Abstract
Streptococcus suis serotype 2 is known to be a major pathogen of swine, causing mainly meningitis. It is also a zoonotic agent leading predominantly to meningitis in humans working in close contact with pigs. In this study, we investigated the ability of S. suis to up-regulate the expression of adhesion molecules involved in inflammation, using an enzyme-linked immunosorbent assay. S. suis serotype 2 stimulated the up-regulation of the expression of intercellular adhesion molecule-1 (ICAM-1, CD54), CD11a/CD18 and CD11c/CD18 on human THP-1 monocytes, but did not change that of ICAM-1, vascular cell adhesion molecule-1 (VCAM-1, CD106) and E-selectin (CD62E) on human endothelial cells. The up-regulation of adhesion molecules was time- and bacterial concentration-dependent, and cell wall components were largely responsible for such stimulation. To a lesser extent, purified haemolysin of S. suis also stimulated adhesion molecule expression. Stimulation of monocytes with strains of different origin showed that there was no clear tendency for human strains to induce a higher expression of adhesion molecules than strains from diseased pigs. Finally, monocytes stimulated with S. suis also showed an increase in adherence to endothelial cells. Hence, S. suis is capable of up-regulating important adhesion molecules involved in inflammation, which may result in an increased leucocyte recruitment into sites of infection, thus providing a possible mechanism for some of the inflammatory features of meningitis caused by this pathogen.
Keywords: adhesion molecules, endothelial cells, meningitis, monocytes, Streptococcus suis
INTRODUCTION
Streptococcus suis is a well-known swine pathogen causing a wide range of infections such as meningitis, septicaemia, arthritis and pneumonia [1]. S. suis can also cause meningitis in individuals who work in close proximity to pigs, often leading to serious sequelea such as hearing loss [2]. To date, 35 serotypes have been identified, of which serotype 2 is considered the most virulent and most frequently isolated from diseased animals and humans [3]. Knowledge of virulence factors of S. suis serotype 2 is limited. So far, the only proven critical virulence factor is the polysaccharidic capsule (CPS) [4]. Cell wall and extracellular proteins, including a haemolysin (named suilysin), are also associated with virulence; however, most virulent North American strains do not possess these factors [5–7].
The pathogenesis of meningitis caused by S. suis serotype 2 is largely unknown; however, several mechanisms have been proposed recently [8]. S. suis may be transmitted via the respiratory route, breaching the mucosal epithelia in the upper respiratory tract by as yet unknown mechanisms [8]. Once in the blood, bacteria would come into contact with phagocytes. An early theory suggested uptake of bacteria by monocytes, intracellular survival and invasion of the central nervous system (CNS) by the ‘Trojan horse theory’[9,10]. However, only a low number of monocytes were shown to actually contain bacteria (<2%), and most bacteria remained extracellular [10]. In fact, recent studies using isogenic mutants defective in capsule production demonstrated the antiphagocytic properties of the CPS, as only non-encapsulated mutants were phagocytosed readily [4,11]. Although S. suis may travel mainly as free bacteria, other alternative mechanisms may also take place. A recent study demonstrated a high level of adhesion (without phagocytosis) of S. suis to monocytes, which led to the proposition of a ‘modified’ Trojan horse theory, where bacteria may travel externally in association with monocytes [8,12].
Survival of S. suis in the bloodstream as free bacteria would lead to septicaemia and invasion of the meninges and other tissues. S. suis has been shown to adhere preferentially to human brain microvascular endothelial cells, a single layer of specialized cells forming the blood–brain barrier (BBB) [13]. One consequence of this adhesion is the up-regulation in the production of proinflammatory cytokines by these cells [14], which might be responsible for inducing an acute inflammatory exudate that increases the volume of cerebral spinal fluid (CSF), leading to increased intracranial pressure. S. suis is also able to stimulate the production of proinflammatory cytokines by both human [15] and murine [16] phagocytes.
Leucocyte influx into the subarachnoid space and the increase in BBB permeability are considered hallmarks of bacterial meningitis [17]. Leucocyte recruitment to sites of inflammation is mediated by several families of adhesion molecules present on the surface of leucocytes and endothelial cells [18]. These include the selectins (E- and P-selectin (CD62E and CD62P) expressed on endothelial cells, and L-selectin (CD62L) on leucocytes); the β2 integrins (CD11a/CD18, CD11b/CD18 & CD11c/CD18), exclusively expressed on leucocytes; and members of the immunoglobulin superfamily (IgSF), mainly intercellular adhesion molecule-1 (ICAM-1, CD54) and vascular cell adhesion molecule-1 (VCAM-1, CD106), expressed on endothelial cells; however, ICAM-1 has also been shown to be expressed on monocytes [19]. These molecules work together in mediating leucocyte rolling, firm adhesion and subsequent extravasation [20] by forming multiple receptor-ligand pairs that act either in a sequential and orchestrated fashion [18] or, as proposed more recently, in parallel pathways forming bottlenecks rather than a linear process [21]. The immune response has to be controlled and directed correctly, otherwise excessive trafficking of leucocytes to extravascular locations can lead to serious tissue injury and destruction [22,23]. In fact, adhesion molecules are used by various microorganisms during the pathogenesis of infection [23]. Bacteria or bacterial products can up-regulate the surface expression of adhesion molecules on leucocytes and/or on endothelial cells, which would in turn promote leucocyte adhesion [24,25].
Hence, in this study, we examined the effect of S. suis serotype 2 and several of its purified components on the surface expression of ICAM-1, VCAM-1 and E-selectin by endothelial cells, and of ICAM-1, CD11a/CD18, CD11b/CD18 and CD11c/CD18 by THP-1 monocytes. The increase in THP-1 monocyte adherence to endothelial cells following stimulation with S. suis was also studied.
MATERIALS & METHODS
Bacterial strains and growth conditions
The S. suis serotype 2 virulent strain 31533, isolated originally from a pig with meningitis, was used as the reference strain in this study [26]. Seven other porcine strains and 14 strains isolated from human cases of infection were also used (Table 1), together with the avirulent, non-encapsulated isogenic transposon mutant strain 2A, derived from the wild-type strain S735 [4]. Bacteria were maintained as stock cultures in Todd-Hewitt broth (THB; Difco Laboratory, Detroit, MI, USA) containing 50% glycerol at −80°C. The THB was supplemented with tetracycline (10 µg/ml; Sigma-Aldrich, Oakville, Ontario, Canada) for growing mutant strain 2A [4]. Bacteria were grown overnight on bovine blood agar plates at 37°C, and isolated colonies were used as inocula for THB; these cultures were incubated for 18 h at 37°C. Working cultures for cell stimulation were made by inoculating 200 µl of these cultures into 10 ml of THB and incubating at 37°C with agitation until the mid-log phase (6 h of incubation; final optical densities at 540 nm, 0·4–0·5). Bacteria were washed twice in phosphate-buffered saline (PBS), pH 7·4 and diluted to approximately 2 × 109 colony-forming units (cfu)/ml in PBS. An accurate determination of the cfu/ml in the final suspension was made by plating onto THB agar.
Table 1.
Strains of S. suis serotype 2 used in this study
| Strain | Origin | Geographical origin |
|---|---|---|
| 31533a | Diseased pig | France |
| S735 | Diseased pig | the Netherlands |
| D282 | Diseased pig | the Netherlands |
| 94–623 | Healthy pig | France |
| 89–1591 | Diseased pig | Canada |
| 90–1330 | Diseased pig | Canada |
| 89–999 | Diseased pig | Canada |
| AAH4 | Diseased pig | USA |
| Reims | Human; spondylodiscitis | France |
| EUD95 | Human; meningitis | France |
| Biotype 2 | Human; endocarditis | France |
| HUD Limoge | Human; septic shock | France |
| FRU95 | Human; meningitis | France |
| LEF95 | Human; meningitis | France |
| 96–52466 | Human; arthritis | France |
| H11/1 | Human; meningitis | UK |
| AR770353 | Human; meningitis | the Netherlands |
| AR770297 | Human; meningitis | the Netherlands |
| 91–1804 | Human; endocarditis | Canada |
| 94–3037 | Human; meningitis | Canada |
| 98–3634 | Human; endocarditis | Canada |
| 99–734723688 | Human; septicaemia | Canada |
Strain used as reference in this study.
Preparation of killed bacteria
Bacteria were heat-killed by incubating the organisms at 60°C for 45 min, the minimal experimental condition required for S. suis killing [16]. The killed cultures were subcultured on blood agar plates at 37°C for 48 h to confirm that no organisms remained viable. Heat-killed bacterial preparations were stored at 4°C and resuspended in cell culture medium just before stimulation assays were performed. For comparative studies, live bacteria (from 6 h cultures as prepared above) were used at initial infectious concentration of 105 cfu/ml.
Purified bacterial components
CPS and bacterial cell wall were purified as described previously [16,27]. S. suis haemolysin, purified as described previously [28], was kindly provided by Dr A. A. Jacobs (Intervet, Boxmeer, the Netherlands). The haemolysin was reactivated by addition of 0·1% 2-mercaptoethanol (2-ME; Bio-Rad, Mississauga, Ontario, Canada) to cell culture medium. Concentrations used in this study were not toxic to cells (data not shown), as measured by the lactate dehydrogenase cellular injury assay [13].
Cell cultures
THP-1 monocytes were purchased from ATCC (TIB 202) and maintained in RPMI medium with l-glutamine (Gibco, Burlington, Ontario, Canada) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), 0·1% 2-ME and penicillin–streptomycin (5000 U/ml) (Gibco). Cells were cultured in flasks (Sarstedt, Newton, NC, USA) and in 96-well tissue culture plates (Becton Dickinson, Bedford, MA, USA). Human umbilical vein endothelial cells (HUVEC) were purchased from ATCC (CRL-1730). Cells were grown in F-12K medium (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS, 30 µg/ml of endothelial cell growth supplement (ECGS; Becton Dickinson) and penicillin–streptomycin (5000 U/ml). Flasks (Becton Dickinson) and 96-well tissue culture plates were precoated with gelatin to support these cells. All cultures of cells were incubated at 37°C, with 5% CO2 in a humid atmosphere.
Stimulation of cells
Prior to cell stimulation, 48 h cultures of THP-1 monocytes and HUVEC were plated at 100 µl/well of 96-well culture plates at 5 × 105 cells/ml and 105 cells/ml, respectively. Different S. suis strains, as well as different concentrations of purified cell wall, CPS or haemolysin that had been prepared in the appropriate cell culture medium, were then added to cells. At different time intervals, stimulants were removed, and THP-1 monocytes were fixed by the addition of 50 µl of 100% ethanol and left to dry; HUVEC were fixed with 1% paraformaldehyde for 20 min and then washed with PBS. In our hands, these conditions were shown to preserve adhesion molecule detection without cell loss (data not shown). Lipopolysaccharide (LPS) from Escherichia coli 0127:B8 (1 µg/ml; Sigma-Aldrich) served as a positive control for HUVEC and THP-1 monocyte stimulation assays. Cells with medium alone served as controls for the basal expression of adhesion molecules. All solutions and S. suis preparations used in this study were tested for the presence of endotoxin by a Limulus amebocyte lysate (LAL) gel-clot test (Pyrotell STV, Cape Cod, Falmouth, MA, USA) with a sensitivity limit of 0·03 endotoxin units (EU)/ml. Parallel assays with polymixin B (PmB, 1 µg/ml; Sigma-Aldrich) were performed during the stimulation of cells with bacteria or purified bacterial products to confirm the absence of endotoxin contamination during the test.
Enzyme-linked immunosorbent assays (ELISA) for adhesion molecules
Expression of ICAM-1, VCAM-1, E-selectin, CD11a/CD18, CD11b/CD18 and CD11c/CD18 was measured by an ELISA. Wells containing fixed THP-1 or HUVEC cells in 96-well culture plates were blocked with 1% bovine serum albumin (Boehringer Mannheim, Germany) in PBS, and one of the following monoclonal antibodies against the adhesion molecules were added to different wells: anti-ICAM-1 (0·1 µg/ml) and anti-VCAM-1 (1 µg/ml), purchased from R&D Systems (Minneapolis, MN, USA); anti-E-selectin (1 µg/ml), anti-CD11a/CD18 (10 µg/ml) and anti-CD11b/CD18 (15 µg/ml), kindly provided by Dr C. Wayne Smith (Baylor College of Medicine, Houston, TX, USA); and anti-CD11c/CD18 (10 µg/ml), purchased from BD Biosciences (Mississauga, ON, Canada). For each antibody, different concentrations were tested in order to find the optimal one. Thereafter, plates were washed three times and exposed to a horseradish peroxidase-conjugated polyclonal goat antimouse IgG and IgM (Jackson Immunoresearch laboratories, Inc., West Grove, PA, USA). Bound enzyme was detected by adding a 1 : 1 solution of 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide (Intergen, St Milford, MA, USA) for 10–20 min. Optical density (OD) was read at 450 nm, using a microplate reader (UVmax; Molecular Devices, Sunnyvale, CA, USA). Each condition was tested in triplicate and results represent the mean of at least three experiments. The basal expression of each adhesion molecule was subtracted from all presented results.
Adhesion assay
The adherence of THP-1 monocytes to HUVEC was semiquantified following methylene blue dye staining, as described by Oliver et al.[29]. Monocytes were stimulated for 48 h with heat-killed S. suis strain 31533 (109 cfu/ml) or with LPS (1 µg/ml as a positive control) and added to non-stimulated HUVEC. In addition, non-stimulated monocytes were added to HUVEC that had been stimulated for 24 h with strain 31533 or with LPS, or to non-stimulated HUVEC. The adherence of non-stimulated monocytes to non-stimulated HUVEC represents the basal adhesion of monocytes to HUVEC. Monocytes were allowed to adhere for 40 min at 37°C. Monocytes were also co-incubated with HUVEC for 24 h in the presence of strain 31533. At the end of the incubation time, wells were washed five times with PBS, then fixed with 100% ethanol and left to dry. Staining of cells was carried out by adding 0·1% methylene blue (in 0·1 m borate buffer, pH 8·7) followed by incubation for 10 min at room temperature. Wells were then washed three times with borate buffer (0·01 m). The methylene blue dye bound by the cells was solubilized with 100 µl HCL (0·1 N) per well for 30 min at 37°C. The amount of methylene blue was determined colourimetrically by the microplate reader, at 650 nm. Experiments were performed at least three times in triplicate wells.
Statistics
Differences were analysed for significance by using the Student's t-test (two-tailed P-value). A P-value < 0·05 was considered significant. Differences between strains of each origin and between strains of the same group were analysed for significance using general linear models (GLM), followed by Tukey–Kramer post-hoc tests for differences between strains. The SAS software (SAS, Cary, NC, USA) was used for these analyses.
RESULTS
S. suis does not up-regulate surface expression of ICAM-1, VCAM-1 and E-selectin on HUVEC
The basal expression of adhesion molecules was subtracted from results, thus all values obtained throughout this study represent the up-regulated expression of adhesion molecules after bacterial or LPS stimulation. ICAM-1 was present on non-stimulated HUVEC, whereas low levels of VCAM-1 and E-selectin were detected (ICAM-1: 0·49 ± 0·06; VCAM-1: 0·05 ± 0·04; E-selectin: 0·11 ± 0·03). Stimulation of HUVEC with 109 cfu/ml of heat-killed bacteria of S. suis serotype 2, of porcine or human origin, did not induce the surface expression of E-selectin and VCAM-1 and did not increase the basal expression of ICAM-1 (Fig. 1). Stimulation with live bacteria of S. suis serotype 2 of porcine or human origin also did not yield changes in adhesion molecule expression on HUVEC. On the other hand, stimulation with LPS increased the expression of all three adhesion molecules (Fig. 1). E-selectin expression reached maximal levels at an earlier time-point in comparison to the increase in expression of ICAM-1 and VCAM-1. ICAM-1 peaked at a later time-point, whereas VCAM-1 continued to increase with time. Both ICAM-1 and E-selectin expression gradually declined.
Fig. 1.
Kinetics of the up-regulated expression of ICAM-1(⋄), VCAM-1(▪) and E-selectin (▴) on HUVEC stimulated by LPS. Results obtained after stimulation with heat-killed (109 cfu/ml) or live (108−104 cfu/ml) S. suis strains of porcine and human origin included in this study (Table 1) are represented by (•) for all three adhesion molecules. Basal expression of adhesion molecules measured on non-stimulated cells was subtracted from all results. Data are expressed as mean ± standard deviation and represent at least three separate experiments.
Heat-killed bacteria of S. suis up-regulate ICAM-1, CD11a/CD18 & CD11c/CD18 on THP-1 monocytes in a time-dependent manner
THP-1 monocytes expressed negligible levels of ICAM-1 and CD11b/CD18, whereas basal levels of CD11a/CD18 and CD11c/CD18 were present on non-stimulated cells (ICAM-1: 0·04 ± 0·014; CD11a/CD18: 0·372 ± 0·076; CD11b/CD18: 0·10 ± 0·041; CD11c/CD18: 0·515 ± 0·057). These values of basal expression were subtracted from those obtained following stimulation for each adhesion molecule. Stimulation with heat-killed bacteria of S. suis serotype 2 strain 31533 (109 cfu/ml) up-regulated the expression of ICAM-1, CD11a/CD18 and CD11c/CD18 in a time-dependent manner (Fig. 2). The up-regulation of CD11a/CD18 and CD11c/CD18 was gradual and lower than that observed with ICAM-1. ICAM-1 expression peaks and levels off with time, whereas both CD11a/CD18 & CD11c/CD18 expression continue to increase with time. A slight non-significant up-regulation of CD11b/CD18 was detected on cells stimulated by S. suis (data not shown). LPS-stimulated monocytes yielded similar results to S. suis-stimulated cells with respect to the kinetics of ICAM-1 and CD11a/CD18 expression, but not to the kinetics CD11c/CD18 expression. Stimulation with S. suis results in an early peak in the up-regulation of CD11c/CD18, followed by a second burst of up-regulation; however, stimulation with LPS leads to an early peak in expression that is constant with time. LPS-stimulated monocytes also expressed a slight but non-significant up-regulation of CD11b/CD18 (data not shown). Results from the LAL test demonstrated no significant levels of endotoxin contamination in bacterial preparations. Cell culture medium contained less than 0·03 EU/ml (<0·005 ng/ml of endotoxin). Data from parallel experiments, in which PmB was present to neutralize any endotoxin contamination, revealed similar results (data not shown).
Fig. 2.
Kinetics of the expression of ICAM-1 (a), CD11a/CD18 (b) and CD11c/CD18 (c) on THP-1 monocytes stimulated by heat-killed bacteria of S. suis serotype 2 strain 31533 (109 cfu/ml) (•) and by LPS (1 µg/ml) (▴) measured at different times of incubation. Basal expression of adhesion molecules measured on non-stimulated cells was subtracted from all results. Data are expressed as means ± standard deviations from at least three separate experiments.
Up-regulation of ICAM-1, CD11a/CD18 & CD11c/CD18 on THP-1 monocytes is bacterial concentration-dependent
Stimulation of THP-1 monocytes for 48 h with decreasing concentrations of heat-killed bacteria of S. suis strain 31533 showed that the up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 was bacterial concentration-dependent (Fig. 3). The up-regulation of CD11a/CD18 and CD11c/CD18 expression was more sensitive to decreasing concentrations of S. suis in comparison to ICAM-1 up-regulation. ICAM-1 expression decreased more gradually than the integrins, but was also not significant at bacterial concentrations lower than 107 cfu/ml (P > 0·1).
Fig. 3.
Effect of different concentrations of bacteria of heat-killed S. suis serotype 2 strain 31533 on the expression of ICAM-1 (•), CD11a/CD18 (▪) and CD11c/CD18 (▴) on THP-1 monocytes, measured following 48 h of stimulation. Basal expression of the appropriate adhesion molecule measured on non-stimulated cells was subtracted from each result. Data are expressed as mean ± standard deviation and represent at least three separate experiments.
Heat-killed versus live bacteria
In comparative experiments, live bacteria at an initial infectious concentration of 105 cfu/ml were used to induce the up-regulation of adhesion molecules on THP-1 monocytes. Because the number of bacteria increased rapidly from 105 to ∼108−109 in the culture medium, heat-killed bacteria at the latter concentration were used for comparison purposes. Under these conditions, levels of ICAM-1, CD11a/CD18 and CD11c/CD18 up-regulation were similar for live and heat-killed bacteria (P > 0·1) after an incubation time of 24 h (Fig. 4). Higher concentrations of bacteria caused significant cytotoxicity (data not shown).
Fig. 4.
Comparison of the expression of ICAM-1, CD11a/CD18 and CD11c/CD18 on THP-1 monocytes stimulated for 24 h with heat-killed (H, □) bacteria of S. suis serotype 2 (109 cfu/ml) strain 31533 versus live (L, ▪) bacteria of the same strain (105 cfu/ml). Basal expression of adhesion molecules measured on non-stimulated cells was subtracted from all results. Data are expressed as mean ± standard deviation and represent at least three separate experiments.
Up-regulation of ICAM-1 and integrins on THP-1 monocytes is independent of the porcine or human origin of S. suis strains
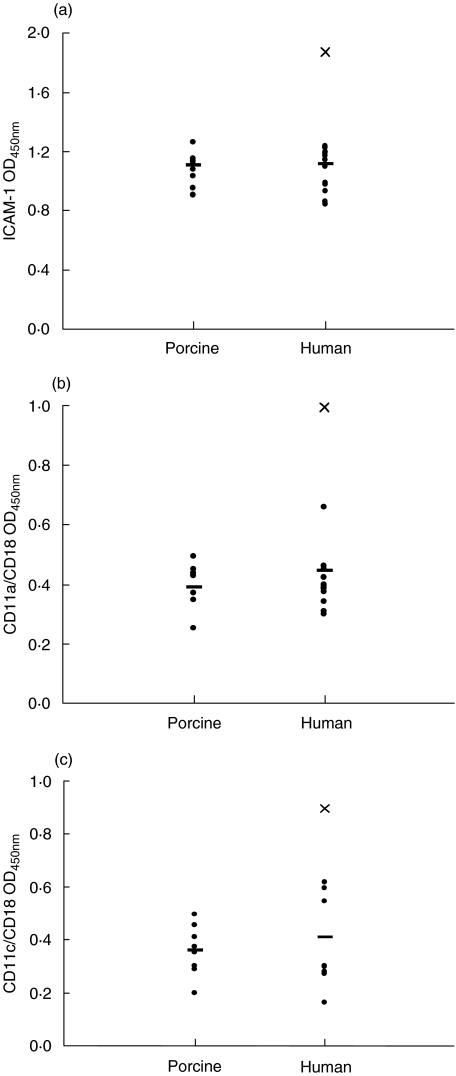
Strains of porcine or human origin were compared for their capacity to stimulate adhesion molecule expression on monocytes following 48 h of stimulation. Statistical analysis revealed no significant differences in the up-regulation of ICAM-1 (P = 0·9), CD11a/CD18 (P = 0·4) and CD11c/CD18 (P = 0·9) induced by strains of different origins. On the other hand, there were significant differences between strains within each group (P < 0·001) in their ability to induce the up-regulation of adhesion molecule expression. Interestingly, strain ‘Reims’, originating from a human case of spondylodiscitis [30], constantly showed the highest up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 compared to all other strains (Fig. 5).
Fig. 5.
Expression of ICAM-1 (a), CD11a/CD18 (b) and CD11c/CD18 (c) on THP-1 monocytes stimulated with heat-killed bacteria of porcine and human strains of S. suis serotype 2 (109 cfu/ml) for 48 h (see Table 1). The average of each group (porcine or human) is indicated (–). Each point represents one strain and is the average of at least three separate experiments. Human strain ‘Reims’ is indicated in all three graphs as (X). Basal expression of adhesion molecules measured on non-stimulated cells was subtracted from all results.
Role of bacterial components in the up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 on THP-1 monocytes
Purified components of S. suis were used to stimulate monocytes for 48 h, in order to discern which bacterial components contributed to the increase in surface expression of adhesion molecules. Stimulation of monocytes with CPS did not result in any significant up-regulation of ICAM-1, CD11a/CD18 or CD11c/C18, even when concentrations as high as 200 µg/ml were used (data not shown). On the other hand, cell stimulation with purified cell wall material resulted in a high up-regulation of all three adhesion molecules (Fig. 6). As observed for whole bacteria, the up-regulation was dependent on the concentration of cell wall material. Upon stimulation of cells with purified haemolysin, the expression of all three adhesion molecules was up-regulated (Fig. 6). The level of up-regulation was very significant, especially at 1 and 0·5 µg/ml of haemolysin, but lower than that induced by the cell wall. It is important to mention that washed heat-killed bacteria suspensions do not contain any haemolytic activity.
Fig. 6.
The effect of different concentrations of purified cell wall (checkered bars) and haemolysin (empty bars) of S. suis serotype 2 on the expression of ICAM-1 (a), CD11a/CD18 (b) and CD11c/CD18 (c) on THP-1 monocytes, following 48 h of stimulation, compared to the stimulation observed with whole bacteria (solid bar) (heat-killed strain 31533, 109 cfu/ml). Basal expression of adhesion molecules measured on non-stimulated cells was subtracted from all results. Data are expressed as mean ± standard deviation and represent at least three separate experiments.
Non-encapsulated mutant strain versus wild-type strain
The non-encapsulated mutant strain was compared to its wild-type porcine strain S735 with respect to capacity to stimulate ICAM-1, CD11a/CD18 and CD11c/CD18. The mutant strain induced significantly higher up-regulation (P < 0·001) of these adhesion molecules with respect to the wild-type strain: ICAM-1: 1·55 ± 0·17 versus 1·03 ± 0·14; CD11a/CD18: 0·65 ± 0·08 versus 0·25 ± 0·07; CD11c/CD18: 0·66 ± 0·09 versus 0·37 ± 0·07, respectively.
S. suis-stimulated THP-1 monocytes increase their adherence to HUVEC
THP-1 monocytes stimulated with heat-killed strain 31533 demonstrated an increased adherence to HUVEC (P < 0·001) in comparison to non-stimulated THP-1 monocytes (Fig. 7). Because bacteria of S. suis were unable to stimulate the expression of adhesion molecules on HUVEC, it would be expected that no increase in adhesion of monocytes would be detected on S. suis-stimulated HUVEC. Indeed, when HUVEC were stimulated with bacteria, no significant increase in adherence of monocytes was observed (P > 0·05). Stimulation of THP-1 monocytes or HUVEC with LPS, as a positive control, resulted in a significant increase in adherence of monocytes (P < 0·001). Co-culture of THP-1 monocytes and HUVEC in the presence of S. suis also resulted in an increased monocyte adherence (P < 0·001).
Fig. 7.
Adherence of THP-1 monocytes to endothelial cells following stimulation with LPS (1 µg/ml) or heat-killed bacteria of S. suis (109 cfu/ml) strain 31533. Monocyte stimulation: THP-1 monocytes that have been stimulated for 48 h by LPS or S. suis were incubated for 40 min with non-stimulated HUVEC. HUVEC stimulation: non-stimulated THP-1 monocytes were incubated for 40 min with LPS- or S. suis-stimulated HUVEC. Co-culture: THP-1 monocytes and HUVEC were co-incubated for 24 h in the presence of S. suis. Wells were washed, fixed and stained with methylene blue. HCL was added to solubilize the stain and the OD was read at 650 nm. Results were compared to the value representing basal adhesion of non-stimulated THP-1 monocytes on non-stimulated HUVEC. Increase in monocyte adherence upon stimulation, in comparison to non-stimulated cells, is represented by vertical bars. *P < 0·05. Data are expressed as mean ± standard deviation and represent three separate experiments.
DISCUSSION
Pathogenesis of meningitis caused by S. suis is not well understood, and little is known about the role of the virulence factors that have been described to date. Recent work has shown that S. suis interacts with phagocytes and stimulates the production of proinflammatory cytokines [15,16]. In this study, we characterized this interaction further by demonstrating that stimulation of THP-1 monocytes with S. suis serotype 2 up-regulates the expression of ICAM-1, CD11a/CD18 and CD11c/CD18. These adhesion molecules play an important role in leucocyte adherence and extravasation into inflammatory sites [18]. During experimental meningitis, inflammatory leucocytes were shown to be the major cause of the BBB injury and cerebral oedema [31]. Blocking antibodies against CD18 or ICAM-1 reduced leucocytosis into the CSF which in turn reduced brain oedema [19,32]. In addition, up-regulation of the expression of integrins has been correlated with an influx of inflammatory cells into the CSF [33,34] and in an increase in the adherence capacity of monocytes in vitro[35].
The up-regulation of adhesion molecules on S. suis-stimulated monocytes is time- and bacterial concentration-dependent, with a specific pattern of expression for each adhesion molecule. LPS was used as a positive control as it is a well-known immunomodulator that is capable of up-regulating several adhesion molecules, including ICAM-1 and β2 integrins, on the surface of monocytes [36–38]. Most previous studies report the kinetics of expression of these adhesion molecules between 0 and 24 h. In this study, we report the kinetics of adhesion molecule up-regulation by S. suis and LPS until 96 h of stimulation. The rapid up-regulation of CD11c/CD18 compared to ICAM-1 and CD11a/CD18 was due probably to the fact that this molecule is stored in intracellular granules, whereas ICAM-1 and CD11a/CD18 are not [39]. It has been shown previously that, under activation, monocytes could mobilize this pool within a few minutes, translocating CD11c/CD18 to the cell surface [40]. Stimulation with LPS resulted in a different pattern of kinetics, where only a first up-regulation at 2 h was detected, and remained constant with time, as has been shown previously [37]. S. suis-activated monocytes, on the other hand, showed a second increase of CD11c/CD18 expression between 24 h and 72 h after infection. This second increase on this adhesion molecule expression may be related to newly synthesized protein. Finally, S. suis and LPS were unable to significantly up-regulate the expression of CD11b/CD18. LPS has been previously shown to up-regulate CD11b/CD18 on fresh blood monocytes [37], thus this difference in response may be due to the relative immaturity of THP-1 monocytes [41]. Nevertheless, an up-regulation was observed after treatment of these cells with other stimulating agents, such as phorbol myristate acetate (PMA) (unpublished observations). Other bacteria have been shown to up-regulate the expression of adhesion molecules. For example, Mycobacterium tuberculosis, similarly to S. suis, up-regulates ICAM-1 expression on THP-1 monocytes in a time-dependent fashion and causes a minimal increase in the expression of CD11b/CD18, noted only at 24 h [24]. On the other hand, this bacterium is unable to up-regulate the expression of CD11a/CD18 [24]. Other bacteria, including Staphylococcus aureus and Streptococcus pneumoniae, do significanlty increase CD11b/CD18 expression on leucocytes [42,43]. Thus, bacterial-stimulated monocytes exhibit a specific pattern of adhesion molecule expression.
The kinetics of the three up-regulated adhesion molecules was also dependent on bacterial concentration. A high concentration of heat-killed bacteria was needed to obtain high levels of adhesion molecule expression. Similarly, monocytes and macrophages stimulated with S. suis also require a high bacterial concentration for maximal levels of cytokine release [15,16]. In fact, the presence of high levels of bacteria in the bloodstream of diseased animals is correlated with the presence of clinical signs and symptoms in these animals [44]. Heat-killed and viable bacteria of S. suis induced similar levels of adhesion molecule expression on THP-1 monocytes. This is in agreement with a previous study, which demonstrated that heat-killed or viable S. suis have the capacity to induce similar levels of proinflammatory cytokines by stimulated THP-1 monocytes [15].
One consequence of the up-regulation of adhesion molecules is an increase in leucocyte rolling, firm adhesion and subsequent extravasation [20]. In this study, we demonstrated a correlation between the up-regulation of adhesion molecules and the increase in adherence of monocytes to endothelial cells. S. suis-stimulated monocytes, expressing increased amounts of adhesion molecules, bound to HUVEC in higher numbers compared to non-stimulated monocytes. LPS-stimulated monocytes also caused an increase in monocyte adherence to HUVEC.
HUVEC constitutively express ICAM-1, and ICAM-1 is a ligand for β2 integrins, that are themselves expressed exclusively on leucocytes. Hence, the up-regulated expression of β2 integrins upon stimulation by S. suis could account for the observed increase in monocyte–endothelial adhesion. However, the role of other adhesion molecules, such as P-selectin, cannot be ruled out. Indeed, P-selectin is also considered of great importance in leucocyte adhesion to and migration through the endothelial barrier [20]. High basal expression of P-selectin was observed on HUVEC under our experimental conditions. However, no further increase in P-selectin expression was observed after S. suis infection, and only a slight up-regulation of its expression was observed after LPS activation (unpublished observations).
As a ligand for β2 integrins, ICAM-1 appears to participate in leucocyte–leucocyte, leucocyte–endothelial and leucocyte–epithelial cell interactions, transendothelial migration and adhesion-dependent respiratory burst. In addition, it has been shown that the binding of CD11a/CD18 and ICAM-1 is important in the activation of T cell signal transduction pathways, which in turn would enhance the host inflammatory response [45,46]. Thus, the induced expression of ICAM-1 on S. suis-stimulated monocytes could play an important role in enhancing cellular migration and in the interaction of T lymphocytes with antigen-presenting cells. Similarly, it was reported that continuous up-regulation of the expression of ICAM-1 on mononuclear phagocytes induced by M. tuberculosis may mediate the recruitment of monocytes and enhance the antigen presentation of M. tuberculosis, thus permitting the generation and maintenance of the host response [24]. On the other hand, the modulation of macrophage adhesion may influence the trafficking of M. tuberculosis-infected macrophages within the host, with increases in levels of CD11a/CD18 and ICAM-1 enhancing the adhesive properties of the macrophage and consequent decrease in phagocytic receptors as well as in the phagocytic capacity of an already-infected cell [47].
In order to identify possible bacterial candidates responsible for the monocyte cell adhesion molecule activation, different purified components of S. suis were tested. Results showed that the cell wall is largely responsible for the up-regulation of adhesion molecules. Stimulation with cell wall components resulted in increased ICAM-1, CD11a/CD18 and CD11c/CD18 expressions that were as high as those obtained with whole bacteria. The purified haemolysin was also able to stimulate the expression of ICAM-1, CD11a/CD18 and CD11c/CD18 on THP-1 monocytes, but to lower levels in comparison to cell wall components. This toxin belongs to the family of antigenetically related cholesterol-binding toxins that form transmembrane pores and possess a ‘multihit’ mechanism of action [48]. The mechanism used by this toxin to induce adhesion molecule expression may involve calcium ion concentration changes in cells and the subsequent phosphotydilinositol response activation, as already reported for listeriolyin, which also belongs to the same family of toxins [49]. Thus, a similar mechanism may be apply to suilysin activation of host cells. This hypothesis remains to be elucidated.
In contrast to the above purified factors, the CPS of S. suis does not cause any significant up-regulation of adhesion molecule expression on monocytes. Furthermore, the non-encapsulated mutant strain stimulated a higher level of up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 than the parent strain. These results are in agreement with other studies from our laboratory that have shown the important contribution of the S. suis cell wall to cytokine production by endothelial cells and by murine macrophages, the capacity of the haemolysin to stimulate cytokine production by endothelial cells and the inability of CPS to stimulate any cytokine production by both endothelial cells and phagocytes [14,16]. The capsule may, at least in part, mask cell wall components that can contribute to the up-regulation of adhesion molecules. Similar to the case of S. suis, a cell wall component of M. tuberculosis up-regulated the same level of ICAM-1 expression as whole bacteria [24] and a non-encapsulated mutant of Neisseria meningitidis has also been shown to cause a different pattern in adhesion molecule expression on leucocytes in comparison to the parent strain [50].
The porcine and human origin of S. suis strains does not seem to influence the degree of up-regulation of adhesion molecules on cells of human origin (THP-1). Genetic comparisons between human and porcine isolates have been performed recently and have placed these isolates in the same group [51,52]. These results, along with the present study, agree with the concept of S. suis being a zoonotic agent. Interestingly, a strain of human origin, ‘Reims’, induced the highest up-regulation of adhesion molecule expression in comparison to all other strains. This strain also induced higher levels of cytokine production by THP-1 monocytes [15]. Ongoing studies in our laboratory focus on the characterization of this strain. Preliminary results indicate that ‘Reims’ is a low-capsulated strain, which would confirm results obtained with the non-encapsulated mutant strain.
It can be argued that the up-regulation of adhesion molecules on THP-1 monocytes stimulated with S. suis is an indirect result of the cytokines that are produced by bacterial stimulation. This hypothesis cannot be ruled out, as it has been shown that S. suis-stimulated THP-1 monocytes induce the production of proinflammatory cytokines. These molecules are potent activators of cells and increase the surface expression of adhesion molecules, including the expression of ICAM-1 and of β2 integrins on monocytes [35,53]. In addition, activated adhesion molecules themselves have the ability to stimulate the production of cytokines [54,55]. Hence, further studies need to be conducted in order to understand the cause and effect of the up-regulated expression of adhesion molecules on THP-1 monocytes stimulated by S. suis.
Important meningeal pathogens, including N. meningitidis, Listeria monocytogenes and S. pneumoniae, have the capability of increasing the expression of adhesion molecules on endothelial cells [25,56, 57]. In the case of L. monocytogenes, this up-regulation is accompanied by an increase in leucocyte adherence [25]. However, under the conditions used in this study, S. suis was incapable of increasing the surface expression of ICAM-1, VCAM-1 and E-selectin on HUVEC. In addition, these endothelial cells stimulated by S. suis do not support an increase in monocyte adherence. LPS, on the other hand, increased both adhesion molecule expression and monocyte adherence to endothelial cells. This reinforces further the correlation between the up-regulation of adhesion molecules and the increase in monocyte adherence. Interestingly, preliminary work in our laboratory has shown that stimulation of endothelial cells with medium originating from a culture of THP-1 monocytes that had been stimulated previously with S. suis led to an increased expression of ICAM-1, E-selectin and VCAM-1 molecules (unpublished results). This indirect up-regulation may be due to cytokines released by S. suis-stimulated THP-1 monocytes.
In conclusion, this study demonstrates the ability of S. suis to up-regulate the expression of important adhesion molecules involved in inflammation. This activation may be responsible, at least in part, for the increase in adherence of monocytes to endothelial cells, thus providing a mechanism for some of the inflammatory features of meningitis caused by this pathogen.
Acknowledgments
We gratefully acknowledge Dr M. Kobisch (Centre National d'Études Vétérinaires et Alimentaires, Ploufragan, France), Dr U. Vecht (DLO-Institute for Animal Science and Health, Lelystad, the Netherlands), Dr T. Alexander (University of Cambridge, UK), Dr L. Brasme (Centre Hospitalier Universitaire de Reims, France), Dr G. Grise (Hôpital des Feugrois, Elbeuf, France), Dr B. Cattier (Centre Hospitalier Universitaire Bretonneau, Tours, France), Dr B. François (Dupuytren Hospital, Limoges Cedex, France) and Dr P. Norton (Institute for Animal Health, Compton, UK) for providing some of the Streptococcus suis type 2 strains. We would also like to thank Dr C. Wayne Smith for kindly providing anti-E-selectin, anti-CD11a/CD18 and anti-CD11b/CD18 monoclonal antibodies. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) grant no. 0680154280, by the Fonds pour la Formation des Chercheurs et l'Aide à la Recherche du Québec (FCAR-équipe) grant no. 99-ER-0214, and by the Canadian Research Network on Bacterial Pathogens of Swine.
REFERENCES
- 1.Higgins R, Gottschalk M. Streptococcal diseases. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of swine. Ames: Iowa State University Press; 1999. [Google Scholar]
- 2.Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–7. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Higgins R, Gottschalk M. Distribution of Streptococcus suis capsular types in 1997. Can Vet J. 1998;39:299–300. [PMC free article] [PubMed] [Google Scholar]
- 4.Charland N, Harel J, Kobish M, Lacasse S, Gottschalk M. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology. 1998;144:325–32. doi: 10.1099/00221287-144-2-325. [DOI] [PubMed] [Google Scholar]
- 5.Gottschalk M, Lebrun A, Wisselink H, Dubreuil JD, Smith H, Vecht U. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can J Vet Res. 1998;62:75–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Segers RP, Kenter T, de Haan LA, Jacobs AA. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol Lett. 1998;167:255–61. doi: 10.1111/j.1574-6968.1998.tb13236.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith HE, Wisselink HJ, Stockhofe-Zurwieden N, Vecht U, Smits MM. Virulence markers of Streptococcus suis type 1 and 2. Adv Exp Med Biol. 1997;418:651–5. doi: 10.1007/978-1-4899-1825-3_152. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol. 2000;75:59–71. doi: 10.1016/s0378-1135(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 9.Williams AE. Relationship between intracellular survival in macrophages and pathogenicity of Streptococcus suis type 2 isolates. Microb Pathog. 1990;8:189–96. doi: 10.1016/0882-4010(90)90046-s. [DOI] [PubMed] [Google Scholar]
- 10.Williams AE, Blakemore WF. Pathogenesis of meningitis caused by Streptococcus suis type 2. J Infect Dis. 1990;162:474–81. doi: 10.1093/infdis/162.2.474. [DOI] [PubMed] [Google Scholar]
- 11.Smith HE, Damman M, Van der Velde J, et al. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–6. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segura M, Gottschalk M. Streptococcus suis interactions with the murine macrophage cell line J774: adhesion and cytotoxicity. Infect Immun. 2002;70:4312–22. doi: 10.1128/IAI.70.8.4312-4322.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charland N, Nizet V, Rubens C, Kim KS, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–43. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadeboncoeur N, Segura M, Al-Numani D, Gottschalk M. Streptococcus suis serotype 2 stimulates the release of pro-inflammatory cytokines by human brain microvascular endothelial cells. FEMS Immunol Med Microbiol. 2003;35:49–58. doi: 10.1111/j.1574-695X.2003.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 15.Segura M, Vadeboncoeur N, Gottschalk M. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin Exp Immunol. 2002;127:243–54. doi: 10.1046/j.1365-2249.2002.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segura M, Stankova J, Gottschalk M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect Immun. 1999;67:4646–54. doi: 10.1128/iai.67.9.4646-4654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang T, Frenette PS, Hynes RO, Wagner DD, Mayadas TN. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J Clin Invest. 1996;97:2485–90. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etzioni A. Adhesion molecules in leukocyte endothelial interaction. Adv Exp Med Biol. 1996;408:151–7. doi: 10.1007/978-1-4613-0415-9_17. [DOI] [PubMed] [Google Scholar]
- 19.Weber JR, Angstwurm K, Burger W, Einhaupl KM, Dirnagl U. Anti ICAM-1 (CD 54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J Neuroimmunol. 1995;63:63–8. doi: 10.1016/0165-5728(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 20.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 21.Ley K. Pathways and bottlenecks in the web of inflammatory adhesion molecules and chemoattractants. Immunol Res. 2001;24:87–95. doi: 10.1385/IR:24:1:87. [DOI] [PubMed] [Google Scholar]
- 22.Lowe JB, Ward PA. Therapeutic inhibition of carbohydrate–protein interactions in vivo. J Clin Invest. 1997;100:S47–51. [PubMed] [Google Scholar]
- 23.Kerr JR. Cell adhesion molecules in the pathogenesis of and host defence against microbial infection. Mol Pathol. 1999;52:220–30. doi: 10.1136/mp.52.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez Ramirez GM, Rom WN, Ciotoli C, et al. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun. 1994;62:2515–20. doi: 10.1128/iai.62.6.2515-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krull M, Nost R, Hippenstiel S, Domann E, Chakraborty T, Suttorp N. Listeria monocytogenes potently induces up-regulation of endothelial adhesion molecules and neutrophil adhesion to cultured human endothelial cells. J Immunol. 1997;159:1970–6. [PubMed] [Google Scholar]
- 26.Kobisch M, Gottschalk M, Morvan P, Cariolet R, Bénévent G, Joly JP. Experimental infection of SPF piglets with Streptococcus suis serotype 2. Journ Rech Porcine En France. 1995;27:97–102. [Google Scholar]
- 27.Sepulveda EMD, Altman E, Kobisch M, Dallaire S, Gottschalk M. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect elisa. Vet Microbiol. 1996;52:113–25. doi: 10.1016/0378-1135(96)00056-9. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs AA, Loeffen PL, van den Berg AJ, Storm PK. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–8. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci. 1989;92:513–8. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- 30.Caumont H, Gerard N, Depernet B, Brasme L, Eschard JP, Etienne JC. L3–L4 spondylodiscitis in a butcher [letter] Streptococcus suis Presse Med. 1996;25:1348. [PubMed] [Google Scholar]
- 31.Bohr VA, Rasmussen N. Neurological sequelae and fatality as prognostic measures in 875 cases of bacterial meningitis. Dan Med Bull. 1988;35:92–5. [PubMed] [Google Scholar]
- 32.Tuomanen EI, Saukkonen K, Sande S, Cioffe C, Wright SD. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989;170:959–69. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson EC, Christensen JP, Marker O, Thompson AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–45. [PubMed] [Google Scholar]
- 34.Rowin ME, Xue V, Irazuzta J. Integrin expression on neutrophils in a rabbit model of Group B streptococcal meningitis. Inflammation. 2000;24:157–73. doi: 10.1023/a:1007085627268. [DOI] [PubMed] [Google Scholar]
- 35.Tiisala S, Majuri ML, Carpen O, Renkonen R. Enhanced ICAM-1-dependent adhesion of myelomonocytic cells expressing increased levels of beta 2-integrins and CD43. Scand J Immunol. 1994;39:249–56. doi: 10.1111/j.1365-3083.1994.tb03368.x. [DOI] [PubMed] [Google Scholar]
- 36.Heinzelmann M, Mercer-Jones MA, Gardner SA, Wilson MA, Polk HC. Bacterial cell wall products increase monocyte HLA-DR and ICAM-1 without affecting lymphocyte CD18 expression. Cell Immunol. 1997;176:127–34. doi: 10.1006/cimm.1997.1089. [DOI] [PubMed] [Google Scholar]
- 37.Darcissac EC, Bahr GM, Parant MA, Chedid LA, Riveau GJ. Selective induction of CD11a,b,c/CD18 and CD54 expression at the cell surface of human leukocytes by muramyl peptides. Cell Immunol. 1996;169:294–301. doi: 10.1006/cimm.1996.0121. [DOI] [PubMed] [Google Scholar]
- 38.Heinzelmann M, Polk HC, Jr, Chernobelsky A, Stites TP, Gordon LE. Endotoxin and muramyl dipeptide modulate surface receptor expression on human mononuclear cells. Immunopharmacology. 2000;48:117–28. doi: 10.1016/s0162-3109(00)00195-8. [DOI] [PubMed] [Google Scholar]
- 39.Carlos TM, Harlan JM. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 40.Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987;80:535–44. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrink M, Gobl AE, Huang R, Nilsson K, Hellman L. Human cell lines U-937, THP-1 and Mono Mac 6 represent relatively immature cells of the monocyte–macrophage cell lineage. Leukemia. 1994;8:1579–84. [PubMed] [Google Scholar]
- 42.Carratelli CR, Nuzzo I, Bentivoglio C, Galdiero M. CD11a/CD18 and CD11b/18 modulation by lipoteichoic acid, N-acetyl- muramyl-alpha-alanyl-D-isoglutamine, muramic acid and protein A from Staphylococcus aureus. FEMS Immunol Med Microbiol. 1996;16:309–15. doi: 10.1111/j.1574-695X.1996.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 43.Kragsbjerg P, Fredlund H. The effects of live Streptococcus pneumoniae and tumor necrosis factor-alpha on neutrophil oxidative burst and beta 2-integrin expression. Clin Microbiol Infect. 2001;7:125–9. doi: 10.1046/j.1469-0691.2001.00216.x. [DOI] [PubMed] [Google Scholar]
- 44.Berthelot-Herault F, Cariolet R, Labbe A, Gottschalk M, Cardinal JY, Kobisch M. Experimental infection of specific pathogen free piglets with French strains of Streptococcus suis capsular type 2. Can J Vet Res. 2001;65:196–200. [PMC free article] [PubMed] [Google Scholar]
- 45.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 46.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 47.DesJardin LE, Kaufman TM, Potts B, Kutzbach B, Yi H, Schlesinger LS. Mycobacterium tuberculosis-infected human macrophages exhibit enhanced cellular adhesion with increased expression of LFA-1 and ICAM-1 and reduced expression and/or function of complement receptors, FcgammaRII and the mannose receptor. Microbiology. 2002;148:3161–71. doi: 10.1099/00221287-148-10-3161. [DOI] [PubMed] [Google Scholar]
- 48.Gottschalk MG, Lacouture S, Dubreuil JD. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology. 1995;141:189–95. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 49.Sibelius U, Rose F, Chakraborty T, et al. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–6. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein NJ, Ison CA, Peakman M, et al. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J Infect Dis. 1996;173:172–9. doi: 10.1093/infdis/173.1.172. [DOI] [PubMed] [Google Scholar]
- 51.Berthelot-Herault F, Marois C, Gottschalk M, Kobisch M. Genetic diversity of Streptococcus suis strains isolated from pigs and humans as revealed by pulsed-field gel electrophoresis. J Clin Microbiol. 2002;40:615–9. doi: 10.1128/JCM.40.2.615-619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol. 1999;37:362–6. doi: 10.1128/jcm.37.2.362-366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada A, Hara A, Inoue M, Kamizono S, Higuchi T, Itoh K. Beta 2-integrin-mediated signal up-regulates counterreceptor ICAM-1 expression on human monocytic cell line THP-1 through tyrosine phosphorylation. Cell Immunol. 1997;178:9–16. doi: 10.1006/cimm.1997.1117. [DOI] [PubMed] [Google Scholar]
- 54.Walzog B, Weinmann P, Jeblonski F, Scharffetter-Kochanek K, Bommert K, Gaehtgens P. A role for beta (2) integrins (CD11/CD18) in the regulation of cytokine gene expression of polymorphonuclear neutrophils during the inflammatory response. FASEB J. 1999;13:1855–65. doi: 10.1096/fasebj.13.13.1855. [DOI] [PubMed] [Google Scholar]
- 55.Cuzzola M, Mancuso G, Beninati C, et al. Beta 2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J Immunol. 2000;164:5871–6. doi: 10.4049/jimmunol.164.11.5871. [DOI] [PubMed] [Google Scholar]
- 56.Dixon GL, Heyderman RS, Kotovicz K, et al. Endothelial adhesion molecule expression and its inhibition by recombinant bactericidal/permeability-increasing protein are influenced by the capsulation and lipooligosaccharide structure of Neisseria meningitidis. Infect Immun. 1999;67:5626–33. doi: 10.1128/iai.67.11.5626-5633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freyer D, Manz R, Ziegenhorn A, et al. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J Immunol. 1999;163:4308–14. [PubMed] [Google Scholar]