Abstract
X-linked agammaglobulinaemia (XLA) is a primary immunodeficiency disease characterized by very low levels or even absence of circulating antibodies. The immunological defect is caused by deletions or mutations of Bruton's tyrosine kinase gene (Btk), whose product is critically involved in the maturation of pre-B lymphocytes into mature B cells. Btk is expressed not only in B lymphocytes but also in cells of the myeloid lineage, including dendritic cells (DC). These cells are professional antigen presenting cells (APC) that play a fundamental role in the induction and regulation of T-cell responses. In this study, we analysed differentiation, maturation, and antigen-presenting function of DC derived from XLA patients (XLA-DC) as compared to DC from age-matched healthy subjects (healthy-DC). We found that XLA-DC normally differentiate from monocyte precursors and mature in response to lipopolysaccharide (LPS) as assessed by de novo expression of CD83, up-regulation of MHC class II, B7·1 and B7·2 molecules as well as interleukin (IL)-12 and IL-10 production. In addition, we demonstrated that LPS stimulated XLA-DC acquire the ability to prime naïve T cells and to polarize them toward a Th1 phenotype, as observed in DC from healthy donors stimulated in the same conditions. In conclusion, these data indicate that Btk defect is not involved in DC differentiation and maturation, and that XLA-DC can act as fully competent antigen presenting cells in T cell-mediated immune responses.
Keywords: X-linked agammaglobulinaemia, dendritic cells, maturation, T cell polarization
INTRODUCTION
X-linked agammaglobulinaemia (XLA) is an X-linked recessive genetic disease characterized by lack of circulating mature B cells resulting in a primary immunodeficiency with severe hypogammaglobulinaemia [1,2]. The B-cell defect is due to mutations in the gene for Bruton's tyrosine kinase (Btk) which lead to a block in the pro-B to pre-B cell transition during B-cell ontogeny [3–5]. Affected males undergo severe and recurrent bacterial infections after the sixth month of age when levels of maternal Ig decline, and show an increased susceptibility to enteroviral infections often resistant to intravenous immunoglobulin (IVIG) administration [1,2]. Furthermore it has been reported that Th1-orientated diseases, such as nonseptic, rheumathoid-arthritis or type 1 diabetes mellitus frequently occur in XLA patients [6,7].
Btk is a non-receptor associated tyrosine kinase which belongs to Tek family together with Itk, Tec and Bmx [8,9]. This family of protein tyrosine kinases is involved in a vast array of signal transduction pathways [10–13]. In particular, Btk plays a pivotal role in lymphohaematopoietic growth and differentiation. It is expressed in both B and myeloid cells, but its functional role has been examined so far mainly in the context of B cell activation [14–16]. The finding that activation of Btk is regulated by a wide variety of receptors, including BCR, Fc-R, IL-3R, IL-5R and IL-6R [17,18] further suggests a Btk role in the differentiation or function of cell lineages other than B lymphocytes. In a previous study, it has been shown that Btk is involved in mast-cell signalling via Fc∈ receptors [19] More recently, macrophages from xid mice have been shown to produce IL-12 in a significantly higher amount than wild-type mice [20], and it has been demonstrated that these mice mount a relatively more dominant Th1- T cell immune response against filarial antigens as compared with their normal counterparts [21]. These findings have suggested a putative role for Btk in regulating macrophage APC function in T cell priming.
Dendritic cells (DC) are professional APC that play a critical role in priming and polarization of naïve T cells [22,23]. Immature DC are localized in non-lymphoid organs where they take up and process foreign antigens [24,25]. In response to local inflammatory stimuli, they undergo phenotypic and functional maturation and migrate to secondary lymphoid tissues where they stimulate naïve T cells and drive their polarization towards a Th1 or Th2 cytokine profile [23]. Despite their pivotal role in T cell-mediated immune response, as well as in the pathogenesis of autoimmune diseases and cancer, the differentiation process and function of DC from XLA patients has not been previously investigated. In this paper, we analysed the capacity of XLA-DC to undergo a correct differentiation and maturation program, to prime and properly polarize naïve T cells.
MATERIALS AND METHODS
Subjects
Five unrelated male patients diagnosed as XLA, according to the WHO classification of primary immunodeficiences, and 11 age-matched healthy controls were included in this study, after obtaining ethical approval. Clinical and molecular data of XLA patients are summarized in Table 1. Analysis of Btk mutations was performed by direct sequencing on cDNA samples as already described [26]. All patients were receiving regular intravenous immunoglobulin (IVIG) replacement therapy (400 mg/kg) at 3–4 week intervals and were free of any serious infections at the time of blood sampling. Peripheral venous blood was collected after informed consent, and for the patient's group, this was immediately before their routine intravenous immunoglobulin infusions were started.
Table 1.
Clinical and molecular data of XLA patients
| Patient | Age at DC analysis (years) | IgG levels (mg/dl) | IgA levels (mg/dl) | IgM levels (mg/dl) | IgE levels (Ui/ml) | Peripheral B cells (%) | Mutation |
|---|---|---|---|---|---|---|---|
| XLA A | 6 | 179 | 9 | 34 | 6 | 0 | R641C |
| XLA B | 10 | 0 | 8 | 6 | 1 | 0 | P190 fs X198 |
| XLA C | 3 | 5 | < 5 | < 5 | 21 | 0·1 | R525P |
| XLA D | 6 | 229 | 11 | 23 | 39 | 1 | DelF98-Q103 |
| XLA E | 6 | < 7·6 | < 6 | 41 | 4 | 0·05 | V568 fs X569 |
Media and reagents
The medium used throughout the study was RPMI 1640 supplemented with 2 mm l-glutamine, 1% nonessential aminoacids, 1% sodium pyruvate, 50 µg/ml kanamycin (Gibco- BRL, Paisley, UK) and 10% FBS (Hyclone Laboratoires, Logan, UT, USA). Human rGM-CSF was purchased from Shoering Plough/Sandoz; human rIL-4, produced by PCR cloning and expression in the myeloma expression system, was a generous gift of Prof A. Lanzavecchia. LPS from E. coli was purchased from Sigma Chemicals Co. (St Louis, MO, USA).
Generation of dendritic cells
PBMC from patients or healthy donors were isolated on Lymphropep cushions (Nycomed Pharma AS, Oslo, Norway). Monocytes were purified by positive sorting using anti-CD14 conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The recovered cells were 99% CD14+ as determined by flow cytometry using the FITC-labelled anti-CD14 mAb (Becton Dickinson). The cells were cultured at 3 × 105/ml in RPMI-10% FBS supplemented with 50 ng/ml GM-CSF and 1000 U/ml IL-4 for 4–6 days to allow differentiation into DC. DC were stimulated with 1 µg/ml LPS for 24 h to induce their maturation.
FACS analysis on DC
Surface staining of DC was performed using FITC-conjugated mAbs anti-B7·1, anti-B7·2, anti-CD83 and PE-conjugated mAbs anti-CD14, anti-CD1a, anti-DR, all produced by Pharmingen/BD. For detection of intracytoplasmatic Btk protein, DC were fixed with 2% paraformaldehyde for 15 min at +4°C and then permeabilized with 0.5% saponin for 30 min at room temperature. After washing, cells were stained with anti-Btk mAb 48–2H (2 µg/ml), for 20 min at room temperature and subsequently incubated with FITC-conjugated anti-mouse IgG1 (Southern Biotechnology Associates, Birmingham, AL, USA) for 20 min at room temperature. The cells were analysed on a FACScalibur (Becton Dickinson).
Cytokine assays
On the 5th day of culture XLA and healthy DC were washed, counted and replated (3 × 105) in a final volume of 1 ml of RPMI + 10%FCS in the presence or absence of 1 µg/ml LPS for an additional 24 h. Culture supernatants were assayed for IL-12p70 and IL-10 by Endogen Elisa kits, according to manufacturer instructions and expressed in pg/ml. The limit of detection was 15 pg/ml.
Mixed leukocyte reaction (MLR)
DC from either XLA patients or control subjects were stimulated with 1 µg/ml LPS for 24 h and then extensively washed. Thereafter, they were irradiated at 3000 rads and cultured at different concentrations in 96 well plates in the presence of 1 × 105 unfractioned cord blood cells. Cell cultures were in part tested on day 5 for [3H]-thymidine incorporation, in part expanded with 500 U/ml human rIL-2 to obtain polyclonal T cell lines to be analysed for cytokine production.
Intracellular staining for cytokine detection
T cell lines were stimulated with 50 ng/ml PMA plus 1 µg/ml ionomycin (Sigma) for 4 h. Brefeldin A (Sigma) at 10 µg/ml was added during the last 2 h of culture. Cells were fixed with 2% paraphormaldehyde, permeabilized with PBS containing 1% FBS and 0.5% saponin, stained with FITC-conjugated anti-IFN-γ and PE-conjugated anti-IL-4 mAbs (Becton Dickinson), and then analysed on a FACScalibur.
RESULTS
DC differentiation and maturation are uninpaired in XLA patients
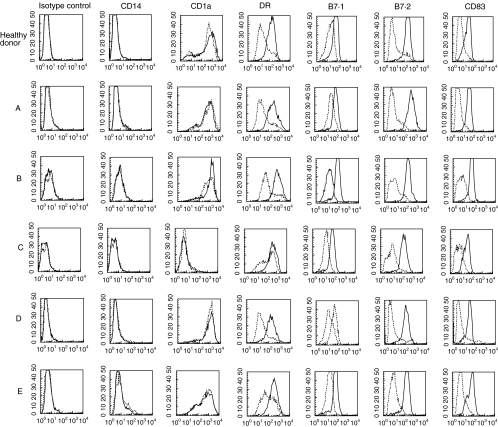
DC were generated in vitro from purified blood monocytes of five XLA patients and 11 age-related healthy donors in the presence of GM-CSF and IL-4. As shown in Fig. 1, XLA-DC were CD14− and CD1a+, apart from one patient whose DC were CD1a−. These data indicated a normal differentiation from monocytes to DC. After 24 h of stimulation with LPS XLA-DC acquired a mature phenotype similar to healthy DC as assessed by up-regulation of MHC class II, B7·1 and B7·2 costimulatory molecules and expression of CD83, a classical marker of DC maturation.
Fig. 1.
Flow cytometric analysis of surface phenotype of DC from five XLA patients and one representative of 11 age-related healthy donors. DC were generated from blood monocytes and stimulated with LPS for 24 h to induce their maturation. Dotted histograms represent immature DC and solid histograms identify LPS-stimulated DC. Fluorescence values are measured on gated large cells. The data are from one representative experiment out of three performed.
Btk detection by intracellular staining
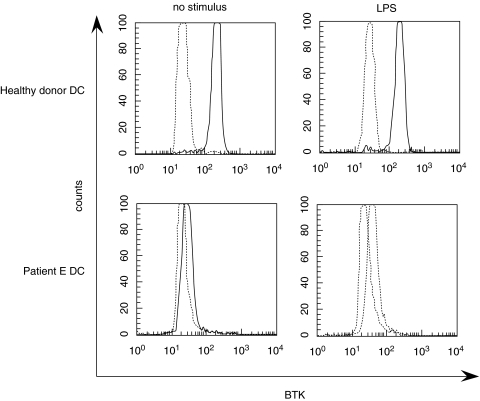
To analyse BTK expression in XLA-DC, we performed an intracellular staining of this protein in all five XLA patients and in two healthy donors by the use of mAb 48–2H. This monoclonal antibody has been shown to constitute a rapid and sensitive approach for evaluation of BTK expression in monocytes and a useful method for detection of XLA patients and female carriers [27]. A representative experiment is shown in Fig. 2: immature as well as LPS-stimulated DC from one healthy donor expressed the intracellular protein, as it could be expected by their myeloid origin, while DC from patient E were defective of Btk. Similar results were obtained with DC from all other patients (data not shown).
Fig. 2.
Intracellular staining for BTK detection in immature DC and LPS-stimulated DC from 1 XLA patient and 1 healthy donor. Cells were fixed, permeabilized and stained with an anti-BTK IgG1 mAb (48–2H) followed by FITC-conjugated antimouse IgG1. Expression of BTK is shown as a solid line and the negative control stained with a mouse IgG1 mAb followed by FITC-conjugated antimouse IgG1 is shown as a dotted line. This staining was performed twice for all XLA patients and three healthy donors.
XLA DC produce IL-12 and IL-10 after LPS stimulation
Next we asked whether the mature phenotype of XLA-DC was associated or not with a normal function in term of cytokine production. Therefore, we analysed IL-12 and IL-10 production by LPS-treated XLA- or healthy DC. As shown in Table 2, DC from XLA patients produced levels of IL-12 (median 496 pg/ml) similar to their normal counterparts (median 442 pg/ml). IL-10 production by XLA-DC was higher (median 136 pg/ml) than that produced by healthy DC (median 31 pg/ml), suggesting a trend toward a higher production in these patients. Immature DC from both groups did not produce either IL-12 or IL-10 (data not shown). These results indicate that the mature phenotype of XLA-DC is associated with functional maturation as assessed by cytokine production.
Table 2.
Cytokine production by LPS-stimulated DC from five XLA patients and 11 healthy donors
| IL-12 (pg/ml) | IL-10 (pg/ml) | |
|---|---|---|
| Patients | ||
| A | 33* | 161 |
| B | 324 | 17 |
| C | 496 | 118 |
| D | 776 | 136 |
| E | 748 | 233 |
| Median | 496 | 136 |
| Healthy donors | ||
| 1 | 764 | 131 |
| 2 | 845 | < 15† |
| 3 | 442 | < 15 |
| 4 | 36 | < 15 |
| 5 | < 15 | 65 |
| 6 | 370 | 31 |
| 7 | 616 | < 15 |
| 8 | 1210 | 158 |
| 9 | 415 | 166 |
| 10 | 288 | nd |
| 11 | 1077 | nd |
| Median | 442 | 31 |
DC, at day 5th of culture, were washed, adjusted to 3 × 105 c/ml and cultured in the presence or absence of 1 µg|ml LPS for an additional 24 h. Supernatants were examined for cytokines by ELISA
Values expressed as the mean of two independent experiments.
Detection limit of the assay: 15 pg/ml. nd, not determined.
XLA-DC are fully competent APC for the stimulation of naïve T cells
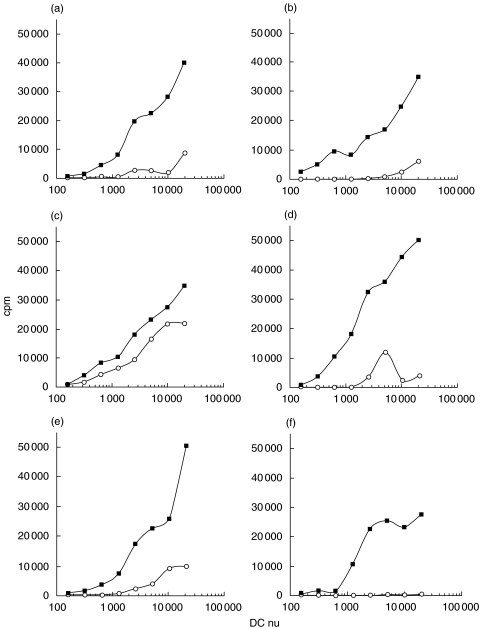
The fundamental role of DC is to prime naïve T cells and to determine their polarization toward a specific cytokine production pattern. To verify whether XLA-DC could fulfil this function despite the lack of Btk, unfractionated cord blood cells were stimulated with immature or LPS-stimulated DC derived from four XLA patients and from two age-matched healthy donors. As shown in Fig. 3, XLA- and healthy-DC induced a comparable proliferative response of naïve allogeneic T cells. As expected, LPS-stimulated DC from both groups were more efficient in T cell priming as compared to immature DC which do not possess a high immunostimulatory capacity. CD1a− immature DC from patient C induced a particularly high proliferative response of naïve T cells, which could be caused by a spontaneous maturation occurred in vitro.
Fig. 3.
Stimulation of naïve T cells by XLA DC. Immature DC (○) were left untreated or stimulated with LPS for 24 h (▪). A mixed leucocyte reaction (MLR) was then set up where irradiated DC from four XLA patients (a–d) and two healthy donors (e, f) were cultured at different cell numbers with 1 × 105 unfractioned cord blood cells. The proliferative response was measured after 5 days by thymidine incorporation. The background proliferation of cord blood cells alone was < 100 cpm. The data shown are from 1 representative experiment out of 2 performed.
T cell polarization by XLA-DC
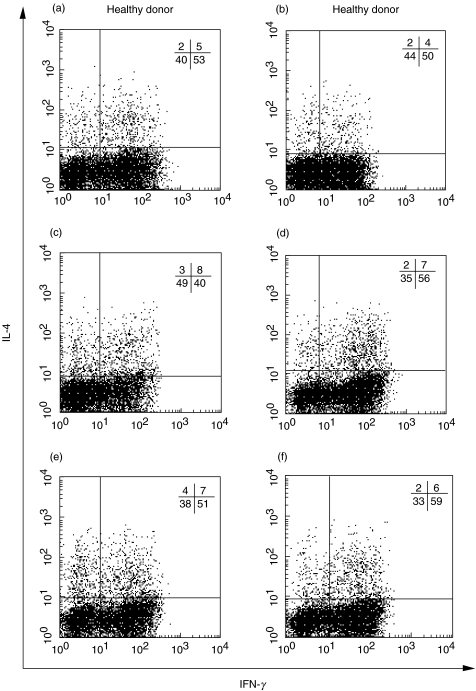
The pattern of IL-4 and IFN-γ production by cord blood T lymphocytes primed by LPS stimulated-DC from four XLA patients and two age-matched healthy donors was also analysed. As shown in Fig. 4, the percentage of IFN-γ or IL-4 producing T cells stimulated by LPS-matured DC from three different patients was not significantly different from healthy donors, with prevalence of Th1 lymphocytes. Immature DC from patients A, B, D, and from healthy donors did not induce T cell polarization while immature DC from patient C primed 13% IFN-γ producing cells. (data not shown). Interestingly, DC from patient C, which lacked CD1a expression, induced a weaker polarization of naïve T cells. Collectively, these data indicate that XLA-DC analogously to healthy DC drive a preferential Th1 cell polarization of naïve T cells when stimulated with LPS.
Fig. 4.
Intracellular staining for IL-4 and IFN-γ produced by cord blood T cells. T cells (90–95% CD4+, data not shown) expanded from the MLR assays shown in Fig. 3 (a–d, XLA patients; e–f, healthy donors) were stimulated with PMA and ionomycin for 4 h. Cells were fixed, permeabilized and stained with FITC-conjugated anti-IFN-γ and PE-conjugated anti-IL-4 mAbs. The numbers indicate the percentage of cells in each quadrant.
DISCUSSION
Btk is a non-receptor protein tyrosine kinase that is crucial for B lymphocytes to maturate into antibody-producing cells [13,14]. Mutations in the Btk gene are responsible for X-linked immunodeficiency (xid) in mouse and X-linked agammaglobulinaemia in humans [3,4]. The resulting human disease is characterized by an enhanced susceptibility to either bacterial or nonbacterial infections that require early treatment with intravenous immunoglobulins (IVIG). Furthermore these patients show an increased prevalence of autoimmune diseases [6,7]. Btk is normally expressed not only in B-cells, but also in cells of the myeloid lineage, but is absent on T cells and plasma cells [3,4]. In female XLA carriers not only B cells manifest the skewed inactivation of the mutated X-chromosome but also monocytes undergo random inactivation of the normal and mutated X-chromosomes [28,29]. However, very few data are available on the role of this enzyme in myeloid cell biology and function. In this paper, we investigated if Btk defect could affect differentiation, maturation or function of human DC from XLA-patients, considering that these myeloid cells play a pivotal role in the antigen presentation to specific CD4+ and CD8+ T cells and in skewing the polarization of these cells toward a prevalent Th/Tc-1 or Th/Tc-2 phenotype [23].
Circulating monocytes from XLA patients were found to fully differentiate into immature DC as defined by the expression of CD1a surface molecule and by downmodulation of CD14 expression upon culture with GM-CSF and IL-4. This indicates that Btk, which has been shown to be involved in a wide range of signal transduction processes in B cells, is not implicated in the first step of DC differentiation. Of note, in one out of the five patients tested, DC were CD1a−. Studies are in progress to assess if this alteration may be present also in other XLA patients and its functional meaning.
In response to local inflammatory stimuli such as IL-1 and TNF-α or to bacterial products such as lipopolysaccharide (LPS) [22,25] or viral double-stranded RNA [30], immature DC undergo phenotypic and functional maturation which allows these cells to become very efficient immunostimulatory APC. Therefore, we investigated if this maturation process was normally accomplished by XLA-DC. As shown in Fig. 1, LPS -treated XLA-DC were found to normally up-regulate MHC class II, B7·1 and B7·2 costimulatory molecules and they expressed CD83, all findings indicating that XLA-DC are able to acquire the classical phenotype of mature APC.
We further investigated another important function of these cells, namely their capacity to produce cytokines in response to maturation stimuli. IL-12 is the key determinant of Th1 polarization and it is produced by DC in response to microbial as well as cell-derived stimuli such as CD40L. IL-10 has been identified as a major factor that blocks IL-12 production by DC in an autocrine manner and impairs their ability to generate Th1 responses [31]. Therefore the balance between these two cytokines is critical for maintaining immune system regulation. As shown in Table 2 LPS-treated XLA-DC produced IL-12 without any significant difference with healthy DC, in contrast with the report of higher IL-12 production by macrophages from xid mice which has been shown to depend indirectly on the reduced production of nitric oxide [20]. A trend toward a higher production of IL-10 by XLA-DC has been observed but, in our experimental setting, it did not correlate with any alteration of DC cell function. Our results clearly show that cytokine production by DC in humans is not affected by Btk defect.
The major function of DC is to prime naïve T cells and to drive their polarization toward a Th1 or Th2 phenotype [23,24]. When tested in MLR assays, mature XLA-DC were found to efficiently induce the proliferation of naïve T cells (Fig. 3). This evidence demonstrates the normal antigen presenting capacity of these cells. The analysis of cytokine production by these allogeneic T cell lines showed that XLA-DC are capable to prime IFN-γ and IL-4 producing cells with the same efficiency than healthy DC. These results are in agreement with recent data demonstrating a normal specific Th1/Th2 response to the hepatitis B virus envelope antigen (HBenvAg) in XLA patients vaccinated against hepatitis B [32]. Another human study has suggested with analysis at clonal level that tetanus toxoid-specific T cell response induced in XLA patients is skewed toward a Th-1 phenotype [33]. In our DC maturation we induced DC maturation with LPS that is a classical Th-1 promoting stimulus so we can not establish if XLA-DC in different conditions might be potentially defective in their Th-2 stimulatory activity.
In conclusion, our study demonstrates for the first time that DC are fully competent APC in XLA patients, accounting for a normal T cell priming and polarization. The lack of Btk in XLA-DC does not alter their behaviour, as it could be suggested by the results obtained in xid mice or by the clinical observation of an increased prevalence of Th1-orientated diseases, supporting the hypothesis that this cell population does not contribute to any immunological alteration in XLA patients. These findings may have important implications for setting up T-cell based vaccination protocols against intra- and extra-cellular pathogens and they could suggest a wider diffusion of such vaccines in XLA patients.
Acknowledgments
We would like to thank Roberto Nisini and Maria Teresa De Magistris for critical reading the manuscript and in particular Marino Paroli for his helpful comments and suggestions during all the work.
REFERENCES
- 1.Sideras P, Smith CI. Molecular and cellular aspects of X-linked agammaglobulinemia. Adv Immunol. 1995;59:135–223. doi: 10.1016/s0065-2776(08)60631-8. [DOI] [PubMed] [Google Scholar]
- 2.Ochs HD, Smith CI. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine (Baltimore) 1996;75:287–99. doi: 10.1097/00005792-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Saffran DC, Rawlings DJ, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X- linked agammaglobulinemia. Cell. 1993;72:279–90. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 4.Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings DJ, Saffran DC, Tsukada S, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–61. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 6.Rosen FS, Cooper MD, Wedgwood RJ. The primary immunodeficiencies. N Engl J Med. 1995;333:431–40. doi: 10.1056/NEJM199508173330707. [DOI] [PubMed] [Google Scholar]
- 7.Timmers E, de Weers M, Alt FW, Hendriks RW, Schuurman RK. X-linked agammaglobulinemia. Clin Immunol Immunopathol. 1991;61:S83–93. doi: 10.1016/s0090-1229(05)80042-x. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada S, Rawlings DJ, Witte ON. Role of Bruton's tyrosine kinase in immunodeficiency. Curr Opin Immunol. 1994;6:623–30. doi: 10.1016/0952-7915(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 9.Yamada N, Kawakami Y, Kimura H, et al. Structure and expression of novel protein-tyrosine kinases, Emb and Emt, in hematopoietic cells. Biochem Biophys Res Commun. 1993;192:231–40. doi: 10.1006/bbrc.1993.1404. [DOI] [PubMed] [Google Scholar]
- 10.Liao XC, Littman DR, Weiss A. Itk and Fyn make independent contributions to T cell activation. J Exp Med. 1997;186:2069–73. doi: 10.1084/jem.186.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saharinen P, Ekman N, Sarvas K, Parker P, Alitalo K, Silvennoinen O. The Bmx tyrosine kinase induces activation of the Stat signaling pathway, which is specifically inhibited by protein kinase Cdelta. Blood. 1997;90:4341–53. [PubMed] [Google Scholar]
- 12.Weil D, Power MA, Smith SI, Li CL. Predominant expression of murine Bmx tyrosine kinase in the granulo- monocytic lineage. Blood. 1997;90:4332–40. [PubMed] [Google Scholar]
- 13.Kitanaka A, Mano H, Conley ME, Campana D. Expression and activation of the nonreceptor tyrosine kinase Tec in human B cells. Blood. 1998;91:940–8. [PubMed] [Google Scholar]
- 14.Desiderio S. Role of Btk in B cell development and signaling. Curr Opin Immunol. 1997;9:534–40. doi: 10.1016/s0952-7915(97)80107-0. [DOI] [PubMed] [Google Scholar]
- 15.Khan WN, Sideras P, Rosen FS, Alt FW. The role of Bruton's tyrosine kinase in B-cell development and function in mice and man. Ann N Y Acad Sci. 1995;764:27–38. doi: 10.1111/j.1749-6632.1995.tb55802.x. [DOI] [PubMed] [Google Scholar]
- 16.Khan WN, Alt FW, Gerstein RM, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–99. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Katagiri T, Takaki S, et al. IL-5 receptor-mediated tyrosine phosphorylation of SH2/SH3-containing proteins and activation of Bruton's tyrosine and Janus 2 kinases. J Exp Med. 1994;180:2101–11. doi: 10.1084/jem.180.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda T, Takahashi-Tezuka M, Fukada T, et al. Association and activation of Btk and Tec tyrosine kinases by gp130, a signal transducer of the interleukin-6 family of cytokines. Blood. 1995;85:627–33. [PubMed] [Google Scholar]
- 19.Hata D, Kawakami Y, Inagaki N, et al. Involvement of Bruton's tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–47. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, George A, Bal V, Ravindran B, Rath S. Bruton's tyrosine kinase deficiency in macrophages inhibits nitric oxide generation leading to enhancement of IL-12 induction. J Immunol. 1999;163:1786–92. [PubMed] [Google Scholar]
- 21.Mukhopadhyay S, Sahoo PK, George A, Bal V, Rath S, Ravindran B. Delayed clearance of filarial infection and enhanced Th1 immunity due to modulation of macrophage APC functions in xid mice. J Immunol. 1999;163:875–83. [PubMed] [Google Scholar]
- 22.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 23.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschese V, Orlandi P, Plebani A, et al. X-chromosome inactivation and mutation pattern in the Bruton's tyrosine kinase gene in patients with X-linked agammaglobulinemia. Mol Med. 2000;6:104–13. Italian XLA Collaborative Group. [PMC free article] [PubMed] [Google Scholar]
- 27.Futatani T, Miyawaki T, Tsukada S, et al. Deficient expression of Bruton's tyrosine kinase in monocytes from X- linked agammaglobulinemia as evaluated by a flow cytometric analysis and its clinical application to carrier detection. Blood. 1998;91:595–602. [PubMed] [Google Scholar]
- 28.Conley ME, Puck JM. Carrier detection in typical and atypical X-linked agammaglobulinemia. J Pediatr. 1988;112:688–94. doi: 10.1016/s0022-3476(88)80683-8. [DOI] [PubMed] [Google Scholar]
- 29.Puck JM, Nussbaum RL, Conley ME. Carrier detection in X-linked severe combined immunodeficiency based on patterns of X chromosome inactivation. J Clin Invest. 1987;79:1395–400. doi: 10.1172/JCI112967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 32.Paroli M, Accapezzato D, Francavilla V, et al. Long-lasting memory-resting and memory-effector CD4+ T cells in human X- linked agammaglobulinemia. Blood. 2002;99:2131–7. doi: 10.1182/blood.v99.6.2131. [DOI] [PubMed] [Google Scholar]
- 33.Amedei A, Romagnani C, Benagiano M, et al. Preferential Th1 profile of T helper cell responses in X-linked (Bruton's) agammaglobulinemia. Eur J Immunol. 2001;31:1927–34. doi: 10.1002/1521-4141(200106)31:6<1927::aid-immu1927>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]