Abstract
Rheumatoid arthritis is a chronic inflammatory disease of unknown aetiology predominantly affecting cells and tissues of synovial joints. Here we show that the two important complement regulators FHL-1 and factor H play a protective anti-inflammatory role in rheumatoid arthritis. Expression analyses at the mRNA- and protein level show in vitro expression and secretion of both regulators by synovial fibroblasts derived from patients with rheumatoid arthritis. Similarly the two regulators are synthesized in vivo in diseased synovial tissue, and in particular synovial lining cells express high levels of FHL-1. The anti-inflammatory role of these regulators in rheumatoid arthritis is highlighted by their induction with IFN-γ and dexamethasone, whilst the pro-inflammatory cytokine TNF-α had no effect. Transient transfection experiments with various FHL-1/factor H promoter-luciferase reporter constructs into cells of distinct origin show independent cell and tissue specific promoter regulated transcription of these two regulators. The inducible expression, specifically of FHL-1 has physiological consequences. By binding directly to surfaces the released proteins protect cells from inflammatory damage and complement-mediated cell lysis. This study shows a novel protective and anti-inflammatory role of the two important complement regulators FHL-1 and factor H in rheumatoid arthritis and suggests a disease controlling role of the two proteins.
Keywords: FHL-1/, Reconectin, Factor H, rheumatoid arthritis, inflammation, complement
INTRODUCTION
The complement system, a central part of the innate immunity protects organisms against invading microorganisms, removes debris from plasma and modulates humoral and cell-mediated immune responses. Activation of the complement system induces and enhances inflammatory reactions, which are initiated and mediated by newly generated anaphylatoxins and other inflammatory peptides. In addition the activated system opsonizes particles with complement components and generates a potentially cytolytic membrane attack complex on foreign targets [1–3]. Complement activation occurs via three major pathways: (i) the classical (ii) the lectin and (iii) the alternative pathway. The alternative pathway, which is initiated spontaneously, continuously and independently of antibodies, attacks all particles and cells. Consequently, host cells need to provide specific protection mechanisms against the deleterious effects of alternative pathway activation [2–4].
The plasma proteins factor H-like protein-1 (FHL-1), which is also termed reconectin, as a regulator of complement and fibronectin-like adhesion protein [5], factor H, and the three membrane bound molecules complement receptor type 1, CR1 (CD35), decay accelerating factor, DAF (CD55) and membrane cofactor protein, MCP (CD46) regulate AP activation at the level of C3b and C3bBb. Factor H is an abundant 150 kD single chain plasma glycoprotein (400–500 µg/ml) composed of 20 separately folding protein domains, termed short consensus repeats (SCR). An alternatively processed factor H gene transcript encodes FHL-1, a 42-kD protein that contains the first seven SCRs of factor H and four additional amino acids at the C-terminal end. FHL-1 is found in plasma at a concentration of 10–50 µg/ml [5,6].
FHL-1 and factor H are central complement regulators. Both proteins compete with factor B for binding to C3b, support the dissociation of the C3bBb complex (decay-accelerating activity) and act as cofactors for the cleavage of C3b by factor I [7–9]. Although both proteins are highly structurally related and have overlapping regulatory activities and binding functions, they also display functional differences. For example, FHL-1 uniquely acts as an adhesion protein and has cell-spreading activity, which is mediated by an exposed RGD domain located within SCR 4 which binds to an integrin-type receptor [5,10].
Increased complement activation occurs during inflammatory processes, such as in rheumatoid arthritis, where complement activation products are generated in the synovium [11]. Rheumatoid arthritis (RA) is a common human autoimmune disease with a prevalence of about 1%. Despite recent progress in defining the aetiology and pathogenesis, the pathophysiology of the disease is still incompletely understood. Rheumatoid arthritis is characterized by a chronic inflammation of synovial joints [12,13] and a consequent progressive destruction of cartilage and bone. Cytokines are major mediators of inflammation in RA [14] and a myriad of cytokines are found within the rheumatoid joint. Some of these immune mediators appear to exacerbate disease (e.g. TNF-α) [12], whereas others may down-regulate inflammation (e.g. IL-4, IL-10) [15,16]. In many cases the precise roles of the effector proteins are poorly defined (e.g. IFN-γ) [17–20]. There is also evidence of a systemic inflammation. For example, the levels of acute phase proteins and activation products of complement are elevated in blood and synovial fluids of patients suffering from active RA [21–23]. The complement activation profile shows that in RA complement activation is induced mainly by the alternative pathway [22,24]. Cartilage-specific collagen type II serves as an activator surface of the alternative pathway, thus indicating a direct and local role of the exposed matrix in the production and perpetuation of RA [25]. In affected joints of RA patients immune complexes, which also activate complement are generated and which may further enhance the inflammatory reactions [26–29]. Use of the soluble complement regulator CR1 has shown to reduce complement activation and tissue injury in a rat model of RA [30–32]. Moreover the resistance of C5-deficient mice to collagen-induced arthritis indicates an important role for complement in the pathogenesis of arthritis [33].
The aim of the present study was to investigate the role of the human complement regulators FHL-1 and factor H in RA. We demonstrate that both proteins are expressed in vitro and in vivo by synovial fibroblasts derived from patients with RA, and characterized the cell- and tissue-specific expression of both regulators as well as the effects of the cytokines IFN-γ, TNF-α and the therapeutic steroid dexamethasone on their expression levels. A physiological relevance is suggested by the fact that both released proteins bound to the cell surface. Thus by acting in an autocrine fashion FHL-1 and factor H protect synovial cells from complement-mediated cell cytoxicity and stimulation of this activity could offer new therapeutic aspects in RA.
MATERIALS AND METHODS
Cells
The human cell lines MRC-5 (lung fibroblast), 293-T (epithelial kidney) and HUH7 (hepatoblastoma) were cultured by standard methods in RPMI 1640 containing 10% heat inactivated fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 µg/ml) and Amphotericin B (0·25 µg/ml) (Life Technologies, Heidelberg, Germany). Human synovial fibroblasts cell lines GDR, HWL and SOZ were established from patients with active rheumatoid arthritis and were cultured for 3–5 passages [34,35]. After reaching confluency cells were treated for 24 h with human recombinant interferon-γ (Pharmingen, Heidelberg, Germany) at a working concentration of 100 U/ml, human recombinant tumour necrosis factor-α (Sigma-Aldrich, Heidelberg, Germany) at 10 ng/ml or dexamethasone (Serva, Heidelberg, Germany at 0·1 µM.
Antibodies and other reagents
The monoclonal antifactor H antibody 196X cell line was kindly obtained from Dr J. Tamerius (Quidel Corp., San Diego, CA, USA), and the Mab VIG8 obtained from Prof W. Prodinger has been reported previously [36]. Polyclonal rabbit antisera anti-SCR 1–4 (raised against the N-terminal SCRs 1–4 of FHL-1 and factor H) and anti-SCR 19–20 (raised against the C-terminal SCRs of factor H) have been described [37,38]
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cells using Trizol LS Reagent (Life Technologies, Heidelberg, Germany). RNA from human liver tissue was isolated with guanidine thiocyanate and purified by CsCl centrifugation [38]. Isopropyl alcohol precipitated RNA (1 µg) dissolved in water was denatured at 70°C for 10 min and then immediately chilled on ice. Reactions were carried out in a volume of 20 µl containing 10 mM dithiotreitol (Gibco), 500 µM dNTP Mix (Amersham Biotech, Freiburg, Germany), 25 µg/ml oligo(dT)12–18 (Amersham Biotech) and 200 U Moloney murine leukaemia virus reverse transcriptase (Gibco) in a reverse transcriptase buffer (Gibco). The reaction was allowed to proceed for 1 h at 37°C.
Each PCR reaction was carried out in a 100-µl volume containing 200 µM dNTP (Amersham Biotech), 10 pmol of each of the specific primers and 2·5 U Taq polymerase (Amersham Biotech) in an PCR buffer containing 1·5 µM MgCl2 (Amersham Biotech). The samples were denatured at 94°C for 5 min and amplification was performed for 30 cycles with denaturation at 94°C for 1 min, annealing at 46°C for 1 min and extension at 72°C for 1 min The final reaction step was followed by a 10-min extension step at 72°C to ensure that the amplified DNA was double-stranded. Amplified products (10 µl for each PCR sample) were electrophoresed by agarose gel. The identities of the PCR products were confirmed by cloning into pCRII cloning vector (Invitrogen, Groningen, the Netherlands) and sequence analysis. Primers used are shown in Table 1.
Table 1.
Primers used in RT-PCR
| Protein | Sense | Antisense | Position |
|---|---|---|---|
| FHL-1 | 5’CAG AAG TTC AGA GGG TAA AGC T 3’ | 5’TAC TGG CTG GAT ACC TGC TCC G 3’ | 1006–1430 |
| Factor H | 5’TCT GCA TGT TGG CCT TCC TGT C 3’ | 5’CTT CCT TGT AAA TCT CCA CCT G 3’ | 2861–3215 |
| CD46 | 5’TTG GGG GCT TAC GGC TCC AAA T 3’ | 5’TGT GAG GAG CCA CCA ACA TTT G 3’ | 137–500 |
| CD55 | 5’GTG TTA CAT GAG AAG GAG ATG G 3’ | 5’TGA CTG TGG CCT TCC CCC AGA T 3’ | 168–640 |
| CD59 | 5’GGG ATG AAG GCT CCA GGC TGC T 3’ | 5’CTG CAG TGC TAC AAC TGT CCT A 3’ | 139–447 |
| β-actin | 5’TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA 3’ | 5’CTA GAA GCA TTG CGG TGG ACG ATG AG GG 3’ | 478–1128 |
Immunoblotting
Supernatants from synovial fibroblasts grown in RPMI without FCS were concentrated 30-fold with the Millipore Ultrafree®-15 Centrifugal Filter Device. Aliquots were electrophoresed on a 10% SDS-PAGE gel under nonreducing conditions and transferred to a nitrocellulose filter (Schleicher & Schuell, Dassel, Germany) [38]. After blocking nonspecific binding sites with 5% (w/v) dried milk in PBS for 30 min the filter was incubated with the rabbit antifactor H SCR 1–4 antibody (raised against the N-terminal SCRs 1–4 of factor H and FHL-1 (as described in 37) overnight at 4°C, washed, and incubated with peroxidase-conjugated goat anti-rabbit IgG (diluted 1 : 400) (Dako, Glostrup, Denmark) for 3 h at 22°C. The bound secondary antibody was detected following addition of 0·3% (w/v) 4-chloro-1-naphthol in 10% (v/v) methanol in PBS.
Plasmids and transfection
The FHL-1/factor H promoter luciferase constructs has been described [39]. For transient transfections of the cell lines, 4 × 105 cells were transfected with 2 µg of reporter plasmid together with 1 µg of pRL-TK vector (Promega, Heidelberg, Germany) to control for transfection efficiency. After 24 h, cells were washed twice with PBS and harvested by lysing in 250 µl of lysis reagent (Promega) for 8 min at room temperature and centrifuged for 10 s at maximum speed in an Eppendorf microfuge. Cell supernatant (50 µl) was mixed with 50 µl of luciferase assay reagent (Promega) and the light emission was measured immediately at 25°C using a luminometer (Berthold Biolumat, Bad Wildbad, Germany LB 9500C). The initial 10 s integral of light emission was recorded. All assays were performed five times.
Flow cytometry
For flow cytometric analysis synovial fibroblasts were detached from culture dishes with 0·02% EDTA, washed twice and suspended into 0·5% BSA/PBS. To study the binding of FHL-1 and factor H to cell membranes, 5 × 105 cells were first incubated for 1 h at 37°C with 25% NHS, with purified factor H (500 µg/ml, obtained from Calbiochem, Schwalbach, Germany) or with recombinant FHL-1 (50 µg/ml) expressed in insect cells as described [37] for 30 min at 37°C. Following a 30-min incubation with polyclonal anti-SCR 1–4 or anti-SCR 19–20 antibody at 4°C, cells were washed carefully and incubated with secondary antibody (FITC-conjugated swine anti-rabbit IgG (Pharmingen) for 30 min at 4°C, washed and analysed using a Becton Dickinson FACS-SCAN 440 flow cytometer. Control staining was performed using a nonspecific mouse or rabbit IgG as the primary antibody (Sigma).
Immunoperoxidase staining
For immunoperoxidase staining, fresh tissue samples were frozen in liquid nitrogen and stored at −70°C. Serial cryostat sections (4–5 µm) were transferred to poly L-lysine coated slides, dried, fixed in cold (−20°C) acetone for 5–10 min and incubated in PBS, pH 7·4 with 0·1% phenylhydrazine for 30 min to inhibit endogenous peroxidase. The sections were blocked with 1% BSA in PBS for 30 min The primary monoclonal antibodies (VIG8, 196X) were applied at optimal concentrations on the sections and incubated for 60 min at room temperature. Irrelevant mouse IgG (Coulter Immunology, Hialeah, FL, USA) was used as a negative control. The sections were then washed three times in PBS/0·1% Tween and incubated for 60 min with HRP-conjugated rabbit antimouse immunoglobulins (Dako). After three washes with PBS/0·1% Tween, the tissues were treated with aminoethylcarbazol as a chromogen and were finally counterstained with haemalum (Merck, Darmstadt, Germany).
Complement-mediated lysis of cells
Synovial fibroblasts were detached from culture flasks with EDTA 0·02%, counted and washed with RPMI. 2 × 106 cells were labelled with 100 µCi of 51Cr (Amersham Biotech) in 1 ml RPMI-10% FCS for 2 h at 37°C, washed twice with RPMI, incubated for a further 30 min to release loosely bound 51Cr and washed again twice. Duplicate aliquots of 51Cr-labelled cells (105 cells/50 µl) were treated with different concentrations of anti-SCR-1–4 or anti-SCR 19–20 antibodies (20 min, 22°C) and 25% NHS as a complement source (30 min, 37°C) in a total volume of 200 µl. The amount of cell lysis was determined as percentage of 51Cr release into the supernatant (experimental radioactivity minus basal radioactivity). 51Cr release in the absence of antibodies was taken as basal radioactivity, and release by 0·1% Nonidet P40 (BDH Laboratory Supplies, Poole, UK) as total lysis. All experiments were performed in duplicate and the results are expressed as mean ± SD values.
RESULTS
Synthesis of complement regulators FHL-1 and factor H by synovial fibroblasts
We have previously demonstrated the presence of the two complement regulators FHL-1 and factor H in synovial fluid of RA patiens [35]. It was therefore of interest to compare at an RNA level the expression and regulation of FHL-1 and factor H with that of the additional regulators CD46, CD55 and CD59. In synovial fibroblast cell lines GDR, HWL and SOZ, derived from patients with RA, constitutive expression of FHL-1, CD46, CD55 and CD59 was found, while factor H expression was undetectable. Representative data are shown for the GDR cell line (Fig. 1a,b). In addition, we tested the effect of inflammatory mediators on the expression of complement regulators in these cells. The pro-inflammatory cytokine TNF-a did not influence the expression level of any of the investigated regulatory proteins (Fig. 1a, lane 2). In contrast interferon-g (IFN-g) and the anti-inflammatory agent dexamethasone (DXM) induced FHL-1 and factor H expression in all three cell lines, but did not affect expression of CD46, CD55 or CD59. Representative data for GDR cells are shown in Fig. 1a, lane 3 and lane 4.
Fig. 1.
Expression of complement regulators in synovial fibroblasts derived from rheumatoid arthritis patients. (a) Expression of complement regulators factor H, FHL-1, CD46, CD55 and CD59 in synovial fibroblasts from rheumatoid arthritis patients was analysed at the mRNA level by RT-PCR. mRNA was isolated from synovial fibroblasts, which were either untreated (control) (lane 1), treated for 24 h with TNF-α (10 ng/ml) (lane 2), or IFN-γ (100 U/ml) (lane 3) or for 48 h with dexamethasone (0·1 µM) (lane 4). After reverse transcription sequence specific primers were used for PCR analysis. Actin-β was used as a positive control. PCR fragments were separated by agarose gel electrophoresis. (b) Analysis of FHL-1 and factor H expression at the protein level. Culture supernatants were isolated from synovial fibroblasts cultivated either in serum free medium (control) (lane 1), from cells treated for 24 h with TNF-α (10 ng/ml) (lane 2), IFN-γ (100 U/ml) (lane 3) or from cells treated for 48 h with dexamethasone (0·1 µM) (lane 4). The supernatants were separated by SDS-PAGE, transferred to nitrocellulose membranes and proteins were visualized by Western blotting using antiserum specific for factor H and FHL-1.
Expression and regulation of the soluble factors FHL-1 and factor H was further confirmed on the protein level by immunoblotting. When synovial fibroblasts were grown in serum-free medium FHL-1, but not factor H was detected in the supernatant of untreated synovial fibroblasts (Fig. 1b, lane 1), whereas IFN-γ and DXM, but not TNF-α increased the expression of both proteins (Fig. 1b, lane 3 and lane 4). Thus synovial fibroblasts express several complement regulators, and expression of FHL-1 and factor H is up-regulated by the mediator IFN-γ and the anti-inflammatory steroid dexamethasone, but not by the pro-inflammatory cytokine TNF-α.
In situ expression of FHL-1 and factor H in diseased synovial tissue
Next we confirmed these results by analysing FHL-1 and factor H expression in vitro, in diseased synovial villous tissue isolated from metacarpal joints of an RA patient during surgical therapy. Synovial tissue derived from a patient suffering from osteoarthritis, without signs of inflammation served as a control. Since there is no specific antibody available to detect FHL-1 two antibodies were used in a subtractive approach to identify unique FHL-1 expression. Monoclonal antibody 196X detects both FHL-1 and factor H as it reacts with an epitope located within SCR 1 [5,35]. This mAb showed particularly strong staining of the cells lining the synovium and revealed additional expression in the interstitial spaces and connective tissue, as well as in blood vessels (Fig. 2b). In contrast, the factor H-specific mAb VIG8, which binds to the unique C-terminal end of this protein [36], revealed staining of the interstitial spaces, which contain synovial fibroblasts, macrophages and connective tissue, as well as blood vessels (Fig. 2a). The expression of FHL-1 can be judged indirectly, by comparing the patterns and intensities of the common mAb 196X and of the factor H-specific mAb VIG8. Stronger staining by the 196X mAB suggests unique expression of FHL-1 in synovial lining cells. In addition, macrophages stained for CD68 were predominantly located in the synovial lining (data not shown). The tissue sections from the patient with osteoarthritis without inflammation did not stain positively with either the VIG8 nor the 196X mAbs (Fig. 2c,d).
Fig. 2.
In situ expression of FHL-1 and factor H in the synovia of rheumatoid arthritis tissue. Immunohistochemistry of a section prepared from a rheumatoid arthritis patient (a,b) was compared with a respective tissue obtained from a patient with osteoarthritis, with no signs of inflammation and which was consequently used as control (c,d). Monoclonal antibody VIG8 (which recognizes an epitope located within SCR 20 of factor H) was used to locate factor H (a,c) and mAb 196X was used to detect both FHL-1 and factor H as this mAb recognizes a common epitope within SCR 1 (b,d). Staining of the synovial lining cells (especially fibroblasts and to a lesser degree macrophages) and of the interstitial spaces is seen in the RA patient (b), but not in the osteoarthritis control (d). Factor H, visualized with the specific VIG8 mAb is expressed in the interstitial spaces of the RA patient (a), while the FHL-1 and factor H recognized by mAb 196X stained the synovial lining cells, which consist particularly out of fibroblasts. No expression of the two proteins is detected in the material obtained from the osteoarthritis patients (c,d) (×40 magnification).
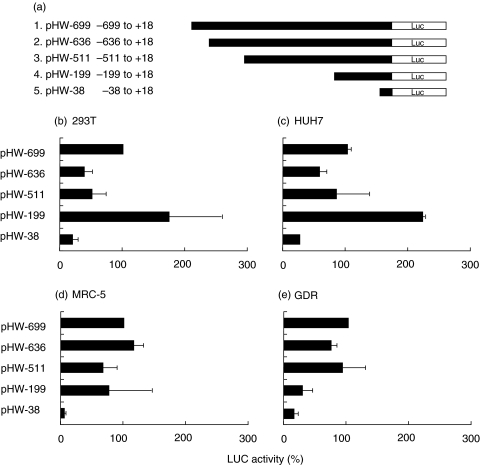
Identification of regulatory elements within the FHL-1/factor H gene promoter
Having demonstrated specific expression of the two related complement regulators in synovial fibroblasts in vitro and in cells comprising the synovial lining in vivo, we next defined further parameters responsible for this cell-specific patterns and responsiveness to inflammatory mediators. The mRNAs encoding FHL-1 and factor H are transcribed from the same human gene. The transcripts initiate at the same transcriptional start site, are regulated by the same promoter, but are alternatively processed. To identify regulatory promoter elements we generated several deletion constructs of the FHL-1/factor H gene promoter [39] and used the constructs for transient transfections analysis (Fig. 3a). Transcriptional regulation of the various FHL-1/factor H promoter constructs were analysed in distinct cell types such as nonfibroblast cell lines (293T and HUH7), in human lung fibroblasts (MRC-5) and in the synovial cell line GDR. The wild type promoter construct (pHW-699) which spans position − 699 to +18 showed different transcriptional activities in the tested cell lines. Promoter activity determined by luciferase activity ranged from 1022 000 RLU in 293T cells to 14, 300 RLU in the GDR cells (Table 2). This substantial difference in promoter activity shows that the analysed wild type FHL-1/factor H gene promoter includes elements responsible for cell- and tissue-specific activity. The promoter domains mediating this cell type-specific responsiveness were identified by the use of promoter-deletion constructs. These truncated constructs revealed the presence of cell specific regulatory elements, e.g. the presence of a repressive element within the domain −511 to −199, which is active in 293T and HUH7 cells (Fig. 3b,c), but not in the analysed fibroblasts (fig. 3d,e).
Fig. 3.
Tissue specific expression of FHL-1/factor H gene-promoter constructs. (a) Schematic diagram of the FHL-1/factor H gene promoter reporter constructs generated for reporter gene activity assays. Gene promoter activity was analysed following transient transfection of the indicated cell lines: (b) 293T kidney cells, (c) HUH7 liver cells, (d) MRC-5 lung fibroblasts and (e) GDR synovial fibroblasts. The activity was normalized to that obtained with the wild type promoter construct (pHW-699), which was set to 100%. Each column represents the mean of at least five independent experiments; the mean value and standard deviations are shown. (Results are summarized in Table 2)
Table 2.
Transcriptional activity of the FHL-1/factor H promoter in various human cell lines
| Cell line | Transcriptional activity of pHW-699 (RLU) |
|---|---|
| 293T | 1022 028 |
| HUH7 | 118 810 |
| MRC-5 | 15 995 |
| GDR | 14 305 |
In addition, the effect of inflammatory mediators which induce FHL-1 and factor H expression (compare Fig. 1a and b) on promoter activity was determined. Again a cell specific response to IFN-γ was demonstrated. IFN-γ increased transcription in HUH7 and MRC-5 cells, but not in 293T and GDR (Fig. 4) nor in SOZ cells (data not shown). In the responsive cells IFN-γ increased transcriptional activity between 5 and 8 fold. Different promoter elements mediate this induction in these cells, again showing a tissue specific-effect of this response.
Fig. 4.
Cell specific effect of IFN-γ on the FHL-1/factor H promoter activity. The various FHL-1/factor H promoter constructs were transiently transfected into 293T kidney cells (a), HUH7 liver cells (b), MRC-5 lung fibroblasts (c) and GDR synovial fibroblasts (d). Transfected cells were treated with interferon-γ (100 U/ml) for 24 h. The values show the increase in luciferase activity, as compared to the basal activity obtained for each construct. Each column represents the mean of at least five independent experiments; the mean value and standard deviations are shown.
The tissue-specific effect of the mediators is further highlighted by the effect observed using dexamethasone. This anti-inflammatory steroid showed an almost 10 fold induction, specifically in the synovial cell lines GDR (Fig. 5) and SOZ (not shown). The various deletion constructs allowed the location of the potential regulatory sites, e.g. of the element responsible for DXM induction in GDR fibroblasts to an area between position −199 and −39 (Fig. 5a). In contrast the pro-inflammatory mediator TNF-α had no effect on the FHL-1/factor H gene promoter activities in synovial GDR-fibroblasts (Fig. 6). These results are in agreement with the lack of an effect of this mediator on FHL-1 and factor H on mRNA or protein expression.
Fig. 5.
Cell specific effect of dexamethasone on the FHL-1/factor H promoter activity. FHL-1/factor H promoter constructs were transiently transfected into 293T kidney cells (a), HUH7 liver cells (b), MRC-5 lung fibroblasts (c) and GDR synovial fibroblasts (d). Transfected cells were treated with dexamethasone (0·1 µM) for 24 h. The values show the increase in luciferase activity, as compared to the basal activity obtained for each construct. Each column represents the mean of at least five independent experiments; the mean value and standard deviations are shown.
Fig. 6.
Effect of TNF-α on the FHL-1/factor H promoter activity in synovial fibroblasts. FHL-1/factor H promoter constructs were transiently transfected into GDR synovial fibroblasts which were further treated with TNF-α (10 ng/ml) for 24 h. The values represent the increase in luciferase activity compared with the basal activity of each construct (1×). Each column represents the mean of at least five independent experiments; the mean value and standard deviations are shown.
Binding of FHL-1 and factor H to the surface of synovial fibroblasts
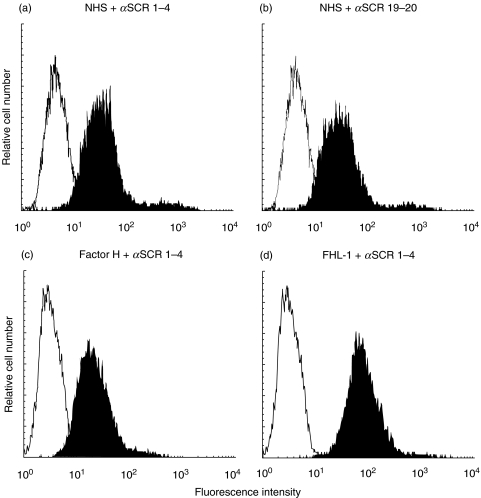
Having demonstrated FHL-1 and factor H expression, secretion by synovial fibroblasts and presence in synovial tissue in RA, we next asked whether these released proteins bind directly to the surface of cells, in an autocrine fashion. To this end synovial fibroblasts were incubated in NHS, which served as a source for FHL-1 and factor H or the cells were directly treated with purified recombinant FHL-1 or factor H. In both cases the presence of the regulators at the cell surfaces, as analysed by FACS analysis showed significant binding of both proteins to cell surfaces (Fig. 7). Upon incubation with NHS and staining with anti-SCR 1–4 or anti-SCR 19–20 antiserum, FHL-1 and factor H were detected on the cells surfaces (Fig. 7a,b). Due to the lack of antiserum specific for FHL-1 a set of two antisera were used: anti-SCR 1–4 antiserum identifies both FHL-1 and factor H, while SCR 19–20 antiserum is specific for factor H. Eighty seven percent of the cells stained positive with the anti-SCR 1–4 Ab and 81% of the cells with the anti-SCR 19–20 Ab (Fig. 7a,b). Analysis of the two antisera showed binding of both complement regulators to the surface of human synovial fibroblasts. Binding is considered specific as similar staining is observed after treatment with physiological amounts of purified factor H (500 µg/ml). In the latter case 84% of the cells stained positive (Fig. 7c). In addition, purified, recombinant FHL-1, also used at its physiological plasma concentration of approximately 50 µg/ml showed significant binding. A high fraction of the cells (99·4%) stained positive (Fig. 7d). In the absence of the primary antibodies no staining reactions were observed (data not shown).
Fig. 7.
Binding of FHL-1 and factor H to the surface of synovial fibroblasts. Binding of FHL-1 and factor H to synovial fibroblasts was analysed by flow cytometry. Cells cultured in serum free medium were used directly (control) or incubated with 25% NHS (a,b), purified complement factor H (500 µg/ml) (c) or purified recombinant FHL-1 (50 µg/ml) (d). After washing cells were stained with specific polyclonal antiserum directed against SCR 1–4 (a,c,d) or SCR 19–20 (b), followed by FITC-labelled swine anti-rabbit antiserum (▪). Irrelevant mouse IgG was used as a primary antibody for control staining (□).
Complement-mediated lysis of synovial fibroblasts
As synovial fibroblasts express and secrete FHL-1 and factor H and have the capacity to bind the two complement regulators to their surface, we asked whether this acquisition protects the cells against complement-mediated cell lysis. Consequently we used specific inhibitory antibodies to analyse the protective effect of the individual components on cell lysis. Cell lysis was significantly increased in synovial fibroblasts which were preincubated with antibodies against SCR 1–4 (57%), as compared to untreated controls (36%). The anti-SCR 1–4 antibody, which neutralizes both FHL-1 and factor H increased cell lysis significantly (t-test; p = 0·1 at dilution of 1 : 50; P= 0·01 at dilution 1 : 10), while the anti-SCR 19–20 antibody, which binds to factor H but not to FHL-1 did not affect cell lysis (33%) (Fig. 8).
Fig. 8.
The effect of FHL-1 and factor H neutralization on complement-mediated killing of synovial fibroblasts cells. 51Cr labelled cells were incubated with varying dilutions of anti-SCR 1–4 (•) or anti-SCR 19–20 (○) antibodies and in 25% NHS as the complement source. Cell lysis was determined after 30 min incubation by 51Cr release. The results represent means ± SD of duplicate experiments.
DISCUSSION
Rheumatoid arthritis a chronic disease of unknown aetiology is characterized by ongoing inflammatory processes which predominantly affect cells and tissues of synovial joints. Here we show that the two central complement regulators FHL-1 and factor H are up-regulated in RA and play a protective, anti-inflammatory effect in the disease. In vitro expression analysis at the mRNA and protein level show that both molecules are synthesized and secreted by synovial fibroblasts derived from patients with RA, and in vivo analysis demonstrated expression of both regulators in synovial tissue. Particularly high levels of FHL-1 are expressed by synovial lining cells. The anti-inflammatory role of the two mediators in RA is highlighted by their up-regulation upon treatment with the cytokine IFN-γ and with dexamethasone, whilst the pro-inflammatory TNF-α is ineffective. Transfection experiments with various FHL-1/factor H promoter reporter constructs into cell lines of distinct origin show a cell and tissue-specific regulation of these two central complement regulators. The inducible expression, specifically of FHL-1 has physiological consequences. By binding directly to the cell surface the released molecules act in an autocrine fashion and protect the secreting cells as well as their directly neighbouring cells from complement-mediated cell lysis. This study shows for the first time a protective role of two important complement regulators FHL-1 and factor H in RA, an activity involved in suppressing inflammation and cell damage. Further up-regulation of these anti-inflammatory factors by IFN-γ and dexamethasone could offer new possibilities for a therapy of RA.
The initial trigger causing inflammation and induction of RA is unknown. Complement-binding, complement-derived inflammatory mediators or immune complexes which initiate and perpetuate the inflammatory response are present within the joint and may contribute to local tissue damage [27]. In general self cells are protected from the damaging effects of the activated complement system by regulatory molecules which are either expressed on the membrane (e.g. MCP (CD46), DAF (CD55), CR1 (CD35) and protectin (CD59)), or which are secreted (e.g. FHL-1, C4 BP and factor H) [4,40,41]. At sites of inflammation and complement activation like RA, tissue cells and specifically synovial fibroblasts must be protected from bystander lysis. These cells must therefore actively down-regulate or inactivate toxic complement activation products. As shown in this study synovial fibroblasts derived from patients with RA are well prepared for such a scenario, as they express a panel of membrane anchored and secreted complement regulators (Fig. 1). This combination of protective molecules enables the cells (i) to actively control complement activation directly at their surface, (ii) to down-regulate newly formed cascade enzymes and (iii) to inactivate toxic activation products. The constitutive and particularly the inducible expression of at least several complement regulators suggests an important protective role in RA. Tissue damage in RA is believed to be complement-mediated and thus could be explained by an insufficient activity of these inducible regulators. Such a scenario indicates a tight balance between disease progressing inflammatory and protective anti-inflammatory reactions.
The two secreted regulators FHL-1 and factor H may play an important protective role in the RA as their expression is up-regulated by IFN-γ, which on one hand acts as a protective cytokine but has also disease exacerbating roles in animal models [15,19], and by dexamethasone (Fig. 1), which is an effective drug in RA treatment [42]. In contrast the expression levels of FHL-1 and factor H are not affected by the pro-inflammatory mediator TNF-α, which is known to play a destructive role in RA and whose toxic effects apparently are suppressed by the infusion of anti-tumour necrosis factor-α mAb [43,44]. In addition, expression of the membrane bound regulator CD59 is down-regulated in synovial lining cells in RA, which results in an insufficient inhibition of the membrane attack complex formation [45] and thus underlines the relevance of FHL-1 and factor H expression and function.
FHL-1 and factor H are encoded by a single human gene and the expression of both molecules is tightly regulated by transcriptional initiation, RNA processing and elongation, alternative splicing, mRNA processing and secretion [5]. By analysing the activity of the FHL-1/factor H gene promoter we identified cell type-specific promoter activity as basal activities ranged from 1022 000 RLU in 293T cells to approximately 15 000 RLU in different fibroblast cell lines. The cell specific regulatory elements include repressor and activation domains, which was revealed by the use of deletion constructs. For example, a repressor element, located between position −511 to −199, is active in 293T and HUH7 cells, but not in fibroblasts. Sequence comparisons reveal within this region consensus binding sites for transcription factors H4TF-1 (− 261 to − 269) and LVa-RS (− 310 to − 314), which were initially reported in the H4 histone gene [46] or the enhancer regions of moloney murine leukaemia virus, and for immediate early genes of cytomegalovirus [46], respectively. The cell and tissue-specific effect of DXM and IFN-γ, and the lack of an effect of the proinflammatory mediator TNF-α on transcriptional regulation confirms the tightly regulated promoter activity of the FHL-1/factor H gene [47].
Functional glucocorticoid response elements (GRE) are identified in this study by transfection analyses and are localized between positions −199 and −39 of the FHL-1/factor H promoter (Fig. 5). The sequences of the potential sites (position − 108 to − 114 and − 143 to − 170) differ from the consensus GRE [48,49]. Glucocorticoids have been used as effective anti-inflammatory agents for the treatment of RA for almost five decades [42]. These synthetic drugs bind to specific receptors in the cytoplasm and upon migration to the nucleus such complexes interact specifically with regulatory promoter elements of inflammatory genes [48]. Glucorticoid binding modulates promoter function and increases or represses promoter activity [48,49]. Thus their up-regulatory effect on FHL-1 and factor H expression may contribute to the anti-inflammatory effects of drugs like dexamethasone in RA treatment/therapy.
Complement activation and predominant activation of the alternative pathway occurs in synovial fluid of RA [22,24]. A recent study shows the importance of complement in the pathogenesis of RA, as C5-deficient mice are highly resistant to the induction of collagen-induced arthritis [33]. The inability to inactivate newly generated complement products contributes to the pathophysiology of the disease. Cells, which are in direct contact with synovial fluid must in particular find means to inactivate toxic complement products [11,22,23]. This scenario is further confirmed by treatment with the soluble inhibitor complement-receptor type 1. Intra-articular administration markedly inhibited inflammation and damage of joint tissue [30,32], while systemic application was ineffective [50]. These data show that synovial fluid represents a separated microenvironment, which uses locally produced complement effector proteins and regulators, such as FHL-1 and factor H. Local cells, such as resident synovial fibroblasts as well as infiltrating polymorphonuclear cells and activated macrophages express FHL-1 and factor H, MCP, DAF and CD59 (Fig. 1) [5,50,51], as well as complement components C1 to C7 [23,52]. Locally synthesized complement effector proteins and regulators contribute significantly to the protection of local cells and tissues [52,53].
In vitro and in vivo expression analysis show a regulated synthesis of FHL-1 and factor H by synovial lining fibroblasts, which can be further increased by anti-inflammatory mediators. The distinct expression patterns of both molecules as shown by immunohistochemistry and their absence in tissue derived from a patient with osteoarthritis illustrates the relevance of these molecules in RA. Following secretion, both regulators attach firmly to the surface of the synovial cells as shown here by FACS analysis (Fig. 7). In this bound configuration the proteins which down-regulate C3 convertases and participate in the inactivation of freshly formed C3b confer resistance to complement-mediated cytotoxicity (Fig. 8).
A particularly important role of FHL-1 in protecting synovial fibroblasts can be postulated, as inactivation of this protein causes significantly increased cell lysis. In contrast, inhibition of both FHL-1 and factor H did not further affect complement cytotoxicity. In addition FHL-1 is present in higher concentrations in synovial fluid than in plasma [35]. This novel protective mechanism, which is described here for an inflammatory human disease RA is highly related to the atypical form of haemolytic uremic syndrome (HUS), where factor H mutants show a role of factor H in protection of endothelial cells [54,55]. By demonstrating an important anti-inflammatory role of the two human complement regulators FHL-1 and factor H in rheumatoid arthritis, we identify new targets for a therapeutic intervention.
Acknowledgments
We thank Eva Kampen for excellent technical assistance. M.A.F. is supported by a scholarship from the Studienstiftung des Deutschen Volkes. This work is part of the doctoral thesis of M.A.F. at the Faculty of Medicine at the University of Hamburg. Funding from the Deutsche Forschungsgemeinschaft DFG (Zi 432), the Thüringer Ministerium für Wissenschaft, Forschung Technologie und Kunst (TMWFK), the DAAD (Projektbezogener Personenaustausch mit Finnland), the Academy of Finland and the State subsidy for University Hospitals in Finland and the National Health and Medical Research Council of Australia is kindly acknowledged.
REFERENCES
- 1.Müller-Eberhard HJ, Schreiber RD. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- 4.Kraus S, Seger R, Fishelson Z. Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis. Clin Exp Immunol. 2001;123:366–74. doi: 10.1046/j.1365-2249.2001.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipfel PF, Skerka C. FHL-1: a human complement and immune regulator with cell-adhesive function. Immunol Today. 1999;20:135–40. doi: 10.1016/s0167-5699(98)01432-7. [DOI] [PubMed] [Google Scholar]
- 6.Friese MA, Hellwage J, Jokiranta TS, et al. FHL-1 and factor H. two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol. 1999;36:809–18. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 7.Whaley K, Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976;144:1147–63. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiler JM, Daha MR, Austen KF, et al. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci USA. 1976;73:3268–72. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pangburn MK, Schreiber RD, Müller-Eberhard HJ. Human complement C3b inactivator. isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977;146:257–70. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellwage J, Kühn S, Zipfel PF. The human complement regulatory factor-H-like protein 1, which represents a truncated form of factor H, displays cell-attachment activity. Biochem J. 1997;326:321–7. doi: 10.1042/bj3260321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corvetta A, Pomponio G, Rinaldi N, et al. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin Exp Rheumatol. 1992;10:433–8. [PubMed] [Google Scholar]
- 12.Harris E. Rheumatoid arthritis; pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 13.Sewell K, Trentham D. Pathogenesis of rheumatoid arthritis. Lancet. 1993;341:283–6. doi: 10.1016/0140-6736(93)92627-6. [DOI] [PubMed] [Google Scholar]
- 14.Horsfall AC, Butler DM, Marinova L, et al. Suppression of collagen-induced arthritis by continous administration of IL-4. J Immunol. 1997;159:5687–94. [PubMed] [Google Scholar]
- 15.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 16.Walmsley M, Katsikis PD, Abney E, et al. Interleukin-10 inhibition of the progression of estabished collagen-induced arthritis. Arthritis Rheum. 1996;39:495–501. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 17.Feldmann M. Pathogenesis of arthritis: recent research progress. Nat Immunol. 2001;2:771–3. doi: 10.1038/ni0901-771. [DOI] [PubMed] [Google Scholar]
- 18.Manoury-Schwartz B, Chiocchia G, Bessis N, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-γ receptors. J Immunol. 1997;158:5501–6. [PubMed] [Google Scholar]
- 19.Vermeire K, Heremans H, Vandeputte M, et al. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J Immunol. 1997;158:5507–13. [PubMed] [Google Scholar]
- 20.Morgan BP, Daniels RH, Williams BD. Measurement of terminal complement complexes in rheumatoid arthritis. Clin Exp Immunol. 1988;73:473–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Kageyama Y, Koide Y, Yoshida A, et al. Reduced susceptibility to collagen-induced arthritis in mice deficient in IFN-γ receptor. J Immunol. 1998;161:1542–8. [PubMed] [Google Scholar]
- 22.Brodeur JP, Ruddy S, Schwartz LB, et al. Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1531–7. doi: 10.1002/art.1780341209. [DOI] [PubMed] [Google Scholar]
- 23.Hogasen K, Mollnes TE, Harboe M, et al. Terminal complement pathway activation and low lysis inhibitors in rheumatoid arthritis synovial fluid. J Rheumatol. 1995;22:24–8. [PubMed] [Google Scholar]
- 24.Aggarwal A, Bhardwaj A, Alam S, et al. Evidence for activation of the alternate complement pathway in patients with juvenile rheumatoid arthritis. Rheumatology. 2000;39:189–92. doi: 10.1093/rheumatology/39.2.189. [DOI] [PubMed] [Google Scholar]
- 25.Hanauske-Abel HM, Pontz BF, Schorlemmer HU. Cartilage specific collagen activates macrophages and the alternative pathway of complement: evidence for an immunopathogenic concept of rheumatoid arthritis. Ann Rheum Dis. 1982;41:168–76. doi: 10.1136/ard.41.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke TD, Hurd ER, Ziff M, Jasin HE. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. II. Preferential localization of antigen-antibody complexes to collagenous tissues. J Exp Med. 1972;135:323–38. doi: 10.1084/jem.135.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasin HE. Mechanism of trapping of immune complexes in joint collagenous tissues. Clin Exp Immunol. 1975;22:473–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Jasin HE, Cooke TD. The inflammatory role of immune complexes trapped in joint collagenous tissues. Clin Exp Immunol. 1978;33:416–24. [PMC free article] [PubMed] [Google Scholar]
- 29.Brauer R, Thoss K, Henzgen S, et al. Significance of cell-mediated and humoral immunity in the acute and chronic phase of antigen-induced arthritis in rabbits. Exp Pathol. 1988;34:197–208. doi: 10.1016/s0232-1513(88)80151-8. [DOI] [PubMed] [Google Scholar]
- 30.Goodfellow RM, Williams AS, Levin JL, et al. Local therapy with soluble complement receptor 1 (sCR1) suppresses inflammation in rat mono-articular arthritis. Clin Exp Immunol. 1997;110:45–52. doi: 10.1046/j.1365-2249.1997.5111408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodfellow RM, Williams AS, Levin JL, et al. Soluble complement receptor one (sCR1) inhibits the development and progression of rat collagen-induced arthritis. Clin Exp Immunol. 2000;119:210–6. doi: 10.1046/j.1365-2249.2000.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linton SM, Williams AS, Dodd I, et al. Therapeutic efficacy of a novel membrane-targeted complement regulator in antigen-induced arthritis in the rat. Arthritis Rheum. 2000;43:2590–7. doi: 10.1002/1529-0131(200011)43:11<2590::AID-ANR29>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Kristan J, Hao L, et al. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J Immunol. 2000;164:4340–7. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 34.Aicher WK, Alexander D, Haas C, Kuchen S, Pagenstecher A, Gay S, Peter HH, Eibel H. Transcription factor early growth response 1 activity up-regulates expression of tissue inhibitor of metalloproteinases 1 in human synovial fibroblastsArthritis. Rheum. 2003;48:348–59. doi: 10.1002/art.10774. [DOI] [PubMed] [Google Scholar]
- 35.Friese MA, Hellwage J, Jokiranta TS, et al. Different regulation of factor H and FHL-1 by inflammatory mediators and in rheumatoid arthritis. Clin Exp Immunol. 2000;121:406–16. doi: 10.1046/j.1365-2249.2000.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodinger WM, Hellwage J, Spruth M, et al. The C-terminus of factor H: monoclonal antibodies inhibit heparin binding and identify epitopes common to factor H and factor H-related proteins. Biochem J. 1998;331:41–7. doi: 10.1042/bj3310041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kühn S, Skerka C, Zipfel PF. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J Immunol. 1995;155:5663–70. [PubMed] [Google Scholar]
- 38.Skerka C, Hellwage J, Weber W, et al. The human factor H-related protein 4 (FHR-4). A novel short consensus repeat-containing protein is associated with human triglyceride-rich lipoproteins. J Biol Chem. 1997;272:5627–34. doi: 10.1074/jbc.272.9.5627. [DOI] [PubMed] [Google Scholar]
- 39.Ward HM, Higgs NH, et al. Cloning and analysis of the human complement factor H gene promoter. Immunol Cell Biol. 1997;75:508–10. doi: 10.1038/icb.1997.79. [DOI] [PubMed] [Google Scholar]
- 40.Jokiranta TS, Zipfel PF, Hakulinen J, et al. Analysis of the recognition mechanism of the alternative pathway of complement by monoclonal anti-factor H antibodies: evidence for multiple interactions between factor H and surface bound Ce3d. FEBS Lett. 1996;393:297–302. doi: 10.1016/0014-5793(96)00905-2. [DOI] [PubMed] [Google Scholar]
- 41.Morgan BP, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15:369–6. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 42.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The arthritis and rheumatism council low-dose glucocorticoid study group. N Engl J Med. 1995;24:181–97. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 43.Elliott MJ, Maini RN, Feldmann M, et al. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344:1125–7. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- 44.Feldmann M, Elliott MJ, Woody JN, et al. Anti-tumour necrosis factor-α therapy in rheumatoid arthritis. Adv Immunol. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- 45.Konttinen YT, Ceponis A, Meri S, et al. Complement in acute and chronic arthritides: assessment of C3c, C9, and protectin (CD59) in synovial membrane. Ann Rheum Dis. 1996;55:888–94. doi: 10.1136/ard.55.12.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dailey L, Hanly SM, Roeder RG, et al. Distinct transcription factors bind specifically to two regions of the human histone H4 promoter. Proc Natl Acad Sci USA. 1986;83:7241–5. doi: 10.1073/pnas.83.19.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams SA, Vik DP. Characterization of the 5′ flanking region of the human complement factor H gene. Scand J Immunol. 1997;45:7–15. doi: 10.1046/j.1365-3083.1997.d01-364.x. [DOI] [PubMed] [Google Scholar]
- 48.Beato M, Truss M, Charez S. Control of transcription by steroid hormones. Ann NY Acad Sc. 1996;784:93–123. doi: 10.1111/j.1749-6632.1996.tb16231.x. [DOI] [PubMed] [Google Scholar]
- 49.Boumpas DT, Chrousos GP, Wilder RL, et al. Glucocorticoid therapy for immune-mediated diseases. basic and clinical correlates. Ann Intern Med. 1993;119:1198–208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 50.Mizuno M, Nishikawa K, Goodfellow RM, et al. The effects of functional suppression of a membrane-bound complement regulatory protein, CD59, in the synovial tissue in rats. Arthritis Rheum. 1997;40:527–33. doi: 10.1002/art.1780400319. [DOI] [PubMed] [Google Scholar]
- 51.Colten HR. Tissue-specific regulation of inflammation. J Appl Physiol. 1992;72:1–7. doi: 10.1152/jappl.1992.72.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Guc D, Gulati P, Lemercier C, et al. Expression of the components and regulatory proteins of the alternative complement pathway and the membrane attack complex in normal and diseased synovium. Rheumatol Int. 1993;13:139–46. doi: 10.1007/BF00301260. [DOI] [PubMed] [Google Scholar]
- 53.deCeulaer C, Papazoglou S, Whaley K. Increased biosynthesis of complement components by cultured monocytes, synovial fluid macrophages and skynovial membrane cells from patients with rheumatoid arthritis. Immunology. 1980;41:37–43. [PMC free article] [PubMed] [Google Scholar]
- 54.Zipfel PF. Hemolytic uremic syndrome. how do factor H mutants mediate endothelial damage. Trends Immunol. 2001;22:345–8. doi: 10.1016/s1471-4906(01)01972-x. [DOI] [PubMed] [Google Scholar]
- 55.Manuelian T, Hellwage J, Meri S, et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111:1181–90. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]