macrophages [31]. Although several studies have focused on the central role of IL-8 in the normal immune response to TB, i.e. through leucocyte recruitment to areas of granuloma formation [32,33], little is known about chemokine production in pulmonary TB patients in terms of their distinct disease statuses or infecting strains. The current data show that IL-8 is elevated significantly in the 30-kDa Ag-stimulated PBMC from N-TB patients compared with those from MDR-TB patients. However, there were no significant differences in the levels of IL-8 secretion between MDR-TB and TF-TB patients. However, our data are inconsistent with the previous finding that IL-8 production by PBMC in response to ex vivo stimulation with PPD was similar in normal blood donors and patients with pulmonary TB [34]. This discrepancy might be due to the disease status of pulmonary TB patients, because the mean duration of anti-TB treatment in the previous studies was approximately 6 months [34], and our data are unique in demonstrating differential IL-8 production in various disease states.
We also found that rhTNF-α directly increased IL-8 secretion by PBMC from HTR or MDR-TB patients. TNF-α is a pluripotent activator of inflammation and induces a proinflammatory cytokine cascade, which is mediated partly by the inducible expression of IL-8 [35]. The previous study also demonstrated that TNF-α-induced IL-8 expression was dependent on nuclear factor-κB through a delayed, reactive oxygen species-dependent signalling pathway [35]. Our data emphasize an additional critical role of TNF-α in the regulation of IL-8 expression, and further definition of this pathway will yield new insights into the mechanisms underlying inflammation that is initiated by TNF-α.
In conclusion, our results demonstrate that 30-kDa Ag-stimulated PBMCs from MDR-TB patients have significantly depressed levels of TNF-α, although the levels of IL-8 in PBMC from these patients are not significantly different from those of TF-TB patients. The depression of TNF-α secretion in MDR-TB patients is partially alleviated by neutralizing antibody to IL-10. In addition, TNF-α may contribute to the innate immune response to TB infection through the regulation of IL-8. Therefore, diminution of TNF-α levels may impair host immune defences critically in MDR-TB patients.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ10-PG6–01 G03-002).
REFERENCES
- 1.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 2.McDyer JF, Hackley MN, Walsh TE, Cook JL, Seder RA. Patients with multidrug-resistant tuberculosis with low CD4+ T cell counts have impaired Th1 responses. J Immunol. 1997;158:492–500. [PubMed] [Google Scholar]
- 3.Lee JS, Song CH, Kim CH, et al. Profiles of IFN-γ and its regulatory cytokines (IL-12, IL-18, and IL-10) in peripheral blood mononuclear cells from patients with multidrug-resistant tuberculosis. Clin Exp Immunol. 2002;128:516–24. doi: 10.1046/j.1365-2249.2002.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 5.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin-1, tumour necrosis factor-α and transforming growth factor-β, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bean AG, Roach DR, Briscoe H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–11. [PubMed] [Google Scholar]
- 8.Mohan VP, Scanga CA, et al YuK. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–55. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–9. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 10.Nunez Martinez O, Ripoll Noiseux C, Carneros Martin JA, Gonzalez Lara V, Gregorio Maranon HG. Reactivation tuberculosis in a patient with anti-TNF-α treatment. Am J Gastroenterol. 2001;96:1665–6. doi: 10.1111/j.1572-0241.2001.03836.x. [DOI] [PubMed] [Google Scholar]
- 11.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene intercrine cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 13.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nature Med. 1996;2:1174–8. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 14.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 15.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 16.Kurashima K, Mukaida N, Fujimura M, et al. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–7. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 17.Sadek IM, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513–21. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 18.Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–80. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Lin Y, Iyer DV, Gong J, Abrams JS, Barnes PF. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–4. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song CH, Kim HJ, Park JK, et al. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect Immun. 2000;68:4477–84. doi: 10.1128/iai.68.8.4477-4484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aung H, Toossi Z, Wisnieski JJ, et al. Induction of monocyte expression of tumor necrosis factor alpha by the 30-kD alpha antigen of Mycobacterium tuberculosis and synergism with fibronectin. J Clin Invest. 1996;98:1261–8. doi: 10.1172/JCI118910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo EK, Kim HJ, Lim JH, et al. Dysregulated production of interferon-gamma, interleukin-4 and interleukin-6 in early tuberculosis patients in response to antigen 85B of Mycobacterium tuberculosis. Scand J Immunol. 2000;51:209–17. doi: 10.1046/j.1365-3083.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 23.Lim JH, Park JK, Jo EK, et al. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect Immun. 1999;67:6187–90. doi: 10.1128/iai.67.11.6187-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedland JS, Hartley JC, Hartley CG, Shattock RJ, Griffin GE. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 1995;100:233–8. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takashima T, Ueta C, Tsuyuguchi I, Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990;58:3286–92. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portales-Perez DP, Baranda L, Layseca E, et al. Comparative and prospective study of different immune parameters in healthy subjects at risk for tuberculosis and in tuberculosis patients. Clin Diagn Laboratory Immunol. 2002;9:299–307. doi: 10.1128/CDLI.9.2.299-307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collart MA, Belin D, Vassalli JD, de Kossodo S, Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986;164:2113–8. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly RP, Freeman SL, Hayes MP. Inhibition of IL-10 expression by IFN-gamma up-regulates transcription of TNF-alpha in human monocytes. J Immunol. 1995;155:1420–7. [PubMed] [Google Scholar]
- 29.Wang CH, Lin HC, Liu CY, Huang KH, Huang TTYuCT, Kuo HP. Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2001;5:283–91. [PubMed] [Google Scholar]
- 30.Zhang Y, Broser M, Cohen H, et al. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–92. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura T, Matsushima K, Tanaka S, et al. Purification of human monocyte derived neutrophil chemotactic factor that shares sequence homology with other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–7. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 33.Ameixa C, Friedland JS. Down-regulation of interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes by interleukin-4 and -10 but not by interleukin-13. Infect Immun. 2001;69:2470–6. doi: 10.1128/IAI.69.4.2470-2476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakaya M, Yoneda T, Yoshikawa M, et al. The evaluation of interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-alpha) level in peripheral blood of patients with active pulmonary tuberculosis. Kekkaku. 1995;70:461–6. [PubMed] [Google Scholar]
- 35.Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–89. [PubMed] [Google Scholar]