Abstract
To study the immunosuppressive and anti-inflammatory effects of combined leflunomide and methotrexate (MTX) therapy on chemokine expression in patients with rheumatoid arthritis (RA), nine patients were enrolled for the combination therapy for 24 weeks. These patients have been on treatment with MTX 15 mg/week for not less than 3 months before entry to the study. A loading dose of l00 mg/day of leflunomide was given for 3 days, followed by 10 mg/day for the rest of the study period. Plasma concentrations of monocyte chemotactic protein-1 (MCP-1), thymus- and activation-regulated chemokine (TARC), and macrophage-derived chemokine (MDC) were assayed before and after combination treatment by ELISA. Gene expression of inflammatory cytokines and chemokines of peripheral blood mononuclear cells was analysed by cDNA expression array. Plasma MCP-1, TARC and MDC concentrations were significantly lower in patients after combination treatment [median (interquartile range) before versus after treatment: MCP-1 of 118·0 (64·0–515·2) versus 3·2 (0·0–22·8) pg/ml, P < 0·01; TARC of 126·1 (27·2–197·4) versus 0·0 (0·0–52·5) pg/ml, P < 0·05; MDC of 503·3 (446·2–600·9) versus 366·8 (337·4–393·4) pg/ml, P < 0·05]. Positive correlations among reductions in plasma chemokines and clinical outcome measures were also found. Expression of chemokine genes including MDC and TARC was suppressed after combination treatment [% suppression of 38·7 (54·3–13·0) and 53·7 (55·9–28·4), respectively]. Combination therapy with leflunomide and MTX exhibits anti-inflammatory activity in the suppression of chemokine expression and subsequent recruitment of inflammatory cells into the inflammatory sites in RA.
Keywords: leflunomide, MCP-1, MDC, rheumatoid arthritis, TARC
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease characterized by symmetric polyarticular inflammation that is frequently accompanied by destruction of joint integrity [1]. The inflamed RA joint shows hyperplasia of the intimal lining layer and increased cellularity of the synovial sublining. Predominant cell types involved in synovial inflammation include activated T cells [2], monocytes/macrophages [3] and neutrophils [4]. Concurrent with the increased cellularity is an increased expression of adhesion molecules that are involved in cell trafficking [5] and of proinflammatory mediators such as cytokines and chemokines [6].
Leflunomide, a novel disease-modifying antirheumatic drug (DMARD) of the isoxazole class, has shown clinical efficacy by effectively reducing joint inflammation in patients with RA [7]. Its primary action is the inhibition of dihydroorotate dehydrogenase, a key enzyme for de novo synthesis of pyrimidines [8,9]. Because pyrimidines are required for proliferation of activated T cells, the net effect of inhibition of pyrimidine synthesis is to halt this process, which is suggested to be a critical step in the pathogenesis of RA. Given the high failure rate of monotherapy and the multi-factorial nature of the pathogenesis of RA, an increasing emphasis is being placed on combinations of therapeutic agents that act to inhibit different pathologic processes of the disease [10]. Considering its mechanism of action, leflunomide can be useful in combination therapy with methotrexate (MTX). MTX is a commonly used DMARD in the treatment of RA, and functions by inhibiting dihydrofolate reductase and hence decreasing the supply of reduced folates for purine biosynthesis [11]. An in vitro study has suggested that MTX promotes apoptosis of activated T cells, an action that would be complementary to the effect of leflunomide to limit T cell proliferation [12].
It is becoming clear that chemokines and their receptors are involved intimately in regulating organ-specific leucocyte trafficking and inflammation in RA [13–15]. Monocyte chemotactic protein-1 (MCP-1) is a monocyte, B and T lymphocyte chemoattractant belonging to the CC subfamily of chemokines, and is produced by macrophages, endothelium, synovial fibroblasts and chondrocytes in the inflamed joint of patients with RA [16–18]. Recent studies have confirmed that MCP-1 is elevated in the synovial fluid and plasma of RA patients, which can act as a marker for RA joint inflammation due to its significant correlation with the number of swollen joints and the Ritchie Articular Index (RAI) [19,20]. Plasma MCP-1 has therefore become the candidate marker for monitoring the clinical efficacy of drug treatment in RA patients.
Thymus- and activation-regulated chemokine (TARC) is another member of CC chemokine family, expressed mainly on mononuclear leucocytes. It is found to be a T cell-specific chemoattractant and functionally related to the macrophage-derived chemokine (MDC) [21]. Expression of MDC mRNA has been shown in macrophages and dendritic cells [22]. MDC induces chemotaxis for monocyte-derived dendritic cells, activated T cells and natural killer (NK) cells [23]. Recent studies have revealed that both TARC and MDC play a role in inflammatory diseases such as allergic asthma and atopic dermatitis [24]. However, expression of TARC and MDC in patients with RA has not yet been investigated.
The anti-inflammatory activity of leflunomide has been shown to be related to the suppression of proinflammatory cytokines, for example tumour necrosis factor (TNF)-induced cellular response and signalling [25] and inhibition of neutrophil migration [26]. It is also found to suppress the activation of NF-κB, a potent intracellular mediator of inflammation [27]. The present study was undertaken to examine the immunosuppressive effect of leflunomide on several chemokines including MCP-1, TARC and MDC in terms of gene and protein expressions. Gene expressions of other chemoattractants such as myeloid progenitor inhibitory factor-1 (MPIF-1), I-309 and interleukin (IL)-15, thymus-expressed chemokine (TECK) and two chemokine receptors (CCR), and clinical responses to the addition of leflunomide to MTX treatment in patients with RA were also studied.
MATERIALS AND METHODS
Patients
All patients recruited in this study fulfilled the 1987 American College of Rheumatology (ACR) criteria for RA [28]. Nine patients with active disease as defined by the presence of six or more swollen or tender joints, based on a 28-joint count [29], who were refractory to treatment with MTX, were recruited into the study. At the time of recruitment, all patients had radiographic erosive diseases and had been on MTX for not less than 3 months at a dosage of not less than 15 mg/week. Consenting patients, aged 18 years and older, were required to use adequate methods of contraception, and pregnant or lactating women were excluded. The study was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong. All subjects gave informed consent before entering the study.
Study design
This 24-week study was an open-label evaluation of the clinical response and suppression of chemokine expression upon the addition of leflunomide to MTX treatment. At enrolment, all patients were on MTX 15 mg/week only. Four weeks before commencement of this open-label study, all other DMARDs were discontinued. Leflunomide was started with a dose of 100 mg daily for 3 days, then 10 mg/day in combination with MTX at 15 mg for the ensuing 24 weeks. Paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), oral or intra-articular corticosteroids were allowed during the trial period.
Clinical assessment of efficacy was performed every 4 weeks. Parameters for assessment included tender and swollen joint counts (28-joint assessment), visual analogue scale (VAS) for pain of 0 (no pain) to 100 (severe pain), duration of early morning stiffness (EMS), patient and physician global assessment scores of RA activity ranging from 0 (very good) to 100 (very poor), functional disability rated by the Health Assessment Questionnaire (HAQ) [30], as well as serum C-reactive protein (CRP) and erythrocyte sediment rate (ESR).
Clinical outcome measures were the swollen and tender joint counts, and the patient's and physician's global assessments of disease activity. In addition, we assessed the proportion of patients showing a clinical response of at least 20% and 50% as defined by the American College of Rheumatology (ACR) criteria [31].
Safety was monitored by physical examination, chest radiography, electrocardiography if required, blood pressure, pulse rate, body temperature and body weight measurements. Standard haematological and serum biochemical tests and urine analysis were also performed. The occurrence of adverse events was documented and included those spontaneously reported by patients as well as those elicited by general questioning.
Blood samples
Twenty ml of heparinized venous peripheral blood was collected from each patient in each of six visits within 24 weeks. Plasma samples were preserved at −70°C for subsequent assays.
Assays for MCP-1, TARC and MDC
Plasma MCP-1, TARC and MDC concentrations of RA patients before and after combination treatment with leflunomide and MTX were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., MN, USA).
cDNA expression array analysis
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by Ficoll-paque density gradient centrifugation (Amersham Pharmacia Biotech Ltd, Uppsala, Sweden). Total RNA was extracted from PBMC using RNeasy Mini Kit (QiagenGmbH, Hilden, Germany) according to the manufacturer's instructions. cDNA expression array analysis was performed using non-radioactive human inflammatory cytokine/receptor GEArray™ Q series Kit (SuperArray Inc., Bethesda, MD, USA), according to the manufacturer's instructions. Brifefly, total RNA was reverse-transcribed with reverse transcriptase and GEAprimer mix in the presence of biotin-16-dUTP. The biotinylated cDNA probes were denatured and added to the hybridization solution. Two identical GEArray™ Q-series membranes dotted with tetra-spot cDNA fragments of 96 human inflammatory cytokine and receptor genes were prehybridized at 60°C for 2 h and then hybridized overnight with the cDNA probes. Membranes were then washed, blocked and incubated with alkaline phosphatase-conjugated streptavidin. The labelled biotin on the membrane was detected by chemiluminescent method and the luminescence intensities of hybridized cDNA probes were analysed using Bio-Rad Quantity One™ software (Bio-Rad Laboratories, CA, USA). The percentage change of gene expression levels before and after treatment with leflunomide was calculated as [(intensity after leflunomide – intensity before leflunomide)/intensity before leflunomide × 100%] using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control.
Statistical analysis
The differences in clinical outcome measures between week 0 and week 24 of leflunomide treatment were assessed by Wilcoxon signed-rank test. The differences in plasma MCP-1, TARC and MDC concentrations in the RA patients before and after leflunomide treatment were assessed by repeated measures test of analysis of variance (anova Friedman test). Results were expressed as either median (interquartile range) or mean ± standard deviation. Spearman's rank correlation test was used to assess the correlations among reductions in plasma chemokines and clinical outcome measures. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) statistical software for Windows, Version 9·0 (SPSS Inc., IL, USA). A probability (P) less than 0·05 was considered significantly different.
RESULTS
Patient demographics and clinical efficacy
The age, sex, duration of diagnosis and clinical outcome measures of the study population are summarized in Table 1. Nine patients (seven females and two males, mean ± SD age of 42·8 ± 11·5 years, range 18–57) were recruited. The mean duration of the diagnosis of RA was 11·3 ± 5·6 years (range 3–17). Eight of the nine patients were positive for rheumatoid factor. Three patients were maintained on low-dose prednisolone between 2·5 and 5 mg daily throughout the study period. Significant reduction was observed in all clinical outcome measures comparing week 0 and week 24 of leflunomide treatment (P < 0·05 for VAS pain score, patient global assessment, physician global assessment, swollen joint score, ESR, CRP, HAQ), except for the duration of morning stiffness (EMS, P = 0·3) (Table 1). Three of the nine patients (responder rate 33·3%) and two of the nine patients (22·2%) treated with leflunomide fulfilled the ACR 20% and ACR 50% response criteria, respectively, after 24 weeks of treatment. No major side effects were encountered.
Table 1.
Demographics and clinical outcome measures of RA patients
| RA patients | P-value | |
|---|---|---|
| Number | 9 | |
| Sex (female/male) | 7/2 | |
| Age, year (mean ± SD, range) | 42·8 ± 11·5 (18–57) | |
| Duration of diagnosis (mean ± SD, range) | 11·3 ± 5·6 (3–17) | |
| Rheumatoid factor (positive/negative) | 8/1 | |
| EMS, min (mean ± SD, range) | ||
| Week 0 | 36·7 ± 99·3 (0–300) | |
| Week 24 | 0·0 ± 0·0 (0–0) | 0·300 |
| VAS pain score (median, IQR) | ||
| Week 0 | 73·0 (24·5–87·0) | |
| Week 24 | 31·0 (22·5–57·5) | 0·027 |
| Patient global assessment (median, IQR) | ||
| Week 0 | 67·0 (62·0–81·5) | |
| Week 24 | 40·0 (17·5–63·5) | 0·004 |
| Physician global assessment (median, IQR) | ||
| Week 0 | 65·0 (61·0–81·5) | |
| Week 24 | 40·0 (17·5–63·5) | 0·004 |
| Swollen joint score (median, IQR) | ||
| Week 0 | 11·0 (5·5–12·5) | |
| Week 24 | 6·0 (2·0–11·0) | 0·023 |
| ESR, mm/ h (median, IQR) | ||
| Week 0 | 72·0 (60·0–96·0) | |
| Week 24 | 59·0 (24·0–74·5) | 0·004 |
| CRP, mg/l (median, IQR) | ||
| Week 0 | 57·5 (36·1–67·7) | |
| Week 24 | 19·4 (4·1–53·9) | 0·039 |
| HAQ score (median, IQR) | ||
| Week 0 | 1·9 (0·5–2·7) | |
| Week 24 | 1·4 (0·4–2·1) | 0·004 |
Plasma concentrations of MCP-1, TARC and MDC
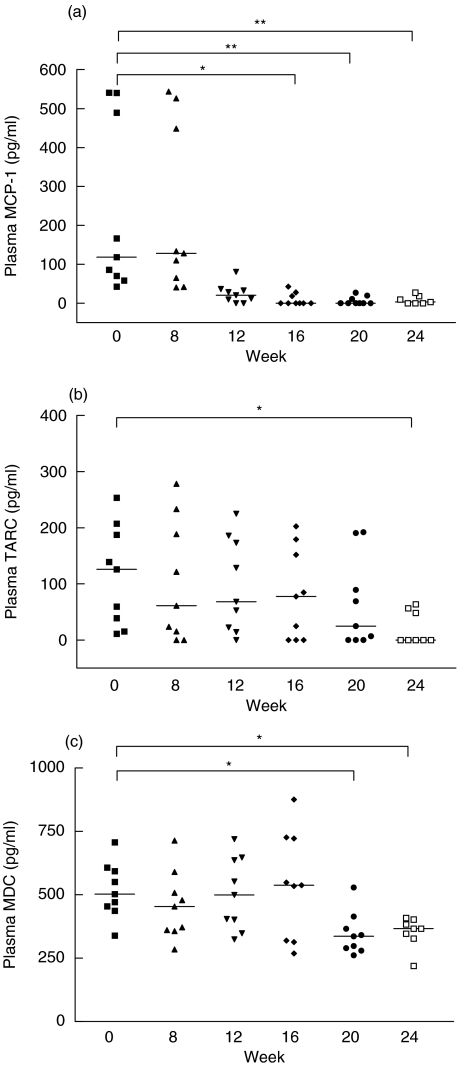
As shown in Fig. 1a, plasma MCP-1 concentrations were lowered significantly after the combination treatment of leflunomide and MTX [median (interquartile range) of MCP-1: week 0 versus week 16, 118·0 (64·0–515·2) versus 0·0 (0·0–23·2) pg/ml, P < 0·05; Week 0 versus week 20, 118·0 (64·0–515·2) versus 0·0 (0·0–15·5) pg/ml, P < 0·01; week 0 versus week 24, 118·0 (64·0–515·2) versus 3·2 (0·0–22·8) pg/ml, P < 0·01]. Plasma TARC concentrations were reduced significantly with the combination treatment only after 24 weeks [week 0 versus week 24, 126·1 (27·2–197·4) versus 0·0 (0·0–52·5) pg/ml, P < 0·05] (Fig. 1b).Figure 1c shows that plasma MDC concentrations was lowered significantly with leflunomide after 20 and 24 weeks [week 0 versus week 20, 503·3 (446·2–600·9) versus 337·4 (284·9–390·6) pg/ml, P < 0·05; week 0 versus week 24, 503·3 (446·2–600·9) versus 366·8 (337·4–393·4) pg/ml, P < 0·05].
Fig. 1.
Plasma concentrations of (a) MCP-1, (b) TARC and (c) MDC in RA patients. The median is indicated by the horizontal bar and each symbol represents an individual. The differences before (week 0) and after (weeks 8, 12, 16, 20, 24) the combination treatment of leflunomide and MTX were determined by anova Friedman Test. *P < 0·05, **P < 0·01.
Expression profile of inflammatory cytokine and chemokine genes
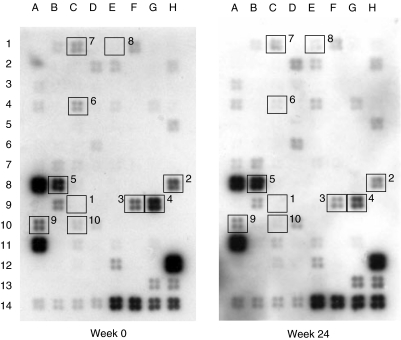
Figure 2 illustrates the effect of leflunomide on gene expression profile of inflammatory cytokines, chemokines and receptors in the PBMC of RA patients treated with MTX. As shown in Table 2, combination treatment of leflunomide and MTX in RA patients suppressed the gene expression of several chemokines including TARC [53·7 (28·4–55·9)%], MDC [38·7 (13·0–54·3)%], MPIF-1 [54·3 (38·6–61·5)%], I-309 [36·4 (7·5–62·2)%] and TECK [44·4 (14·7–46·1)%], although no detectable signal could be observed for MCP-1. Gene expressions of IL-15 [30·5 (5·0–49·2)%], CCR2 [32·0 (−5·7–47·3)%] and CCR4 [30·4 (26·6–53·0)%] were slightly down-regulated by combination treatment. No significant reduction was found in MIP-1 beta gene expression [10·8 (3·5–23·5)%]. For the remaining genes of human inflammatory cytokines, chemokines and receptors, either undetectable signal or no significant change in expression level could be obtained.
Fig. 2.
Expression profile of inflammatory cytokine and receptor genes in PBMC from RA patients before (week 0) and after (week 24) leflunomide and MTX treatment detected by cDNA expression array system. Total RNA was extracted from PBMC, reverse-transcribed and labelled with biotin, and gene expressions were detected using the GEArray™ Q series Kit. Genes with differential expressions in RA patients upon combination treatment were indicated by number-labelled boxes: MCP-1 (1), TARC (2), MDC (3), MPIF-1 (4), I-309 (5), IL-15 (6), CCR2 (7), CCR4 (8), TECK (9) and MIP-1 beta (10). Negative control genes: pUC18 DNA (13 A, 13B, 13C); positive control (housekeeping) genes: GAPDH (13G, 13H), cyclophilin A (14 A, 14B, 14C, 14D), ribosomal protein L13A (RPL13A) (14E, 14F), β-actin (14G, 14H).
Table 2.
Differential expression of human chemokine genes in PBMC of RA patients
| Box | Genes | % Reduction |
|---|---|---|
| 1 | MCP-1 | UD |
| 2 | TARC | 53·7 (28·4–55·9) |
| 3 | MDC | 38·7 (13·0–54·3) |
| 4 | MPIF-1 | 54·3 (38·6–61·5) |
| 5 | I-309 | 36·4 (7·5–62·2) |
| 6 | IL-15 | 30·5 (5·0–49·2) |
| 7 | CCR2 | 32·0 (−5·7–47·3) |
| 8 | CCR4 | 30·4 (26·6–53·0) |
| 9 | TECK (thymus-expressed chemokine) | 44·4 (14·7–46·1) |
| 10 | MIP-1 beta | 10·8 (3·5–23·5) |
The percentage reduction of gene expression level before and after combination treatment with leflunomide and MTX was assessed by the difference in luminescence intensity using human inflammatory cytokine/receptor GEArray™ Q Series Kit (SuperArray), and calculated as [(intensity after leflunomide – intensity before leflunomide)/intensity before leflunomide × 100%] with GAPDH as an internal control. UD = undetectable for the gene expression of MCP-1 in both week 0 (before leflunomide addition) and week 24 (after leflunomide addition). Positions of genes were indicated by number-labelled boxes shown in Fig. 2. Results are expressed as median (interquartile range) of the percentage reduction in the cohort of nine RA patients.
Correlations of reductions in plasma chemokines and clinical outcome measures
As shown in Table 3, suppression in plasma MCP-1 concentration of the RA patients significantly correlated with the reduction in ESR after 24-week combination treatment (r = 0·857, P = 0·024). Despite the absence of statistical significance, plasma MCP-1 reduction showed positive correlations with decreases in both patient and physician global assessment (both r = 0·714), swollen joint score (r = 0·577) and HAQ score (r = 0·618). Plasma TARC reduction also correlated significantly with CRP reduction (r = 0·833, p = 0·008), while plasma MDC correlated positively with swollen joint score reduction without statistical significance (r = 0·614).
Table 3.
Correlations of reductions in plasma chemokines and clinical outcome measures after 24 weeks of combination treatment with leflunomide and MTX in RA patients
| Plasma MCP-1 | Plasma TARC | Plasma MDC | |
|---|---|---|---|
| VAS pain score | 0·469 (0·302) | 0·286 (0·463) | 0·219 (0·581) |
| Patient global assessment | 0·714 (0·088) | −0·183 (0·644) | 0·150 (0·708) |
| Physician global assessment | 0·714 (0·088) | −0·200 (0·613) | 0·217 (0·581) |
| Swollen joint score | 0·577 (0·200) | 0·286 (0·463) | 0·614 (0·086) |
| ESR | 0·857 (0·024)* | −0·117 (0·776) | 0·283 (0·463) |
| CRP | 0·393 (0·396) | 0·833 (0·008)* | −0·233 (0·552) |
| HAQ score | 0·618 (0·139) | 0·362 (0·336) | 0·293 (0·437) |
Results are expressed as the correlation coefficient r (P-value).
P-values less than 0·05 are considered significant using Spearman's rank correlation test.
DISCUSSION
This small open study demonstrated that combination therapy of leflunomide and MTX was effective in patients with refractory RA, achieving ACR 20 and 50 responses of 56% and 33%, respectively (Table 1). Our results are consistent with what has been observed recently [32]. All nine patients, before the addition of leflunomide, had very active disease and were on higher doses of MTX that ranged between 17·5 and 22·5 mg/week. Although we did not have a group of patients continuing on MTX monotherapy as control, none the less, as all the nine patients have been on MTX for many months (range 8–59 months) before the addition of leflunomide, the effects observed were probably not due to delayed MTX-induced effects.
The anti-inflammatory activity of leflunomide has been shown to be associated with the suppression of proinflammatory cytokines and chemokines [33]. The present results indicate that plasma concentrations of several chemokines including MCP-1, TARC and MDC were lowered significantly after combination treatment of leflunomide and MTX (Fig. 1). The reduced circulating chemokines might be responsible for the decreased recruitment of inflammatory cells like lymphocytes and monocytes to the synovial tissue of RA. Because plasma MCP-1 has been suggested to be a marker for joint inflammation in RA [19], the reduced plasma MCP-1 concentrations after 16–24 weeks of leflunomide treatment (Fig. 1a) might result in a decreased migration of monocytes and antigen-driven T lymphocytes to the inflammatory lesions of synovium, and hence the subsequent reduction in joint inflammation, swelling and tenderness (Table 1). These were evidenced by the positive correlations between suppression in plasma MCP-1 and reductions in ESR, patient and physician global assessment, swollen joint score and HAQ score (Table 3). The lack of MCP-1 gene expression in PBMC from RA patients might be explained by the inhibitory effect of MTX on cytokine and chemokine production [11], or other cells such as fibroblast and endothelial cells, but not PBMC, are the major source of MCP-1 in the circulation. There is increasing evidence showing that the elevated MCP-1 in synovial fluid is produced mainly by activated synovial fibroblasts and chondrocytes [19,20].
Both plasma TARC and MDC concentrations were significantly reduced after 24 weeks of combination treatment of leflunomide and MTX (Fig. 1b,c), and their gene expressions were also highly down-regulated (Table 2). The lowered production of TARC might be responsible for the decreased infiltration of antigen-driven T lymphocytes into the rheumatoid synovium, and hence the suppression of RA progression. Synovial T lymphocytes may play a pivotal role in RA by T helper-cell cytokine production, which in turn activates B lymphocytes for the production of rheumatoid factors and destruction of synovial tissues [34]. The lower extent of synovial destruction might be explained by the significantly positive correlation of suppressed TARC production with CRP reduction (Table 3). Meanwhile, the suppressed expression of MDC in the circulation by leflunomide might be related to the decreased migration of dendritic cells and macrophages into synovial fluid. Because MDC is expressed highly by dendritic cells and is also chemotactic for them, it may play an autocrine role in their accumulation at rheumatoid synovium [22], and subsequent joint swelling as evidenced by its positive correlation with swollen joint score (Table 3). In the present study, two CCR genes in PBMC, including CCR2 (MCP-1 receptor) and CCR4 (TARC and MDC), were found to be down-regulated slightly after leflunomide treatment (Table 2), suggesting that the chemotactic gradient for migration of mononuclear leucocytes might be disrupted by both leflunomide and MTX. However, the exact mechanism of chemotaxis suppression by leflunomide and MTX is still not fully understood.
This is the first report demonstrating that gene expressions of three CC chemokines MPIF-1, I-309 and TECK were largely reduced after leflunomide treatment in RA (Table 2). Because MPIF-1 displays chemotactic activity on activated dendritic cells and monocytes [35,36], the suppressed expression might be also related to their reduced infiltration. I-309 is suggested to display an autocrine loop of chemotactic activity on activated T cells and monocytes themselves [37]. TECK also serves as a chemoattractant for thymocytes, dendritic cells and macrophages [38]. In conjunction with the above chemokines, the decreased expression of I-309 and TECK might provide further evidence for which dendritic cells, monocytes and T cells are the major targets of the leflunomide-induced suppression of chemotaxis. In vitro studies of lymphocyte migration in RA indicated that IL-15 accounts for, at least partly, the chemotactic and proinflammatory activity for T cells in synovial fluid, in combination with IL-8 and MCP-1 [39,40]. Our results illustrated that IL-15 gene expression was down-regulated in response to combination treatment of leflunomide and MTX, which proposes that IL-15 is one of the potential therapeutic targets in RA therapy.
In conclusion, the present study has demonstrated that combination therapy of leflunomide and MTX directly or indirectly brings out a significant suppression on the expression of several major chemokines including MCP-1, TARC, MDC, MPIF-1, I-309 and IL-15, and a good clinical response in patients with RA. The down-regulated expression of these chemokines can reflect only the effects of controlling inflammation by leflunomide and MTX, and the underlying mechanism of inhibition on leucocyte migration by leflunomide is not understood fully. Therefore, further mechanistic investigations are required.
Acknowledgments
This study was supported by Aventis Pharma Ltd and a donation from Zindart (De Zhen) Foundation Ltd, Hong Kong.
REFERENCES
- 1.Sharp JT, Wolfe F, Mitchell DM, Bloch DA. The progression of erosion and joint space narrowing scores in rheumatoid arthritis during the first twenty-five years of disease. Arthritis Rheum. 1991;34:660–8. doi: 10.1002/art.1780340606. [DOI] [PubMed] [Google Scholar]
- 2.Fox DA. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997;40:598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- 3.Burmester GR, Stuhlmuller B, Keyszer G, Kinne RW. Mononuclear phagocytes and rheumatoid synovitis: mastermind or workhorse in arthritis? Arthritis Rheum. 1997;40:5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- 4.Chatham WW, Edberg JC, Kimberley RP. The role of neutrophils in rheumatoid arthritis. In: Firestein GS, Panayi GS, Wollheim FA, editors. Rheumatoid arthritis: new frontiers in pathogenesis and treatment. New York: Oxford University Press, Inc.; 2000. pp. 101–12. [Google Scholar]
- 5.Szekanecz Z, Szegedi G, Koch AE. Cellular adhesion molecules in rheumatoid arthritis: regulation by cytokines and possible clinical importance. J Invest Med. 1996;44:124–35. [PubMed] [Google Scholar]
- 6.Koch AE, Kunkel SL, Strieter RM. Cytokines in rheumatoid arthritis. J Invest Med. 1995;43:28–38. [PubMed] [Google Scholar]
- 7.Smolen JS, Kalden JR, Scott DL, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353:259–66. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- 8.Cherwinski HM, Cohn RG, Cheung P, et al. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther. 1995;275:1043–9. [PubMed] [Google Scholar]
- 9.Fox RI. Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol. 1998;25(Suppl. 53):20–6. [PubMed] [Google Scholar]
- 10.Harris ED. Rationale for combination therapy of rheumatoid arthritis based on pathophysiology. J Rheumatol. 1996;23(Suppl. 44):2–4. [PubMed] [Google Scholar]
- 11.Rau R. Methotrexate. In: Firestein GS, Panayi GS, Wollheim FA, editors. Rheumatoid arthritis: new frontiers in pathogenesis and treatment. New York: Oxford University Press, Inc.; 2000. pp. 337–50. [Google Scholar]
- 12.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard J. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katschke KJ, Rottman JB, Ruth JH, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–32. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Katrib A, Tak PP, Bertouch JV, et al. Expression of chemokines and matrix metalloproteinases in early rheumatoid arthritis. Rheumatology (Oxford) 2001;40:988–94. doi: 10.1093/rheumatology/40.9.988. [DOI] [PubMed] [Google Scholar]
- 15.Szekanecz Z, Strieter RM, Kunkel SL, Koch AE. Chemokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:115–32. doi: 10.1007/BF00832002. [DOI] [PubMed] [Google Scholar]
- 16.Harigai M, Hara M, Yoshimura T, Leonard EJ, Inoue K, Kashiwazaki S. Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin Immunol Immunopathol. 1993;69:83–91. doi: 10.1006/clin.1993.1153. [DOI] [PubMed] [Google Scholar]
- 17.Hachicha M, Rathanaswami P, Schall TJ, McColl SR. Production of monocyte chemotactic protein-1 in human type B synoviocytes. Synergistic effect of tumor necrosis factor alpha and interferon-gamma. Arthritis Rheum. 1993;36:26–34. doi: 10.1002/art.1780360106. [DOI] [PubMed] [Google Scholar]
- 18.Pulsatelli L, Dolzani P, Piacentini A, et al. Chemokine production by human chondrocytes. J Rheumatol. 1999;26:1992–2001. [PubMed] [Google Scholar]
- 19.Ellingsen T, Buus A, Stengaard-Pedersen K. Plasma monocyte chemoattractant protein 1 is a marker for joint inflammation in rheumatoid arthritis. J Rheumatol. 2001;28:41–6. [PubMed] [Google Scholar]
- 20.Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lipsky PE. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001;3:118–26. doi: 10.1186/ar149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J Leukoc Biol. 1997;62:634–44. doi: 10.1002/jlb.62.5.634. [DOI] [PubMed] [Google Scholar]
- 22.Godiska R, Chantry D, Raport C, et al. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bochner BS, Bickel CA, Taylor ML, et al. Macrophage-derived chemokine induces human eosinophil chemotaxis in a CC chemokine receptor 3- and CC chemokine receptor 4-independent manner. J Allergy Clin Immunol. 1999;103:527–32. doi: 10.1016/s0091-6749(99)70481-1. [DOI] [PubMed] [Google Scholar]
- 24.Abi-Younes S, Si-Tahar M, Luster AD. The CC chemokines MDC and TARC induce platelet activation via CCR4. Thromb Res. 2001;101:279–89. doi: 10.1016/s0049-3848(00)00402-3. [DOI] [PubMed] [Google Scholar]
- 25.Manna SK, Mukhopadhyay A, Aggarwal BB. Leflunomide suppresses TNF-induced cellular responses. effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J Immunol. 2000;165:5962–9. doi: 10.4049/jimmunol.165.10.5962. [DOI] [PubMed] [Google Scholar]
- 26.Kraan MC, de Koster BM, Elferink JG, Post WJ, Breedveld FC, Tak PP. Inhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double-blind clinical trial in fifteen patients. Arthritis Rheum. 2000;43:1488–95. doi: 10.1002/1529-0131(200007)43:7<1488::AID-ANR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Breedveld FC, Dayer JM. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000;59:841–9. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 29.Smolen JS, Breedveld FC, Eberl G, et al. Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthritis Rheum. 1995;38:38–43. doi: 10.1002/art.1780380106. [DOI] [PubMed] [Google Scholar]
- 30.Koh ET, Seow A, Pong LY, et al. Cross cultural adaptation and validation of the Chinese Health Assessment Questionnaire for use in rheumatoid arthritis. J Rheumatol. 1998;25:1705–8. [PubMed] [Google Scholar]
- 31.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 32.Weinblatt ME, Kremer JM, Coblyn JS, et al. Pharmacokinetics, safety, and efficacy of combination treatment with methotrexate and leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum. 1999;42:1322–8. doi: 10.1002/1529-0131(199907)42:7<1322::AID-ANR4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 33.Fox RI, Herrmann ML, Frangou CG, et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 1999;93:198–208. doi: 10.1006/clim.1999.4777. [DOI] [PubMed] [Google Scholar]
- 34.Yocum DE. T cells: pathogenic cells and therapeutic targets in rheumatoid arthritis. Semin Arthritis Rheum. 1999;29:27–35. doi: 10.1016/s0049-0172(99)80035-3. [DOI] [PubMed] [Google Scholar]
- 35.Nardelli B, Morahan DK, Bong GW, Semenuk MA, Kreider BL, Garotta G. Dendritic cells and MPIF-1: chemotactic activity and inhibition of endogenous chemokine production by IFN-gamma and CD40 ligation. J Leukoc Biol. 1999;65:822–8. doi: 10.1002/jlb.65.6.822. [DOI] [PubMed] [Google Scholar]
- 36.Nardelli B, Tiffany HL, Bong GW, et al. Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J Immunol. 1999;162:435–44. [PubMed] [Google Scholar]
- 37.Haque NS, Zhang X, French DL, et al. CC chemokine I-309 is the principal monocyte chemoattractant induced by apolipoprotein (a) in human vascular endothelial cells. Circulation. 2000;102:786–92. doi: 10.1161/01.cir.102.7.786. [DOI] [PubMed] [Google Scholar]
- 38.Vicari AP, Figueroa DJ, Hedrick JA, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 39.Al-Mughales J, Blyth TH, Hunter JA, Wilkinson PC. The chemoattractant activity of rheumatoid synovial fluid for human lymphocytes is due to multiple cytokines. Clin Exp Immunol. 1996;106:230–6. doi: 10.1046/j.1365-2249.1996.d01-836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McInnes IB, al-Mughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]