Abstract
We previously reported the establishment of a mouse model system of contact hypersensitivity (CHS) to paraphenylemediamine (PPD). In order to analyse the functional contribution of Th2 cytokines, IL-4 and IL-5, in PPD induced CHS, STAT6 deficient (STAT6–/–) and wild-type control (WT) mice (C57BL/6) were immunized by the topical application of a PPD solution, and then the subsequent skin reactions were examined. Ear swelling was significantly reduced with a delayed peak response in STAT6–/– mice as compared with that of WT mice. A histological analysis showed the infiltration of both eosinophils and neutrophils in the skin of STAT6–/– mice challenged 24 h previously to significantly decrease in comparison with that in the WT mice. The expression of Th2 cytokines (IL-4, IL-5) by ELISA in the PPD-challenged skin tissue specimens as well as the IgE and IgG1 response after challenge were also profoundly reduced in the STAT6–/– mice. The adoptive transfer of the serum obtained from sensitized WT mice for the putative IgE transfer induced a peak response at 3 h and 24 h after challenge. To further investigate the role of mast cells in the induction of PPD-CHS, mast cell deficient W/Wv mice were sensitized with PPD and then were challenged. Maximal ear swelling was detected from 12 to 24 h and another small peak response was observed at 1 h in+/+mice, whereas only a small peak response at 24 h was detected in W/Wv mice. These data indicate that not only Th2 cytokines and IgE but also mast cells play an essential role in the induction of PPD-CHS.
Keywords: paraphenylenediamine (PPD), contact hypersensitivity, Th2 cytokines, IgE, mast cells
INTRODUCTION
Sensitization by Paraphenylenediamine (PPD) has been considered in several countries to be so great a hazard that its use in hair dyes was banned in Germany in the early 19·00s; it was subsequently prohibited in France, and in Sweden in 1964, however, in Japan, PPD is still used as a active ingredient of hair dyes [1]. It is well known that PPD induces not only contact hypersensitivity but also immediate-type hypersensitivity [2]. The allergenicity of PPD was first described by Mayer [3], Blohm & Rajka [4] demonstrated that the sensitization with benzoquinone played an important role in sensitization with PPD through an intracutaneous sensitization experiment using guinea pigs.
Magnusson [5] established a guinea pig model of PPD-CHS by both the intradermal injection of PPD and the topical application of PPD. Recently, We recently reported a murine model of contact sensitization to PPD by applying PPD for 3 consecutive days on the abdomens of mice [6]. Th2 like-γδ T cells have been demonstrated to play a role in the induction of CHS to PPD [6]. However, it is not clear whether Th2 cytokines, IgE or mast cells play an essential role in the induction of PPD-CHS or not. Very recently, in order to analyse the functional contribution of Th2 cytokines in CHS, STAT6 deficient (STAT6–/–) and wild-type control (WT) mice (C57BL/6) were contact sensitized with 5% TNCB, 0·5% DNFB or 5% oxasolone (Oxa), and then the subsequent skin reactions were examined [7]. The results indicated that the STAT6 signal plays a critical role in the induction phase of CHS to TNCB, DNFB or Oxa [7].
Delayed hypersensitivity (DH) in mice can be preferentially elicited in anatomical sites rich in mast cells [8]. This observation, combined with experiments showing that phamacological agents such as reserpine to deplete mast cells of vasoactive amines, which are known to inhibit DH, suggests that mast cells play an essential role in the expression of DH reaction [8,9]. Several reports demonstrated that mast cell-deficient mice had an impaired ability to express a DH reaction [10–12]. In contrast to these reports, DH or CHS in mast cell-deficient mice were found to be equivalent to+/+control mice [13–16]. Ptak and colleagues [17,18] demonstrated that a low dose, and not a high dose, of IgE mediated DTH initiation via the release of a small amount of 5-hydroxytryptamine by mast cells. We also demonstrated a small amount of IgE to be detected in CHS induced in the wt mice but not in the STAT6–/– mice [7], however, the exact role of mast cells and IgE in CHS remains unclear.
In this paper, we compared the CHS response to PPD in STAT6–/– mice and mast cell deficient mice with that in wild type mice to elucidate the role of Th2 cytokines, IgE or mast cells in the induction of CHS to PPD. As a result, we found that not only Th2 cytokines and IgE but also mast cells play a crucial role in the induction of PPD-CHS.
MATERIALS AND METHODS
Animals
C57/BL6 mice with a targeted disruption of the gene encoding STAT6 (STAT6–/–) were generated in the Department of Host Defense, Research Institute for Microbial Disease, Osaka University (Suita, Japan), as previously reported [19] and age and sex matched wild-type (WT) littermate controls (C57/BL mice) were purchased from the Sankyo Co., Tokyo, Japan. Female mast cell-deficient WBB6F-W/Wv mice and their congenic normal (+/+) mice were obtained from the Sankyo Co., Japan. All animals were housed under specific pathogen-free conditions, and had free access to a commercial diet and water. They were used at from 8 to 15 weeks of age. Each experimental group consisted of at least five mice.
Reagents
The following reagents were obtained from commercial sources: N-2-hydroxyethyl-piperazine-N,-2-ethanesulphonic acid (Hepes) from the Nakarai Chemical Co., Kyoto; p-phenylenediamine (PPD) from the Wako Junyaku Co., Tokyo, and trypsin (1 : 250) from Difco (Detroit, MI, USA). The following mAb were used. Anti-mouse IgE-epsilon chain antiserum (goat; Bethyl Laboratory Inc, TX, USA), anti-Thy1·2 (IgG2; Caltag Laboratory, CA), anti-Ly2 (Rat; Cedarlane Laboratories Ltd, Canada), antimouse CD4(L3T4) mAb (Rat; Cedarlane Laboratories Ltd, Canada); CNBr-activated-Sepharose 4B from Phamacia (Uppsala, Sweden).
Immunization for induction of contact hypersensitivity to PPD
Female mast cell-deficient WBB6F-W/Wv mice and their congenic normal (+/+) mice were contact sensitized by 3 daily topical applications of 50 µl of 2·5% PPD solution in acetone in olive oil (1 : 4) on shaved abdominal skin. The control mice were treated in the same fashion with vehicle alone.
C57BL/6 mice could not be contact-sensitized by a previously described method [6]. Therefore, C57BL/6 mice and STAT6–/– mice were contact sensitized by 5 daily consecutive topical applications of 50 µl of 2·5% PPD with 3% of H2O2 since we recently reported that the pretreatment of H2O2 was found to enhance the ear swelling response in the induction of PPD-CHS in the BALB/c mice (unpublished data).
Challenge and measurement
At 2 days after the last abdominal application, the mice were challenged by applying 20 µl of 2·5% PPD solution on both sides of one ear and vehicle on both sides of the other ear. The thickness of the ear was then measured at 1 h, 3 h, 6 h, 12 h, 24 h, 48 h, 72 h and 96 h after challenge using an engineer's micrometer (Peacock, Ozaki Engineering, Tokyo, Japan).
Quantification of cytokine levels in the supernatant of skin tissue extracts
Samples of ear tissue extracts for ELISA were prepared as described by Ferguson et al. [20]. Briefly, at 24 h after the application of PPD, the ears were excised and then were immediately homogenized with 10-fold volume of 0·1% Tween-20 in PBS. The samples were quickly frozen in liquid nitrogen, thawed in a 37°C water bath, sonicated for 15 s and centrifuged for 5 min at 13000 × g. The resultant supernatants were used for ELISA. The supernatants were stored at − 80°C. The ELISA for IL-4, IL-5 and IFN-γ was conducted using an ELISA kit (Endogen Inc., MA, USA) according to the manufacturer's instructions.
Measurement of the serum concentration of IgE, IgG1 and IgG2
Blood was drawn from the sinus cavernous, and the serum was obtained by the centrifugation at 1500 × g for 10 min The serum IgE level was determined using a commercial ELISA kit (Yamasa, Chiba, Japan). Concentration of IgG1 and IgG2 was determined using commercial ELISA kits (Bethyl Laboratory Inc., Montgomery, USA) according to the manufacturer's instructions.
Adoptive transfer experiments
Serum obtained from PPD-sensitized WT mice at 24 h after challenged by 2·5% PPD painting on ear and cell suspensions obtained from the peripheral lymph nodes from PPD-sensitized WT mice before challenge were injected into the ear of either naive STAT6–/– mice or WT mice (five mice per group) (20 µl serum, 2 × 105 per 20 µl PBS) [21]. One day after the transfer, the mice were challenged by applying 10 µl of 2·5% PPD in olive oil on both sides of the ear. In these experiments negative controls consisted of groups of mice injected with PBS, s.c., into the ear and challenged with 2·5% PPD or groups of mice injected with serum or peripheral lymph node cells, s.c., in the ear and challenged with olive oil. The ear thickness was measured as described above after 3 h and 24 h.
Elimination of IgE from the serum
The elimination of IgE from the serum was conducted by anti-IgE-epsilon chain antiserum (goat; Bethyl Laboratory INC, TX) bound affinity chromatography. Briefly, 5 µg/ml anti-IgE-epsilon chain antibody was bound on CNBr-activated Sepharose 4B beads according to the manufacturer's instructions. The serum (2 ml/column) from PPD-sensitized WT mice was applied onto the anti-IgE-epsilon chain antiserum bound Sepharose 4B column (1·5 × 5 cm) or isotype goat antiserum bound Sepharose 4B column, incubated at room temparature for 60 min and washed. No IgE was detected in the filtrate from the anti-IgE-epsilon chain antiserum bound column and 10–50 ng/ml IgE was detected in the filtrate from isotype goat antiserum bound column.
Histological examination
Ear skin specimens were excised and fixed in 10% formalin, and then were processed and stained with haematoxylin and eosin (HE), May–Grunwald–Giemsa or Toluidine-blue. The number of mononuclear cells, mast cells and granulocytes, such as neutrophils and eosinophils, infiltrating into the dermis was evaluated by staining the tissue specimens stained with Giemsa's solution. The section was examined at a magnification of ×400. At least 10 fields were examined for each earlobe. The number of cells was counted and expressed at the number of cells per mm2.
Statistical analysis
The experimental data are expressed as the mean ± SD (standard deviation). The statistical analysis was performed using Student's t-test.
RESULTS
Diminished CHS response to PPD in STAT6 –/– mice
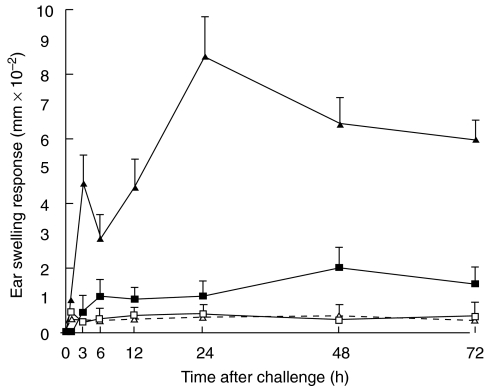
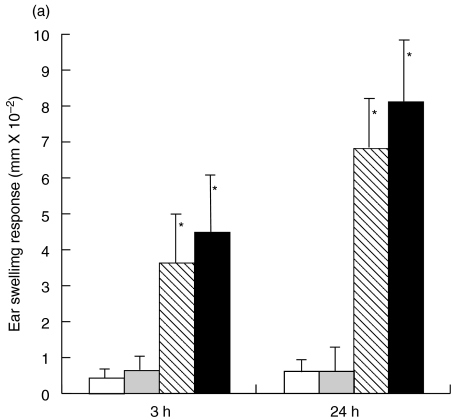
The STAT6–/– mice and wt mice were sensitized with PPD to evaluate the ability of STAT6–/– mice to develop a PPD induced CHS reaction. Two peak responses were detected at 3 h (early phase) and at 24 h (late phase) after the challenge in both the STAT6–/– mice and WT mice. As is shown in Fig. 1, both the early and late phase of CHS to PPD significantly decreased in the STAT6–/– mice in comparison to those in the WT mice (Fig. 1). The peak response of ear swelling was detected at 3 h and 24 h after the challenge in the wt mice whereas a small peak response was observed at 48 h after challenge in the STAT6–/– mice.
Fig. 1.
STAT 6 deficient (STAT6–/–) mice exhibit a reduced CHS elicitation to PPD. STAT6–/– mice (□ Olive-STAT6–/–; ▪ PPD-STAT6–/–) and wild type (WT) mice (▵ Olive-WT; ▴ PPD-WT) were contact sensitized for five days with 2·5% PPD, 2 days after the last immunization, and thereafter both mice were challenged. The maximal ear swelling was detected at 24 h for the wild type mice and at 48 h for the STAT6–/– mice. The data presented are the mean values ± SD of five mice per group and are representative of five experiments. *P < 0·01, significant compared to the results obtained with WT mice
Histopathology of PPD-induced CHS induced in STAT6–/– mice
Since a suppressed CHS response was observed in the STAT6–/– mice, we analysed the histological reaction induced at 3 h and 24 h after PPD challenge in the STAT6–/– mice and WT mice.
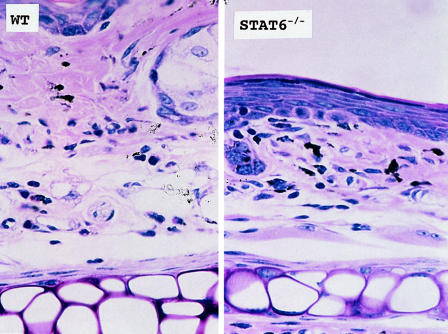
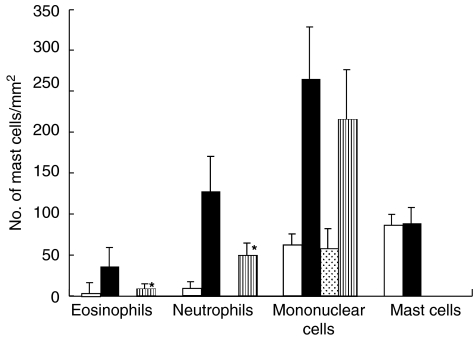
The histological reaction at 3 h revealed slight dermal oedema in WT mice but not in STAT6–/–. The 24 h reaction revealed severe dermal oedema with strong infiltration of mononuclear cells, neutrophils and eosinophils to be detected in the challenged skin of the WT mice (Fig. 2). In contrast, only a mild inflammatory response with a diminished infiltration of mononuclear cells and neutrophils was observed in the challenged skin in the STAT6–/– mice (Fig. 2). Interestingly, the degree of degranulation from mast cells was much less in the STAT6–/– mice (Fig. 2). The number of infiltrated mononuclear cells, neutrophils and eosinophils into the sites were calculated in both the STAT6–/– mice and WT mice. PPD-challenged wt mice had a significantly greater number of eosinophils and neutrophils than the PPD-challenged STAT6–/– mice (P < 0·01) (Fig. 3).
Fig. 2.
Histopathological findings of the contact hypersensitivity induced by PPD painting in WT or STAT 6–/– mice WT mice or STAT6–/– mice were immunized for 5 consecutive days by topical painting with 2·5% PPD with H2O2. Three days after the last abdominal application, the mice were then challenged by applying 2·5% PPD solution. The histological features of 24-h CHS ear skin reaction challenged by PPD in WT mice or in STAT6–/– mice stained with Giemsa's solution. An extremely large degree of oedema was detected in the PPD-challenged skin in the WT mice (STAT6;/;) but not in the STAT6–/– mice (STAT6–/–). A strong infiltration of mononuclear cells, neutrophils and eosinophils was observed in the dermis of the WT mice, however, a diminished infiltration of neutrophils and mononuclear cells was observed in the dermis of the STAT6–/– mice.
Fig. 3.
Effect of STAT6 deficiency on the cellular distribution of the challenged skin. Challenged skin was obtained from the WT and STAT6–/– mice after being challenged with the application of 2·5% PPD or olive oil (Control). □ Olive-WT; ▪ PPD-WT;  Olive-STAT6–/–;
Olive-STAT6–/–;  PPD-STAT6–/–. The columns represent the number of eosinophils, neutrophils, monocytes/macrophages and mast cells which infiltrated the challenged skin. The results shown are from a single experiment representative of three separate experiments.*P-value < 0·01 versus the TNCB-challenged WT mice. **P-value < 0·05 versus the PPD-challenged WT mice.
PPD-STAT6–/–. The columns represent the number of eosinophils, neutrophils, monocytes/macrophages and mast cells which infiltrated the challenged skin. The results shown are from a single experiment representative of three separate experiments.*P-value < 0·01 versus the TNCB-challenged WT mice. **P-value < 0·05 versus the PPD-challenged WT mice.
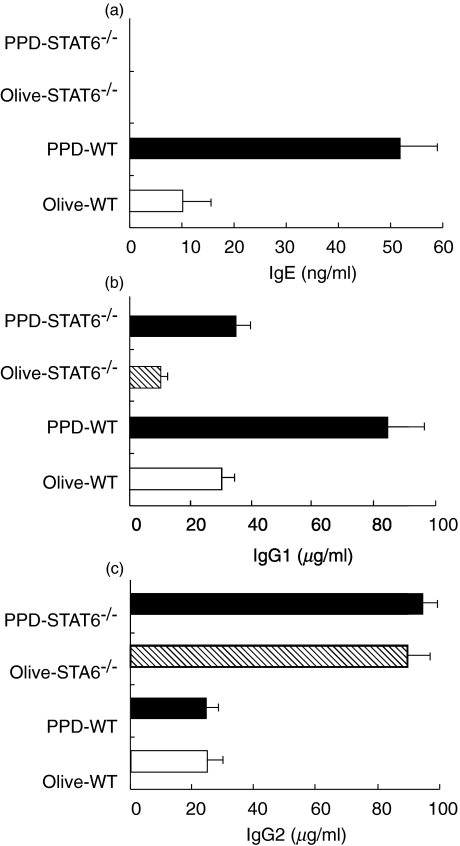
No elevation of the serum IgE and IgG1 in the STAT6–/– mice challenged with PPD
Since IgE has been implicated to play an important role in the pathogenesis of CHS [17,18], STAT6–/– mice and WT mice sensitized with PPD were challenged with PPD or olive oil. The mice were bled at 24 h after PPD challenge, and the serum concentrations of IgE, IgG1 and IgG2a were measured by ELISA. The PPD-challenged WT mice produced significantly higher amounts of serum IgE than the olive oil-challenged littermates. In contrast, neither the olive oil- nor PPD-challenged STAT6–/– mice produced detectable levels of serum IgE (Fig. 4a). The WT mice displayed the expected increase in IgG1 concentration after PPD challenge, whereas the serum IgG1 level was very weakly augmented after the PPD challenge in STAT6–/– mice (Fig. 4b). The serum concentration of IgG2a did not change in either the WT or STAT–/– mice after the PPD challenge, thus indicating that the antigen specific IgG2 production was not influenced by the PPD challenge (Fig. 4c).
Fig. 4.
The serum IgE, IgG1, IgG2a levels of the sensitized WT and STAT6–/– mice challenged with olive oil and PPD. Mice were treated as described in the Methods. The serum IgE, IgG1, IgG2a level was analysed by ELISA. The serum (a) IgE, (b) IgG1 and (c) IgG2a levels in olive oil and PPD-challenged, sensitized WT and STAT6–/– mice. The mice were treated as described in the Methods. The serum was analysed by ELISA. The data represent the mean ± SD for groups of four mice and are representative of three independent experiments. The immunogloblin level was measured by ELISA at 24 h after the challenge. *P-value < 0·01 compared with the other groups.
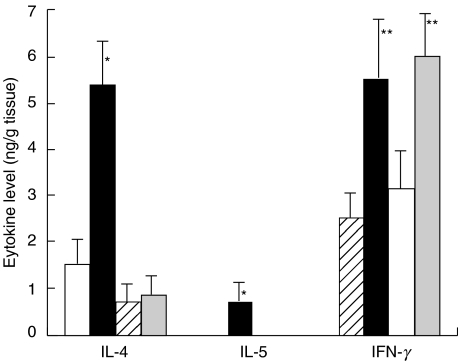
Local production of cytokines in the STAT6–/– mice
In the supernatant from skin tissue obtained from PPD-challenged WT mice, the levels of both IL-4 and IL-5 were significantly higher than those in the supernatant from olive oil-challenged WT mice (Fig. 5). In contrast, no Th2 cytokine was detected in the skin tissue extract from PPD-challenged STAT6–/– mice (Fig. 5). The level of IFN-γ in the STAT6–/– mice was increased in both the WT mice and STAT6–/– mice (Fig. 5).
Fig. 5.
The cytokine levels in the skin tissue supernatants in the olive oil and PPD-challenged, sensitized STAT6–/– mice and WT mice. □ Olive-WT; ▪ PPD-WT;  Olive-STAT6–/–;
Olive-STAT6–/–;  PPD-STAT6–/–. The data represent the mean ± SD for groups of four mice and are representative of three independent experiments. The cytokine levels were measured by comparing them with all other groups. *P-value < 0·01 compared with the other groups, **P-value < 0·01 compared with the negative control groups.
PPD-STAT6–/–. The data represent the mean ± SD for groups of four mice and are representative of three independent experiments. The cytokine levels were measured by comparing them with all other groups. *P-value < 0·01 compared with the other groups, **P-value < 0·01 compared with the negative control groups.
Either lymph node cells or serum from the wt mice induced CHS in the STAT6–/– mice
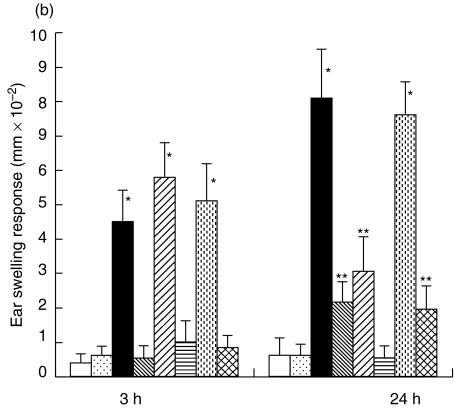
To clarify whether the defect of CHS in STAT6–/– is at the afferent or efferent phase, adoptive cell transfer experiments were conducted. Lymph node cells from the WT mice sensitized with PPD were subcutaneously injected into the ears of naive STAT6–/– mice or WT mice followed by an epicutneous challenge with PPD. Ear swelling was measured at 3 h and 24 h after the challenge. Naive STAT6–/– mice exhibited CHS after the injection of lymph node cells from the sensitized wt mice at both 3 h and 24 h after the challenge (Fig. 6a), however, ear swelling was detected in neither the STAT6–/– mice, which were injected lymph node cells and challenged with olive oil, nor those which were injected with PBS and challenged with PPD (Data not shown). These data thus indicated STAT6 to be an important signal during the induction of CHS. Serum transfer experiments revealed both the early and late responses to be induced by the injection of serum obtained from the sensitized WT mice, however, the late phase responses induced by serum transfer were weaker than those in positive control. Interestingly, both responses diminished after the pretreatment of serum by anti-IgE to antibody coated beads (Fig. 6b). These data indicated to IgE also be a crucial factor in the induction of CHS to PPD.
Fig. 6.
Lymph node cells and serum from sensitized WT mice could induce CHS in the STAT6–/– mice (a) Adoptive transfer experiment were performed. Peripheral lymph node cells from the sensitized wt mice were injected subcutaneously into the ear of STAT6–/– mice. The mice were challenged immediately after the injection of lymph node cells with 2·5% PPD ( PPD) or olive oil (
PPD) or olive oil ( olive oil). Ear swelling was measured at 3 h and 24 h after challenge. As a negative control, mice were challenged immediately after injection of PBS with PPD (□ PBS). As a positive control, the mice were contact sensitized by 5 daily consecutive topical applications of 50 µl of 2·5% PPD on shaved abdominal skin, after 2 days these mice were challenged with 2·5% PPD (▪ PPD painting). The data represent the mean swelling values obtained after 24 h with five mice per group and are representative of three independent experiments. *P-value < 0·01 compared with other negative control groups, **P-value < 0·05 compared with the other negative control groups. (b) Adoptive transfer experiment were performed. The sera from the PPD-sensitized wt mice were injected subcutaneously into the ear of the STAT6–/– mice. As a negative control, naive WT and STAT6–/– mice were challenged immediately after injection of PBS with 2·5% PPD (□ NC-WT,
olive oil). Ear swelling was measured at 3 h and 24 h after challenge. As a negative control, mice were challenged immediately after injection of PBS with PPD (□ PBS). As a positive control, the mice were contact sensitized by 5 daily consecutive topical applications of 50 µl of 2·5% PPD on shaved abdominal skin, after 2 days these mice were challenged with 2·5% PPD (▪ PPD painting). The data represent the mean swelling values obtained after 24 h with five mice per group and are representative of three independent experiments. *P-value < 0·01 compared with other negative control groups, **P-value < 0·05 compared with the other negative control groups. (b) Adoptive transfer experiment were performed. The sera from the PPD-sensitized wt mice were injected subcutaneously into the ear of the STAT6–/– mice. As a negative control, naive WT and STAT6–/– mice were challenged immediately after injection of PBS with 2·5% PPD (□ NC-WT,  NC-STAT6–/–). Ear swelling was measured after 3 h and 24 h. As a positive control, the WT and STAT6–/– mice were contact sensitized by 5 daily consecutive topical applications of 50 µl of 2·5% PPD on shaved abdominal skin, after 2 days these mice were challenged with 2·5% PPD (▪ PPD-WT,
NC-STAT6–/–). Ear swelling was measured after 3 h and 24 h. As a positive control, the WT and STAT6–/– mice were contact sensitized by 5 daily consecutive topical applications of 50 µl of 2·5% PPD on shaved abdominal skin, after 2 days these mice were challenged with 2·5% PPD (▪ PPD-WT,  PPD- STAT6–/–). Naive STAT6–/– mice or PPD-sensitized STAT6–/– mice were challenged immediately after injection of serum from PPD-sensitized wt mice (
PPD- STAT6–/–). Naive STAT6–/– mice or PPD-sensitized STAT6–/– mice were challenged immediately after injection of serum from PPD-sensitized wt mice ( NC-Serum-transfer,
NC-Serum-transfer,  PPD-Serum-transfer). Serum from PPD-sensitized wt mice were pretreated with anti-IgE antibody coated beads column, then were injected subcutaneously to naive STAT6–/– mice or PPD-sensitized STAT6–/– (
PPD-Serum-transfer). Serum from PPD-sensitized wt mice were pretreated with anti-IgE antibody coated beads column, then were injected subcutaneously to naive STAT6–/– mice or PPD-sensitized STAT6–/– ( NC-Serum (IgE-)-transfer,
NC-Serum (IgE-)-transfer,  PPD-Serum (IgE-)-transfer). The data represent the means swelling values obtained after 24 h with five mice per group and are representative of three independent experiments. *P-value < 0·01 compared with the other negative control groups, **P-value < 0·05 compared with the other negative control groups.
PPD-Serum (IgE-)-transfer). The data represent the means swelling values obtained after 24 h with five mice per group and are representative of three independent experiments. *P-value < 0·01 compared with the other negative control groups, **P-value < 0·05 compared with the other negative control groups.
Partially diminished CHS response to PPD in mast cell deficient W/Wv mice
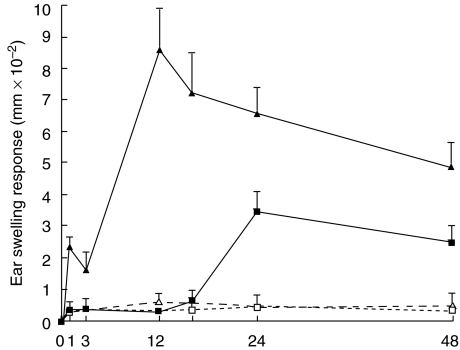
As IgE played an important role in the induction of CHS to PPD, the CHS response to PPD in mast cell deficient W/Wv mice and +/+ mice was examined. As shown in Fig. 7, the CHS response to PPD decreased dramatically and the early phase of CHS to PPD at 1 h after challenge was significantly diminished in the mast cell deficient W/Wv mice as compared with that in the +/+ mice (Fig. 7). The peak response of ear swelling was detected 12 h after the challenge in +/+ mice, whereas the peak response was delayed to 24 h after challenge in mast cell deficient W/Wv mice.
Fig. 7.
WBB6F1-W/Wv mast cell deficient mice exhibit a reduced CHS elicitation to PPD WBB6F1-W/Wv mast cell deficient mice (□ Olive (W/Wv); ▪ PPD (W/Wv)) and wild type (wt) mice (▵ Olive (+/+) ▴ PPD (+/+)) were contact sensitized for five days with 2·5% PPD, 2 days after the last immunization, and thereafter both mice were challenged. The maximal ear swelling was detected at 1 h and 12 h for the wild type mice and at 24 h for the WBB6F1-W/Wv mast cell deficient mice. The data presented are the mean values ± SD of five mice per group and are representative of five independent experiments. *P < 0·01, significant compared to the results obtained with the wild type mice
Histopathology of PPD-induced CHS induced in mast cell deficient W/Wv mice
A histological examination revealed an intense infiltration of mononuclear cells, neutrophils and eosinophils in the CHS response to PPD +/+ mice. In contrast, only a mild inflammatory response with a moderate number of infiltrated cells was observed in the challenged skin in the mast cell deficient W/Wv mice. The number of infiltrated cells into the skin was calculated in both the mast cell deficient W/Wv mice and +/+ mice. No mast cells were detected in the PPD-challenged W/Wv mice. PPD-challenged +/+ mice had a significantly greater number of neutrophils and eosinophils than the PPD-challenged mast cell deficient W/Wv mice (P < 0·01) (Fig. 8).
Fig. 8.
Effect of mast cell deficiency on the cellular dsitribution of the challenged skin. Challenged skin was obtained from WT mice and WBB6F1-W/Wv mast cell deficient mice after being challenged with the application of 2·5% PPD or olive oil (Control). □ Olive (+/+); ▪ PPD (+/+);  Olive (W/Wv);
Olive (W/Wv);  PPD (W/Wv). The columns represent the number of mast cells which infiltrated the challenged skin. The data presented are the mean values ± SD of five mice per group and are representative of three independent experiments. *P-value <0·01 versus TNCB-challenged wt mice. **P-value < 0·05 versus PPD-challenged WT mice.
PPD (W/Wv). The columns represent the number of mast cells which infiltrated the challenged skin. The data presented are the mean values ± SD of five mice per group and are representative of three independent experiments. *P-value <0·01 versus TNCB-challenged wt mice. **P-value < 0·05 versus PPD-challenged WT mice.
DISCUSSION
Considerable progress has been made in elucidating the mechanism of CHS, and CHS is now accepted as a Th1 cell-dependent response [22–24]. In contrast, IL-4 has been demonstrated to be involved in the mechanism of the CHS response [25–28]. Recently, Szczpanik et al. [28] demonstrated that IL-4 production is involved in their down-regulation of the Th 1 cells that mediated CHS. Contrary to this report, IL-4 has been reported to be an essential cytokine during the elicitation phase of CHS [25–27]. The role of IL-4 in CHS is still not clear. We established a murine model system for CHS to PPD in BALB/c mice [6] and demonstrated that Th2 type γδ T cells play a crucial role in the induction of PPD induced CHS through cell transfer experiments, however, it is still unclear as to whether or not Th2 cytokines play an essential in PPD induced CHS. STAT6, a transcription factor of Th2 cytokine, plays a central role in exerting IL-4 and IL-13 mediated biological responses [19,29]. Therefore, we utilized a STAT6 deficient mouse model to study the role of Th2 cytokine, especially IL-4 and IL-5, and mast cell deficient mice to examine the role of mast cells in PPD induced CHS.
The ear swelling response of both the early and late phases was suppressed in STAT6–/– mice after PPD challenge. The oedematous change at the early response and cellular infiltration at the late response were diminished and the local production of both IL-4 and IL-5 was also suppressed in the challenged site of STAT6–/– mice. These findings suggest that the Th2 response is involved in CHS to PPD. These data are consistent with our recent demonstration of STAT6–/– mice which suggested STAT6 to play a major role in the induction of CHS to TNCB, Oxa or DNFB [7]. However, our results did not correlate with those of CHS induced in IL-4–/– mice since the Oxa induced-CHS was not decrease in the IL-4–/– mice [21,30]. It is well known that the Th2 response is completely diminished in STAT6–/– mice but a residual Th2 response has been clearly observed in IL-4–/– mice [19,29]. This difference in the CHS response between the IL-4–/– mice and STAT6–/– mice is attributable to a difference in the type of haptens. PPD is a so-called Th2 hapten which can easily induce a Th2 response, on the other hand, Oxa is a hapten which can induce a Th1 response. The late response of PPD-induced CHS in STAT6–/– mice is much weaker than that by other haptens such as TNCB, DNFB or Oxa. Interestingly, an early response was not observed in the PPD-CHS in STAT6–/– mice. These data indicate that STAT6 signalling plays a crucial role in both the early and late response of CHS to PPD.
A histological analysis revealed a remarkable reduction in the infiltration in both eosinophils and neutrophils in the late response of STAT6–/– mice. In addition, the antigen induced oedematous changes in the dermis was also completely dependent on STAT6 signalling. So far, the exact roles of these neutrophils remain unclear, however, our data indicate that the infiltration of neutrophils is regulated by STAT6 signalling and thus may play a major role in the induction of CHS to PPD.
Not only Th1 cells but also Th2 cells play a role in the induction of hapten specific CHS [31] and STAT6 proteins are essential in Th2 differentiation in vitro[19,29]. STAT6 signalling is essential for a hapten-induced increase in Th2 cytokines production in vivo. A significant elevation of Th2 cytokine, IL-4 and IL5, was noted in the WT mice challenged with haptens. In marked contrast, STAT6–/– mice were unable to produce IL-4 or IL-5 in response to the skin challenge and no detectable Th2 cytokines was detected in the challenged skin of STAT6–/– mice. On the other hand, IFN-γ was increased in both PPD-senssitized WT mice and STAT6–/– mice after challenge. Previously, Saulier et al.[32] demonstrated that the 24-h ear-swelling response of CHS was not significantly reduced by the disruption of IFN γ signalling; however, dermal mononuclear infiltrates were clearly diminished in the IFN-γ receptor –/– mice. In consistent with this paper [32], the number of infiltrated T cells was reduced in the challenged skin of STAT6–/–, however, these infiltrated Th1 cells induced a very small peak at 48 h after challenge in STAT6–/– mice. (Fig. 1). A lack of Th2 cytokine production after a PPD-challenge in STAT6–/– mice reflects the balance of the Th1- and Th2-dependent antibody isotype production in the serum of STAT6–/– mice. STAT6–/– mice have a predominant production of IgG2 antibody without IgE production. A similar observation has been reported in the mouse model of atopic asthma [33–35]. Ptak demonstrated that low dose (10 ng/mouse), and not a high dose (10–100 µg/mouse) of IgE mediated DTH initiation via the release of a small amounts of 5-HT from mast cells [17,18]. PPD-challenged WT mice produced a small amount (10–50 ng/ml) of IgE. IgE can induce the late-phase cutaneous reactions in humans [36,37]. Not only an early response but also a late phase response could be transferred to STAT6–/– mice with serum from PPD-sensitized wt mice and the these responses induced by serum transfer were inhibited by the pretreatment with anti-IgE-epsilon chain antibody coated beads column. However, in consistent with our data, the late phase reaction induced by antigen specific IgE was suppressed in STAT6 deficient mice [38]. These results indicate that the amount of IgE produced by STAT6 signalling in WT mice is a critical factor in the induction of CHS to PPD. Both the early and late response of PPD-CHS could be elicited when the lymph nodes cells from sensitized WT mice were injected into the ears of STAT6–/– mice (Fig. 6a). FACS analysis revealed that γδ T cells were detected from the lymph nodes cells in PPD-sensitized WT mice but not those in STAT6–/– mice (Data not shown) in line with our recent report in which we demonstrated that these γδ T cells belong to Th2 cells and then dependent on IL-4 in growth [6]. These Th2 type γδ T cells may play an important role in the induction of PPD-CHS, however, the exact role of γδ T cell is still unclear now. These data indicate that STAT6 is an important signal during the induction, as has also been suggested previously [7,25].
The early response of CHS to PPD decreased significantly more in the mast cell deficient W/Wv mice than in the+/+mice, however, the late response to PPD also partially decreased. The small peak response of ear swelling was observed at 24 h after challenge in mast cell deficient W/Wv mice. In contrast to our data, DTH or CHS either remained intact [13–16] or decreased [10–12] in previous studies using mast cell-deficient mice. The reason for this discrepancy is that only one point (24 h) observation was performed by these workers [13–16]. They measured ear swelling at only 24 h after challenge in their reports [13–16], however, our finding confirmed that the 24 h CHS response in W/Wv mice decreased slightly, when the responses of the W/Wv mice were assessed at multiple time points in time course study. As a result, a definite defect in CHS was found in W/Wv mice. Interestingly, the response of PPD-CHS in W/Wv mice showed a very similar pattern to those of the STAT6–/– mice. These data indicated that STAT6 signalling in mast calls may play an important role in the induction of PPD-CHS. However, the late response of PPD-CHS in W/Wv was stronger than that in STAT6–/– mice. These results also indicated that other cells except mast cells may release a vasoactive mediator, such as serotonin, and thereby induce a late response. Platelets can provide serotonin for the initiation of CHS [12].
In conclusion, STAT6 signalling was found to play a critical role in the induction of CHS to PPD, while mast cells were also observed to be involved in the initiation of the CHS response to PPD.
Acknowledgments
This work was partially supported by grants (Nos.08670952 and 10670781) from the Japanese Ministry of Education. We would also like to thank Mrs Motoko Sekiya for her excellent technical assistance.
REFERENCES
- 1.Fisher AA. Contact Dermatitis. 3. Philadelphia: Lea & Febiger; 1986. pp. 381–2. [Google Scholar]
- 2.Edward EK, Edward EK., Jr Contact urticaria and allergic contact dermatitis caused by paraphenylenediamine. Cutis. 1984;34:87–8. [PubMed] [Google Scholar]
- 3.Mayer RL. Group-sensitization to compounds of quinone structure and its biochemical basis role of these substances in cancer. Progr Allerg. 1954;4:79.. [Google Scholar]
- 4.Blohm SG, Rajka G. The allergenicity of paraphenylenediamine I, Acta Der matovener (Stockholm) 1970;50:49–51. [PubMed] [Google Scholar]
- 5.Magnusson B. The allegenicity of paraphenelenediamine versus that of paratoluenediamine. Contact Dermatitis Newsletter. 1974;15:432. [Google Scholar]
- 6.Yokozeki H, Watanabe K, Igawa K, Miyazaki Y, Katayama I, Nishioka K. γδ T cells assist αβ T cells in the adoptive transfer of contact hypersensitivity to para-phenylenediamine. Clin Exp Immunol. 2001;125:351–9. doi: 10.1046/j.1365-2249.2001.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokozeki H, Ghoreishi M, Takagawa S, et al. Signal transducer and activator of transcription 6 is essential in the induction of contact hypersensitivity. J Exp Med. 2000;191:995–1004. doi: 10.1084/jem.191.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon RK, Askenase PW, Gershon MD. Requirement for vasoactive amines for production of delayed-type hypersensitivity skin reaction. J Exp Med. 1975;142:732–47. doi: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli SJ, Dvorak AM. What do mast cells have to do with delayed hypersensitivity? Laboratory Invest. 1984;50:365–8. [PubMed] [Google Scholar]
- 10.Askenase P, Van Loven WH, Kraueter-Kops S, et al. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol. 1983;131:2687–94. [PubMed] [Google Scholar]
- 11.Miyachi Y, Imamaura S, Tokura Y, Takigawa M. Mechanisms of contact hypersensitivity in mice. VII. Diminished elicitation by reserpin and defective expression in mast cell-deficient nice. J Invest Dermatol. 1986;87:39–41. doi: 10.1111/1523-1747.ep12523544. [DOI] [PubMed] [Google Scholar]
- 12.Geba GP, Ptak W, Anderson GM, Paliwal V, Ratzlaff RE, Levin J, Askenase PW. Delayed-type hypersensitivity in mast cell-deficient mice. Dependence on platelets for expression of contact sensitivity. J Immunol. 1996;154:557–65. [PubMed] [Google Scholar]
- 13.Thomas WR, Schrader JW. Delayed hypersensitivity in mast cell-deficient mice. J Immunol. 1983;130:2565–7. [PubMed] [Google Scholar]
- 14.Galli SJ, Hammel I. Unequivacal delayed hypersensitivity inmast cell-deficient and beige mice. Science. 1984;226:710–3. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- 15.Ha T-Y, Reed ND, Crowle PK. Immune response potential of mast cell-deficient W/Wv mice. Int Arch Allergy Apppl Immunol. 1986;80:85–94. doi: 10.1159/000234031. [DOI] [PubMed] [Google Scholar]
- 16.Mekori YA, Chang JCC, Wershil BK, Galli S. Studies of the role of mast cells in contact sensitivity response. Cell Immunol 1986. 1987;109:39–52. doi: 10.1016/0008-8749(87)90290-5. [DOI] [PubMed] [Google Scholar]
- 17.Ptak W, Geba GP, Askenase PW. Initiation of delayed-type hypersensitivity by low doses of monoclonal IgE antibody, Mediation by serotonine and inhibition by histamine. J Immunol. 1991;146:3929–36. [PubMed] [Google Scholar]
- 18.Matsuda H, Ushio H, Paliwal V, Ptak W, Askenase PW. Adoptive cell transfer of contact sensitivity-initiation mediated by nonimmune cells sensitized with monoclonal IgE antibodies. J Immunol. 1995;154:5080–92. [PubMed] [Google Scholar]
- 19.Takeda KT, Tanaka W, Shi M, et al. Essential role of Stat6 in IL-4 signaling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson T, Dube P, Griffith TS. Regulation of contact hypersensitivity by interleukin 10. J Exp Med. 1994;179:1597–674. doi: 10.1084/jem.179.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traidl C, Jugert F, Krieg T, Merk H, Hunzelmann N. Inhibition of allergic contact dermatitis to DNCB but not to Oxazolone in Interleukin-4-deficient mice. J Invest Dermatol. 1999;112:476–82. doi: 10.1046/j.1523-1747.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, DiIulio NA, Fairchild RI. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon γ-producing (Tc1) effector CD8+ T cells and interleukin (IL)-4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbrink K, Sorg C, Macher E. Low zone tolerance to contact allergens in mice: a functional role for CD8+ T helper type 2 cells. J Exp Med. 1996;183:759–68. doi: 10.1084/jem.183.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gocinsli BL, Tigelaar RE. Role of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–8. [PubMed] [Google Scholar]
- 25.Dieli F, Asherson GL, Romano GC, Sireci G, Gervasi F, Salerno A. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cell lines. J Immunol. 1994;152:2698–704. [PubMed] [Google Scholar]
- 26.Salerno A, Dieli F, Sireci G, Bellavia A, Asherson GL. IL-4 is a critical cytokine in contact sensitivity. Immunol. 1995;84:404–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Asherson G, Dieli LF, Sireci G, Salerno A. Role of IL-4 in delayed type hypersensitivity. Clin Exp Immunol. 1996;103:1–4. doi: 10.1046/j.1365-2249.1996.845537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szczepanik M, Ptak W, Askenease PW. Role of IL-4 in down-regulation of contact sensitivity by gammadelta T cells from tolerized T cell recptor alpha-/- mice. 1999;98:63–70. doi: 10.1046/j.1365-2567.1999.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol. 1996;157:3220–2. [PubMed] [Google Scholar]
- 30.Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. IL-10 but not IL-4 is a natural suppression of cutaneous inflammatory responses. J Exp Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller KM, Jaunin F, Masouye I, Saurat J-H, Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol. 1993;150:5576–84. [PubMed] [Google Scholar]
- 32.Saulier M, Huang S, Aguet M, Ryffel B. Role of interferon-gamma in contact hypersensitivity assessed in interferon-gamma receprtor-deficient mice. 1995;102:301–12. doi: 10.1016/0300-483x(95)03101-k. [DOI] [PubMed] [Google Scholar]
- 33.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transduction and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;16:939–48. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hypersensitivity in signal transducers and activators of transcription (STAT6)-deficient mice. J Exp Med. 1998;187:1597–42. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyata S, Matsuyama T, Kodama T, Nishioka Y, Kuribayashi K, Takeda K, Akira S, Suguta M. STAT6 deficiency in a mouse model of allergen airway inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin Exp Allergy. 1999;29:114–23. doi: 10.1046/j.1365-2222.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- 36.Solley GO, Gleich GJ, Jordan RE. The late phase of the immediate wheal and flare skin reaction: Its dependence upon IgE antibodies. J Clin Invest. 1976;58:408–20. doi: 10.1172/JCI108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner JA, Pienkowski MM, Plaut M, Norman PS, Lichtenstein LM. Identification of histamine-releasing factor(s) in the late phase of cutaneous IgE-mediated reactions. J Immunol. 1986;136:2583–7. [PubMed] [Google Scholar]
- 38.Malaviya R, Uckun FM. Role of STAT6 in IgE receptor/Fcepsilon RI-mediated late phase allergic responses of mast cells. J Immunol. 2002;168:421–6. doi: 10.4049/jimmunol.168.1.421. [DOI] [PubMed] [Google Scholar]