Abstract
Leptin is a an adipocyte-secreted hormone that regulates weight centrally. However, the leptin receptor is expressed not only in the central nervous system, but also in peripheral tissues, such as haematopoietic and immune systems. Therefore, the physiological role of leptin should not be limited to the regulation of food intake and energy expenditure. Moreover, the leptin receptor bears homology to members of the class I cytokine family, and recent data have demonstrated that leptin is able to modulate the immune response. Thus, the leptin receptor is expressed in human peripheral blood mononuclear cells, mediating the leptin effect on proliferation and activation. In vitro activation and HIV infection in vivo induce the expression of the long isoform of the leptin receptor in mononuclear cells. Also, leptin stimulates the production of proinflammatory cytokines from cultured monocytes and enhances the production of Th1 type cytokines from stimulated lymphocytes. Moreover, leptin has a trophic effect on monocytes, preventing apoptosis induced by serum deprivation. Leptin stimulation activates JAK–STAT, IRS-1-PI3K and MAPK signalling pathways. Leptin also stimulates Tyr-phosphorylation of the RNA-binding protein Sam68 mediating the dissociation from RNA. In this way, leptin signalling could modulate RNA metabolism. These signal transduction pathways provide possible mechanisms whereby leptin may modulate activation of peripheral blood mononuclear cells. Therefore, these data support the hypothesis regarding leptin as a proinflammatory cytokine with a possible role as a link between the nutritional status and the immune response. Moreover, these immunoregulatory functions of leptin could have some relevance in the pathophysiology of obesity.
Keywords: cell activation, cytokine, leptin, leptin receptor, lymphocytes, obesity, PBMC, Th1 response
INTRODUCTION
Leptin, the 16-kDa non-glycosylated protein product of the ob gene [1], is a hormone synthesized mainly in adipose cells [2] to regulate weight control in a central manner, via its cognate receptor in the hypothalamus [3]. Leptin can also be expressed at lower levels in other tissues, such as the placenta and stomach [4,5]. Leptin is released into the circulation, and plasma levels correlate with total body fat mass [6]. When leptin is administered to lean or ob/ob (leptin-deficient) mice it increases the basal metabolic rate and reduces food intake, leading to weight loss [7–9]. On the other hand, there is increasing evidence that leptin has systemic effects apart from those related to energy homeostasis, including regulation of neuroendocrine, reproductive, haematopoietic and immune function [10].
The primary amino acid sequence of leptin indicates that it could belong to the long-chain helical cytokine family [11], such as IL-2, IL-12 and GH. In fact, leptin receptor (Ob-R) shows sequence homology to members of the class I cytokine receptor (gp130) superfamily [12], that includes the receptor for IL-6, leucocyte inhibitory factor and granulocyte colony-stimulating factor. Moreover, Ob-R has been shown to have the signalling capabilities of IL-6-type cytokine receptors [13]. Ob-R expression is not limited to the hypothalamus, but is distributed widely [12,14]. Thus, Ob-R is found in haematopoietic cells [15]. In this context, a role for leptin in haematopoiesis and the immune system at the stem cell level has been proposed [16].
Obese leptin-deficient ob/ob mice and db/db mice, in which the leptin receptor is truncated, display immune dysfunction and lymphoid organ atrophy, affecting thymic size and cellularity similar to that observed in starved animals and malnourished humans [17–19]. Thus, they have reduced levels of peripheral T and B cells [17], suggesting that leptin may have a role in lymphopoiesis. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice [18]. Moreover, human leptin deficiency caused by a missense mutation also produces immune system dysfunction [20]. Leptin can induce proliferation, differentiation and functional activation of haematopoietic cells, and this function may explain the role of adipose tissue present in the marrow cavity [16].
Leptin enhances cytokine production [granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF] in murine peritoneal macrophages [21], and phenotypical abnormalities have been found in macrophages from leptin-deficient obese mice [22]. Furthermore, leptin up-regulates both phagocytosis and the production of proinflammatory cytokines by murine macrophages [23]. In this context, an immunoregulatory role of leptin on macrophage function as a proinflammatory signal has gained physiological importance because inflammatory cytokines [tumour necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1)] can raise mouse leptin levels in vivo, resulting in anorexia and weight loss [24], although leptin has not been proved to be the main signal for the anorectic effects of inflammation. Nevertheless, a role for leptin regulating immunity, inflammation and haematopoiesis has been accepted [25].
In this review we summarize our recent results in leptin modulation of immune cells from peripheral blood in man and the signalling pathways activated by the leptin receptor (Ob-R) that may mediate leptin action in human peripheral blood mononuclear cells.
LEPTIN ACTIVATION OF HUMAN PERIPHERAL BLOOD MONOCYTES
Leptin has been demonstrated to modulate monocyte-machrophage function and to regulate proinflammatory response [22–24]. Moreover, leptin has been shown previously to enhance cytokine production (GM-CSF and G-CSF) by murine peritoneal macrophages [21]. In this context, human leptin has been shown to stimulate in vitro proliferation of human peripheral blood mononuclear cells in a dose-dependent manner [26]. Thus, the proliferative effect of leptin has been assessed by [3H]thymidine and bromodeoxyuridine incorporation (with flow cytometry analysis) at 48 h incubation. The effect of leptin is comparable to that produced by lipopolysaccharide (LPS) (10 ng/ml) or phorbol myristate acetate (PMA) (1 ng/ml). Maximal effect is observed at 10 nm leptin, but a significant effect can be obtained at 0·1 nm leptin. Whether the proliferative effect of leptin is direct or mediated by the increase in the production of cytokines, as observed previously in murine peritoneal macrophages [21], is a question that remains to be investigated. Nevertheless, a direct effect of leptin providing a proliferative signal in haematopoietic cells at the level of multi-lineage progenitor has been shown previously [16].
Leptin stimulation of monocytes has been also checked by measuring thre expression of surface activation markers by flow cytometry: CD25 (IL-2 receptor), HLA-DR, CD38, CD71 (transferrin receptor), CD11b and CD11c [27–29]. Human leptin (10 nm) stimulates the basal expression of CD38, and this effect is comparable to that produced by LPS (10 ng/ml). Moreover, leptin increases dose-dependently the expression of other markers after 72 h culture: CD25 and CD71 [26]. Leptin also enhances dose-dependently the expression of other activation markers that were already present at high levels in non-stimulated monocytes (HLA-DR, CD11b and CD11c). This effect is comparable to that produced by 10 ng/ml LPS. The possible contamination of the leptin obtained from recombinant sources (endotoxin) was ruled out by using polymyxin B, which blocks the interaction of CD14 with LPS. Thus, polymyxin B prevented the effect of of LPS but not that of leptin.
Human leptin also induces the expression of the short-term (12 h incubation) activation marker CD69 in human monocytes [30,31]. Maximal effect is obtained at 100 nm, but a significant effect was observed at 0·1 nm. Also, leptin potentiates the effect of different submaximal concentrations of LPS and PMA on CD69 expression. These data are consistent with those obtained for long-term markers and suggest a direct activation effect of leptin on circulating monocytes. Overall, these findings provide a mechanism whereby leptin may have a role in proinflammatory responses.
LEPTIN STIMULATES CYTOKINE PRODUCTION BY HUMAN CIRCULATING MONOCYTES
Leptin, in addition to the stimulating effect on monocyte activation and proliferation, is able to induce the expression of monocyte cytokines IL-6 and TNF-α[26,32] after 6 h culture, and leptin has been shown previously to enhance cytokine production (GM-CSF and G-CSF) by murine peritoneal macrophages [21].
The effect of human leptin on monocytes is comparable to that of 10 ng/ml LPS and 1 ng/ml PMA [26]. The effect is dependent on the dose, and maximal effect is achieved at 100 nm leptin. Leptin potentiates the effect of submaximal concentrations of LPS (1 ng/ml) or PMA (0·1 ng/ml) [26]. Because LPS administration in mice increases leptin expression and circulating leptin levels these effects of leptin may be physiologically important, functioning as an amplification signal for monocyte activation [24].
Therefore, leptin seems to be a potent stimulatory hormone on human peripheral blood monocytes, suggesting strongly that it may have a role as a proinflammatory cytokine.
LEPTIN ACTIVATION OF HUMAN PERIPHERAL BLOOD LYMPHOCYTES
Leptin has been found to modulate T lymphocyte function in mice [17], but human leptin alone is not able to activate human peripheral blood lymphocytes in vitro[33]. However, when T lymphocytes are co-stimulated with PHA or concanavalin A (Con A), leptin enhances the proliferation and activation of cultured T lymphocytes [33]. Thus, leptin stimulates dose-dependently the expression of CD25 and CD71 in both CD4 and CD8 T cells co-stimulated with submaximal concentrations of PHA (2 mg/ml) or Con A (4 mg/ml) at 48 h culture. Similar dose–response curves can be obtained for CD25 and CD71. Maximal effect is obtained at 10 nm leptin, with an ED50 of about 0·1 nm for CD4 and 0·2 nm for CD8 T lymphocytes [33]. However, when maximal concentrations of PHA and Con A are used, leptin has no further effect. These effects of leptin are observed even in the absence of monocytes, suggesting a direct effect of human leptin on circulating T lymphocytes.
The expression of the early activation marker CD69 in T lymphocytes after 12 h culture is also enhanced by human leptin when only submaximal concentrations of PHA and Con A were used. Thus, leptin alone has no effect on CD69 expression. The same results are obtained in the absence of monocytes, further suggesting a direct effect of leptin on T lymphocytes [33].
Human leptin also the enhances proliferative effect of submaximal concentrations of PHA or Con A in monocyte-depleted lymphocytes. Maximal effect is obtained at 10 nm leptin and the ED50 is 0·5 nm. However, human leptin has no effect when lymphocytes are co-stimulated with maximal concentrations of PHA (10 mg/ml) and Con A (8 mg/ml).
On the other hand, very recent data have shown that leptin modulate differentially proliferation of T cells stimulated with anti-CD3. Thus, leptin seems to inhibit proliferation of memory T cells, whereas it enhanced markedly that of naive cells [34], suggesting further complexity in the actions of leptin on the immune system.
LEPTIN ENHANCES TH1-TYPE CYTOKINE PRODUCTION BY HUMAN T LYMPHOCYTES
Human leptin not only modulates the activation and proliferation of human T lymphocytes but also enhances cytokine production induced by submaximal concentrations of PHA (2 mg/ml). The effect of leptin on lymphocyte function has been assessed by intracellular immunostaining of cytokines using an inhibitor of secretion and flow cytometry analysis [33,35]. Human leptin enhances the production of IL-2 and IFN-γ in stimulated T lymphocytes. Maximal effect of leptin was observed at 10 nm and the dose–response curve showed an ED50 of 0·5 nm[33].
Leptin has been shown to enhance cognate T cell response, skewing cytokine responses towards a Th1 phenotype in mice [17]. The results confirm these effects in non-cognate human T lymphocytes activation [33]. Therefore, leptin seems to be a modulator (enhancer) of T lymphocyte stimulation with a shift towards a Th1 cytokine-production profile. These data are in agreement with the recent observation of the leptin effect on anti-CD3 stimulation of T cells, which increases the production of the proinflammatory cytokine interferon-γ[34].
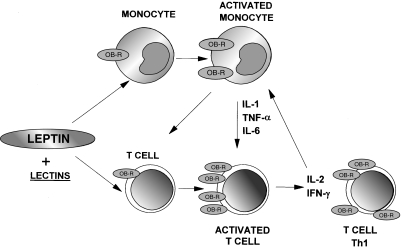
These data concerning leptin modulation of Th1 type cytokine production are consistent with the observed effects stimulating TNF-α and IL-6 production by monocytes, suggesting further the possible physiological role of leptin as a proinflammatory signal. Figure 1 depicts schematically the possible role of human leptin in the regulation of the immune system inducing a proinflammatory response.
Fig. 1.
Model of leptin modulation of the immune system. Leptin directly activates monocytes and further activates co-stimulated T cells, inducing a Th1 response which amplify the proinflammatory response.
LEPTIN RECEPTOR ACTIVATES THE JAK–STAT SIGNALLING PATHWAY IN PERIPHERAL BLOOD MONONUCLEAR CELLS
The presence of the leptin receptor in monocytes and lymphocytes has been shown previously in mice [17,23]. The presence of both the short and long isoforms of the leptin receptor has been confirmed in human peripheral blood T lymphocytes (both CD4 and CD8) by Western blot and flow cytometry analysis [33].
Functional data suggest that Ob-R is a member of the class I cytokine receptor superfamily [12,36]. Thus, similar to other receptors of this class, Ob-R lacks intrinsic tyrosine kinase activity but requires activation of receptor-associated kinases of the Janus family (JAKs) [37], which initiate downstream signalling including members of the STAT (signal transducers and activators of transcription) family of transcription factors [13,38]. After ligand binding, JAKs autophosphorylate and tyrosine phosphorylates various STATs. Activated STATs by leptin stimulation in the hypothalamus dimerize and translocate to the nucleus, where specific gene responses are elicited [38,39]. In this context, we have studied the JAK–STAT signalling pathway triggered by leptin stimulation in human peripheral blood mononuclear cells [40].
To study the activation of JAK kinases by leptin receptor in human peripheral blood mononuclear cells they were stimulated with human leptin, and phosphorylation of immunoprecipitated JAK proteins was analysed by antiphosphotyrosine immunoblot. For immunoprecipitation, antibodies against JAK-2 and JAK-3 were used. A phosphorylated band corresponding approximately to 120 kDa is detected in both immunoprecipitates in response to 10 nm leptin. The effect is transient (5–20 min), and maximal activation of JAK-2 and JAK-3 is observed after 5 min of incubation. Moreover, both JAK isoforms are associated physically with the leptin receptor, as assessed by co-immunoprecipitation of the receptor and JAK-2 or JAK-3. The association is constitutive, as it occurs both in the absence and presence of the ligand. Preassociation of JAK proteins with cytokine receptors has been described for other members of the family [41], and for the leptin receptor itself with JAK-2 [37]. The relative contribution of each JAK isoform in leptin receptor signalling in human peripheral blood mononuclear cells, however, remains to be assessed.
The possible activation of STAT-3 by human leptin in mononuclear cells has been also studied at different time-points. Tyrosine phosphorylation of STAT-3 is observed in reponse to 10 nm human leptin. The effect is maximal at 10 min. At this time, the effect of leptin is dependent on the dose. Maximal effect is achieved at 10 nm leptin, but a significant effect can be observed at 0·1 nm leptin. Another band corresponding to a 70-kDa tyrosine phosphorylated protein is also present in the antiphosphotyrosine immunoblots. Because it has been shown that the RNA binding protein Sam68 [42,43] is Tyr-phosphorylated in stimulated lymphocytes and then associates with various SH2 domain containing proteins [44–47], the identity of the 70 kDa phosphorylated protein was assessed by specific immunoprecipitation and antiphosphotyrosine immunoblot. Thus, human leptin stimulates time-dependently Tyr-phosphorylation of Sam68. Maximal effect can be observed at 10 min, but is still significant after 30 min incubation. At 10 min incubation, the effect of human leptin is dose-dependent. Thus, maximal effect is observed with 10 nm leptin, but some effect is observed with a much lower leptin concentration (0·1 nm). A higher band corresponding to a tyrosine phosphorylated protein of about 85 kDa can also be observed in the immunoblots of anti-Sam68 immunoprecipitates. The molecular weight of this protein is consistent with the expected migration of STAT3, indicating the possible association of Sam68 with STAT3. To assess the leptin-mediated association of Sam68 with STAT-3, both proteins were immunoprecipitated and the the co-precipitation of each protein was checked. Thus, immunoprecipitation of Sam68 shows some STAT3 in response to leptin, whereas immunoprecipitation of STAT3 consistently co-precipitates Sam68. This effect is dependent on the dose of leptin, and maximal effect is again observed at 10 nm.
Leptin has been shown to promote the translocation of STAT-3 to the nucleus in the rat hypothalamus [38,39]. We have shown that human leptin can also promote the translocation of STAT-3 to the nucleus in human peripheral blood mononuclear cells [40]. We incubated mononuclear cells with or without leptin (10 nm) and looked for the presence of STAT-3 in the nucleus. Some STAT-3 can be detected in nuclear lysates in basal conditions, but a significant increase in the amount of STAT-3 is found in the nuclear extract from cells stimulated with human leptin (10 nm) for 15 min. Other STAT isoforms (STAT-1, STAT-5, STAT-6) have also been shown to be activated by the leptin receptor, although only in transfected systems [48,49]. However, other STAT forms have not been assayed in lymphocytes and therefore we cannot rule out the possible implication of STATs other than STAT-3. Nevertheless, STAT-3 is the only STAT that has been shown to be activated by leptin in the hypothalamus [50]. Moreover, the activation of STAT-3 by leptin stimulation has been confirmed recently in a murine macrophage cell line [51].
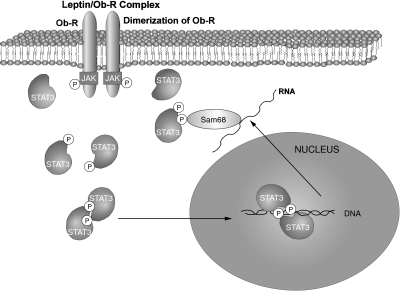
Most members of the cytokine family of receptors stimulate tyrosine phosphorylation of STAT proteins by activating JAK kinases, which are associated with the intracellular part of the transmembrane receptor [52,53]. The binding of the ligand with this kind of receptor promotes the dimerization of the receptor and activation of JAK, which autophosphorylates and tyrosine phosphorylates the receptor and STAT proteins. This sequence of events has already been demonstrated for the leptin receptor, both in vivo[38,39,50,54] and in transfected cells [13,37,48]. A schematic representation of this signalling of the leptin receptor in mononuclear cells is shown in Fig. 2.
Fig. 2.
Leptin receptor signalling via JAK–STAT pathways in PBMC. The leptin receptor is associated with JAKs. Upon leptin stimulation, JAK activity is increased and STAT-3 is tyrosine phosphorylated, dimerized and translocated to the nucleus to activate gene expression. STAT-3 is also associated with tyrosine phosphorylated Sam68 in response to leptin.
THE LEPTIN RECEPTOR ACTIVATES THE PI3K AND MAPK SIGNALLING PATHWAYS IN PERIPHERAL BLOOD MONONUCLEAR CELLS
Different pathways in addition to STATs are known to be involved in leptin receptor signalling in a similar way to other members of the cytokine family. Thus, leptin has been shown to activate mitogen-activated protein kinase (MAPK) [55–58] and phosphatidylinositol 3-kinase (PI3K) [49,59,60].
Tyrosine phosphorylation of the activated leptin receptor has already been reported in other systems [37,55]. In human blood mononuclear cells, we have also found that human leptin stimulates tyrosine phosphorylation of the long form of the leptin receptor [61], as assessed by immunoprecipitation with an antibody against the C-terminus of the protein and immunoblotting with antibodies against phosphotyrosine. This effect is dependent on the dose at 5 min incubation. Maximal phosphorylation can be observed with 10 nm leptin.
Previous studies have shown that leptin activates PI3K in myotubes, β-cells and hepatocytes [49,59,60,62]. This PI3K pathway has also been explored in peripheral blood mononuclear cells (PBMC) in response to human leptin. Thus, PI3K activity associated with tyrosine phosphorylated proteins is found to be increased more than threefold after 10 nm leptin stimulation [61]. PI3K activation is regulated by the association of tyrosine phosphorylated proteins with the SH2 domains of p85 [63]. Thus, in response to leptin, a band corresponding to the molecular mass of the insulin receptor substrate-1 (IRS-1) and several bands of 60–70 kDa are phosphorylated and associated with p85 in a dose–response manner, similar to our previous data in response to insulin [63,64]. Maximal response is observed at 10 nm leptin and 5 min incubation time.
Because we have identified Sam68 previously [65] as one of the 60–70 kDa proteins tyrosine-phosphorylated and associated with p85 in response to insulin [66], the identity of these p85-associated tyrosine phosphorylated proteins was analysed by specific immunoblotting with anti-IRS-1 and anti-Sam68 in antip85 immunoprecipitates. Leptin increased dose-dependently the association of IRS-1 and Sam68 with p85. However, we do not know whether Tyr-phosphorylated Sam68 contributes to the increase in PI3K activity along with IRS-1, or whether it is working only as a docking protein. We know that leptin stimulates PI3K signalling pathway in mononuclear cells, thereby providing some mechanisms for the previously observed leptin activation of human immune cells from peripheral blood [26,33].
Moreover, the effect of leptin on tyrosine phosphorylation of these substrates has been demonstrated further by investigating directly the leptin effect on tyrosine phosphorylation of IRS-1 and Sam68 in PBMC, by specific immunoprecipitation and antiphosphotyrosine immunoblotting. Thus, leptin increases dose- and time-dependently tyrosine phosphorylation of IRS-1 and Sam68. Maximal effect is observed at 10 nm leptin. Whether leptin-stimulated tyrosine phosphorylation is mediated by JAK or another kinase activity remains to be studied.
Tyrosine phosphorylation of Sam68 by the Src family kinase p59fyn has been shown previously to regulate negatively its association with RNA [67]. As leptin stimulation promotes the tyrosine phosphorylation of Sam68 we sought to check the possible regulation of Sam68 association with RNA by leptin. Thus, we used poly(U), as it has been shown previously that Sam68 binds this polymer specifically [42,65]. Leptin stimulation of PBMC inhibited the binding efficiency of Sam68 to poly(U), increasing the tyrosine-phosphorylation level. This effect of leptin was also dependent on the dose. The effect of leptin regulating the RNA binding capacity of Sam68 may be involved in the post-transcriptional modulation of RNA. In this way, Sam68 has been proposed to provide the means for a rapid pathway to regulate protein expression by modifying the mRNA stability and/or mRNA translation. Moreover, Sam68 has been shown to interact with the splicing-associated factor YT521-B in nuclear dots and this interaction is regulated by Tyr-phosphorylation [67]. Thus, Tyr-phosphorylation of Sam68 by leptin stimulation could modulate its association with the splicing machinery in a similar way to that described for p59fyn, and in this way could influence splice site selection. However, these hypotheses remain speculative until investigated.
Because leptin has been found previously to activate MAPK pathways in different systems [55–58], the activation of MAPK by leptin in PBMC has also been assessed by studying its tyrosine/threonine phosphorylation level, which reflects the activation of MEK and, indirectly, all the MAPK pathways. Leptin stimulated tyrosine/threonine phosphorylation of MAPK as assessed by specific immunoblot with the anti-doubly phosphorylated MAPK antibody [61]. Both Erk-1 and Erk-2 were phosphorylated in PBMC after 10 min incubation with human leptin. This effect of leptin was dependent on the dose and maximal effect was observed at 10 nm leptin, and the level of phosphorylated MAPK was decreased subsequently after 15–30 min incubation [61].
Leptin has been found previously to activate MAPK in different systems, mediating a proliferative response [55–58]. Nevertheless, the possible implication of MAPK pathway in the activation and proliferative effect of leptin on PBMC [26,33] needs further investigation to demonstrate a direct link.
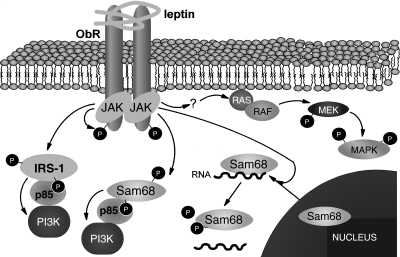
Figure 3 represents schematically these insulin-like signal transduction pathways activated by the leptin receptor.
Fig. 3.
Leptin receptor signalling via PI3K and MAPK in PBMC. Leptin stimulation promotes the tyrosine phosphorylation of the intracellular domain of the leptin receptor, IRS-1 and Sam68, probably by JAK activation. The substrates IRS-1 and Sam68 associate with p85, providing a molecular mechanism for PI3K activation. On the other hand, MAPK signalling cascade is also activated by leptin receptor activation by some unknown mechanism.
ACTIVATION OF PERIPHERAL BLOOD MONO-NUCLEAR CELLS INDUCES THE EXPRESSION OF THE LONG ISOFORM OF THE LEPTIN RECEPTOR
Other cytokine receptors in mononuclear cells, such as the IL-2 receptor, respond to cell activation by increasing their expression level [68]. In order to assess a possible regulation of the long isoform of leptin receptor in mononuclear cells by activation, we have studied the expression of Ob-R by reverse transcription–polymerase chain reaction (RT-PCR) and Western blot in PBMC activated in vitro by lectins (PHA and ConA), and in vivo in HIV-infected patients [69]. We have found that in vitro activation with lectins and in vivo HIV infection correlates with an increase in the leptin receptor expression in PBMC. Considering the stimulatory activity of leptin in PBMC, this effect of activation on leptin receptor expression may be useful as a positive feedback. In this context, lymphocytes have been shown to respond to leptin only when co-stimulated with either PHA or Con A [33], whereas monocytes can be activated directly by leptin. Therefore, the necessity of lymphocytes for co-stimulation may be explained partly by this effect of activation, increasing leptin receptor expression. These data suggest that the leptin receptor may be regulated in a similar way to other cytokine receptors, such as the IL-2 receptor [68].
One of the first signalling steps in leptin receptor activation is its tyrosine phosphorylation [37,55,61]. Thus, the phosphorylation level of the leptin receptor as a test for receptor activation can be used. However, even though the leptin receptor level was up-regulated in PBMC by lectin stimulation, it did not show any increase in tyrosine phosphorylation [69].
On the other hand, we also checked the leptin receptor expression under some in vivo conditions that result in PBMC activation, such as HIV infection. Thus, infection with HIV has been associated with elevated IL-6 levels and production [70,71], and activation of lymphomonocytes is one of the hallmarks of HIV infection [72,73]. In this context, we have found that PBMC from HIV-infected subjects have increased expression of the leptin receptor, in a similar way to in vitro-activated PBMC from healthy controls. Moreover, the leptin receptor is tyrosine phosphorylated in PBMC from HIV+ subjects, suggesting that leptin receptor is not only up-regulated, but also activated. Because PBMC from control donors have increased expression but not activated leptin receptors when stimulated with lectins, a possible explanation is that HIV infection in PBMC may itself induce the activation of leptin receptor.
Although we do not know the role of leptin receptor in the context of HIV infection, these results are consistent with the suggested role of leptin modulating the immune response.
THE ROLE OF LEPTIN AS A TROPHIC FACTOR FOR MONONUCLEAR CELLS
An important property of many cytokines is the protection of cells from apoptosis, i.e. promoting cell survival. Previous work by others have shown that leptin increases the viability and attenuates apoptosis of different cell types, such as osteoblasts, granulosa cells and pancreatic islet cells [74–76]. Moreover, very recently leptin has been found to inhibit stress-induced apoptosis of T lymphocytes in vivo[77]. This effect of leptin is consistent with the reduction in lymphocyte numbers observed normally in fasted and steroid-injected mice [78]. Therefore, leptin may contribute to the recovery of immune suppression in malnutrition. In this context, we have tested the possible effect of leptin on monocyte survival and whether this effect was based on the anti-apoptotic action of leptin, when monocytes are cultured in the absence of serum [78]. Thus, leptin maintained the number of monocytes after 4 days of serum-free incubation, allowing the survival of monocytes. Similar to the action of many cytokines [79,80], the effect of leptin on survival was mediated by the prevention of the apoptosis process (both late and early events) in serum-free cultured monocytes. Therefore, we have demonstrated that leptin promotes dose-dependently survival of blood monocytes prone to apoptosis by culture under serum deprivation, and physiological concentrations of leptin (0·1–1 nm) are sufficient to exert this effect. The effect of leptin on monocyte survival can be reversed completely by blocking p42/44 MAPK activation employing the MEK inhibitor PD98059, whereas it is not affected by PI3K inhibition using wortmannin. Leptin promotes this survival effect by preventing the apoptosis of monocyte cells via MAPK activation. Thus, p42/44 MAPK inhibition using PD98059, but not PI3K inhibition employing wortmannin, is able to block the protective effect of leptin preventing apoptosis of monocytes cultured in the absence of serum. These results are consistent with the recognized role for the p42/44 MAPK pathway in the immune response in general [81], and the anti-apoptotic signal in monocytes in particular [82].
These data support the hypothesis of a role of leptin as an important trophic factor for blood monocytes.
LEPTIN AS A LINK BETWEEN ENERGY STORES AND THE IMMUNE SYSTEM
We believe that there is enough reported evidence on the immunomodulatory effect of leptin to consider this hormone as an important signal that regulates the immune response, with a special role in the up-regulation of inflammation. Thus, leptin could be a link between nutritional status and the immune system. In this context, low leptin levels found in malnourished infants have been associated with suppression of the lymphoproliferative response [83], and weight gain is followed by a significant increase in circulating leptin levels in parallel with a significant increase in Th1 activity [19], supporting further the role of leptin as a nutritional sensor for the immune function. Very recently, this role of leptin has been proved finally in humans by showing the beneficial effect of leptin on T cell hyporesponsiveness of human congenital leptin deficiency [20,84].
Therefore, leptin is the signal that connects the energy stores with the immune system, and may play a role in the immunosuppression of starvation. In fact, the restoration of leptin to normal levels in feeding after starvation is achieved before the body fat returns to normal content, but is sufficient to ameliorate the immune response [83]. On the other hand, leptin levels fall rapidly with starvation before body fat depletion, but producing impairment of the immune system [6]. Thus, leptin administration can restore the immune response towards normal in starved animals [17,18]. Therefore, leptin seems to be a signal for the adaptation of starvation, saving energy for muscle and brain activity. On the other hand, leptin could have played a role in selection, helping the better-nourished to survive under starving conditions and making the starved individuals more prone to die from infection. On the contrary, an excess of leptin levels that correlates with overweight in obese subjects may play a role in pathological conditions mediated by an excess of immune response.
PATHOPHYSIOLOGICAL IMPLICATIONS
In addition to the putative physiological role of leptin in the modulation of the immune response, there is increasing evidence suggesting that leptin may have a role in some immunologically mediated pathophysiological conditions. Thus, leptin has been proved to be necessary for T cell-mediated hepatotoxicity [19] and liver fibrosis [85,86] as well as the induction and progression of autoimmune encephalomyelitis [87,88], intestinal inflammation [89] or experimental arthritis [90]. Although most results have been found in mice, elevated leptin levels, along with other proinflammatory cytokines, have been associated with an increased risk for coronary heart disease [91] and type II diabetes [92,93], probably participating in the immunological activation involved in the pathogenesis of these syndromes. Human leptin has also been related to plasma cytokines in acute pancreatitis [94], sepsis and septic shock [95]. In fact, leptin could be the link connecting the thrifty and the cytokine genotype/phenotype (naturally prone to fat deposit with insulin resistance and high proinflammatory response), which has been suggested to be evolutionarily related [96]. Thus, chronic inflammation may be a trigger for chronic insulin insensitivity [97]. In this context, obesity is a risk factor for type II diabetes mellitus and atherosclerosis, and both complications have been associated with inflammatory and T cell-mediated immune responses [98,99]. Therefore, leptin may play a role in the development of atherosclerosis and type II diabetes in the modern western lifestyle. Leptin, along with other cytokines such as TNF-α and IL-6, may thus contribute to the biochemical and clinical features of the metabolic syndrome X (accelerated atherosclerosis associated with insulin resistance, glucose intolerance and central obesity) [100]. In this context, leptin has been proposed to play a role linking the increased incidence of autoimmune diseases in affluent countries with the increase of adipose tissue in overweight and obese people so common in developed societies [101].
Obesity is a pathological condition with increased leptin levels. In this sense, central versus peripheral leptin resistance may underlie the pathophysiology of obesity. Therefore, the study of signal transduction pathways of the leptin receptor and their alterations both at central and peripheral levels should contribute to a better understanding of the physiological and pathophysiological roles of leptin.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Maffei M, Fei H, Lee GH, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA. 1995;92:6957–60. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell. 1995;80:15–8. doi: 10.1016/0092-8674(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 4.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–33. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 5.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 6.Frederich RC, Löllmann B, Hamann A, et al. Expression of Ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–63. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Scienceet al. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 8.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 10.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 11.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995;373:13–8. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 13.Baumann H, Morella KK, White DW, et al. The full leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 15.Cioffi J, Shafer AW, Zupancic TJ, et al. Novel B219/OB receptor isoforms. Possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–8. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 16.Bennet BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Mathews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–80. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 17.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 18.Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity. Role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–72. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 21.Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93:14564–8. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee F-YJ, Li Y, Yang EK, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Pysiol. 1999;276:C386–C394. doi: 10.1152/ajpcell.1999.276.2.C386. [DOI] [PubMed] [Google Scholar]
- 23.Loffreda S, Rai R, Yang SQ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 24.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 26.Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 27.Scheinbenbogen C, Keilholz U, Richter M, Andressen R, Hunstein W. The interleukin-2 receptor in human monocytes and macrophages: regulation of expression and release of the alpha and beta chains (p55 and p75) Res Immunol. 1992;143:33–7. doi: 10.1016/0923-2494(92)80077-x. [DOI] [PubMed] [Google Scholar]
- 28.Czerniecki BJ, Carter C, Rivoltini L, et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol. 1997;159:3823–37. [PubMed] [Google Scholar]
- 29.Darcissac EC, Bahr GM, Parant MA, Chedid LA, Riveau GJ. Selective induction of CD11a,b,c/CD18 and CD54 expression at the cell surface of human leukocytes by muramyl peptides. Cell Immunol. 1996;169:294–301. doi: 10.1006/cimm.1996.0121. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Cabrera M, Santis AG, Fernández-Ruiz E, et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J Exp Med. 1993;178:537–47. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Maria R, Cifone MG, Trotta R, et al. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen AR, McKinnon KP, Koren HS. Lipopolysaccharide (LPS) stimulates fresh human monocytes to lyse actinomycin d-treated WEHI-164 target cells via increased secretion of a monokine similar to tumor necrosis factor. J Immunol. 1985;135:3978–87. [PubMed] [Google Scholar]
- 33.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 34.Lord GM, Matarese G, Howard JK, Bloom SR, Lechler RI. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol. 2002;72:330–8. [PubMed] [Google Scholar]
- 35.Picker LJ, Singh MK, Zdraveski Z, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 36.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 37.Ghilardi N, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Mol Endocrinol. 1997;11:393–9. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–6. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of STAT3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK–STAT pathway. Cell Immunol. 2001;211:30–6. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 41.Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–4. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 42.Taylor SJ, Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994;368:867–71. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- 43.Fumagalli S, Totti N, Hsuan JJ, Coutneidge SA. A target of Src in mitosis. Nature. 1994;368:871–4. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 44.Richard S, Yu D, Blummer KJ, et al. Association of p62, a multifunctional SH2- and SH3-domain binding protein, with src family tyrosine kinases, Grb2, and phospholipase Cγ1. Mol Cell Biol. 1995;15:186–97. doi: 10.1128/mcb.15.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fusaki N, Iwamatsu A, Iwashima M, Fujisawa JI. Interaction between Sam68 and src family tyrosine kinases Fyn and Lck, in T cell receptor signalling. J Biol Chem. 1995;272:6214–9. doi: 10.1074/jbc.272.10.6214. [DOI] [PubMed] [Google Scholar]
- 46.Jabado N, Pallier A, LeDeist F, Bernard F, Fischer A, Hivroz C. CD4 ligands inhibit the formation of multifunctioonal transduction complexes involved in T cell activation. J Immunol. 1997;158:94–103. [PubMed] [Google Scholar]
- 47.Jabado N, Jauliac S, Pallier A, Bernard F, Fischer A, Hivroz C. Sam68 association with p120GAP in CD4+ T cells is dependent on CD4 molecule expression. J Immunol. 1998;161:2798–803. [PubMed] [Google Scholar]
- 48.Rosenblum CI, Tota M, Cully D et al. Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the fatty leptin receptor in transfected cells. Endocrinology. 1996;137:5178–81. doi: 10.1210/endo.137.11.8895396. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Kuropatwinski KK, White DW, et al. Leptin receptor action in hepatic cells. J Biol Chem. 1997;272:16216–23. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 50.McCowen KC, Chow JC, Smith RJ. Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology. 1998;139:4442–7. doi: 10.1210/endo.139.11.6301. [DOI] [PubMed] [Google Scholar]
- 51.O'Rourke L, Sheperd P. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem J. 2002;364:875–9. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–62. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 53.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 54.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA. 1996;93:6231–5. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjørbaek C, Uotani S, da Silva B, Flier J. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–95. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 56.Bjørbaek C, Buchholz RM, Davis SM, et al. The role of SHP-2 in MAPK activation by leptin receptors. J Biol Chem. 2001;276:4747–55. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi Y, Okimura Y, Mizuno I, et al. Leptin induces mitogen-activated protein kinase-dependent proliferation C3H10T1/2 cells. J Biol Chem. 1997;272:12897–900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- 58.Tanabe K, Okuya S, Tanizawa Y, Matsutani A, Oka Y. Leptin induces proliferation of pancreatic β cell line MIN6 through activation of mitogen-activated protein kinase. Biochem Biophys Res Commun. 1997;241:765–8. doi: 10.1006/bbrc.1997.7894. [DOI] [PubMed] [Google Scholar]
- 59.Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40:1358–62. doi: 10.1007/s001250050832. [DOI] [PubMed] [Google Scholar]
- 60.Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, Ashford MLJ. Essential role of phosphoinositide 3-kinase in leptin-induced KATP channel activation in the rat CRI-G1 insulinoma cell line. J Biol Chem. 2000;275:4660–9. doi: 10.1074/jbc.275.7.4660. [DOI] [PubMed] [Google Scholar]
- 61.Martín-Romero C, Sánchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells. Possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 62.Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling in an hepatic cell line. Proc Natl Acad Sci USA. 2000;97:2344–60. doi: 10.1073/pnas.050580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sánchez-Margalet V, Najib S. Sam68 is a substrate of the insulin receptor and associates with the SH2 domains of p85 PI3K. FEBS Lett. 1999;455:307–10. doi: 10.1016/s0014-5793(99)00887-x. [DOI] [PubMed] [Google Scholar]
- 64.Sung CK, Sanchez-Margalet V, Goldfine ID. Role of p85 subunits of phosphatidylinositol-3-kinase as an adaptor molecule linking the insulin receptor, p62 and GTPase-activating protein. J Biol Chem. 1994;269:12503–7. [PubMed] [Google Scholar]
- 65.Wang LL, Richard S, Shaw AS. p62 association with RNA is regulated by tyrosine phosphorylation. J Biol Chem. 1995;270:2010–3. doi: 10.1074/jbc.270.5.2010. [DOI] [PubMed] [Google Scholar]
- 66.Zhao AZ, Shinohara MM, Huang D, et al. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–54. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- 67.Hartmann AM, Nayler O, Schwaiger FW, Obermaier A, Stamm S. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59 (fyn) Mol Biol Cell. 1999;10:3909–26. doi: 10.1091/mbc.10.11.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai KN, Leung JC, Lai FM. Soluble interleukin 2 receptor release, interleukin 2 production, and interleukin 2 receptor expression in activated T-lymphocytes in vitro. Pathology. 1991;23:224–8. doi: 10.3109/00313029109063570. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez-Margalet V, Martín-Romero C, González-Yanes C, Goberna R, Rodríguez-Baños J, Muniain MA. Leptin receptor expression is induced in activated mononuclear cells in vitro, and in vivo in HIV infected patients. Clin Exp Immunol. 2002;129:119–24. doi: 10.1046/j.1365-2249.2002.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–4. [PubMed] [Google Scholar]
- 71.Berman MA, Zaldivar F, Jr, Imfeld KL, Kenney JS, Sandborg CL. HIV-1 infection of macrophages promotes long-term survival and sustained release of interleukins 1 alpha and 6. AIDS Res Hum Retroviruses. 1994;10:529–39. doi: 10.1089/aid.1994.10.529. [DOI] [PubMed] [Google Scholar]
- 72.Abbate I, Dianzani F, Capobianchi MR. Activation of signal transduction and apoptosis in healthy lymphomonocytes exposed to bystander HIV-1-infected cells. Clin Exp Immunol. 2000;122:374–80. doi: 10.1046/j.1365-2249.2000.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Navikas V, Link J, Persson C, et al. Increased mRNA expression of IL-6, IL-10, TNF-alpha, and perforin in blood mononuclear cells in human HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:484–9. [PubMed] [Google Scholar]
- 74.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stmulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signalling. J Cell Biochem. 2002;85:825–36. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 75.Almog B, Gold R, Tajima K, et al. Leptin attenuates follicular apoptosis and accelerates the onset of puberty in immatures rats. Mol Cell Endocrinol. 2001;183:179–91. doi: 10.1016/s0303-7207(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 76.Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin increases the viability of isolated rat pancreatic islets by suppressing apoptosis. Endocrinology. 2001;142:4827–30. doi: 10.1210/endo.142.11.8494. [DOI] [PubMed] [Google Scholar]
- 77.Fujita Y, Murakami M, Ogawa Y, et al. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–6. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Najib S, Sanchez-Margalet V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol. 2002;220:143–9. doi: 10.1016/s0008-8749(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 79.Caceres-Cortes J, Rajotte D, Dumouchel J, Haddad P, Hoang T. Product of the steel locus suppresses apoptosis in hematopoietic cells. Comparison with pathways activated by granulocyte macrophage colony-stimulating factor. J Biol Chem. 1994;269:12084–91. [PubMed] [Google Scholar]
- 80.Mangan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–12. [PubMed] [Google Scholar]
- 81.Dong C, Davis RJ, Flavel RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 82.Lin H, Chen C, Li X, Chen BD. Activation of the MEK/MAPK pathway is involved in byostatin 1-induced monocytic differentiation and up-regulation of X-linked inhibitor of apoptosis protein. Exp Cell Res. 2002;272:192–8. doi: 10.1006/excr.2001.5417. [DOI] [PubMed] [Google Scholar]
- 83.Palacio A, Lopez M, Perez-Bravo F, Monkeberg F, Schlesinger L. Leptin levels are associated with immune response in malnourished infants. J Clin Endocrinol Metab. 2002;87:3040–6. doi: 10.1210/jcem.87.7.8636. [DOI] [PubMed] [Google Scholar]
- 84.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporresponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–13. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 86.Crespo J, Rivero M, Fabrega E, et al. Plasma leptin and TNF-alpha levels in chronic hepatitis C patients and their relationship to hepatic fibrosis. Dig Dis Sci. 2002;47:1604–10. doi: 10.1023/a:1015835606718. [DOI] [PubMed] [Google Scholar]
- 87.Matarese G, Di Giacomo A, Sanna V, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–16. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 88.Sanna V, Di Giacomo A, La Cava A, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–50. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–25. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 90.Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–82. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 91.Yudkin JS, Yajnik CS, Mohamed-Ali V, Bulmer K. High levels of circulating proinflammatory cytokines and leptin, but not rural, Indians. A potential explanation for increased risk of diabetes and coronary heart disease. Diabetes Care. 1999;22:363–4. doi: 10.2337/diacare.22.2.363. [DOI] [PubMed] [Google Scholar]
- 92.Pickup JC, Chusney GD, Mattock MB. The innate immune response and type 2 diabetes: evidence that leptin is associated with a stress-related reaction. Clin Endocrinol. 2000;52:107–12. doi: 10.1046/j.1365-2265.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 93.Matarese G, Sanna V, Lechler RI, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–61. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 94.Konturec PC, Jaworek J, Maniatoglou A, et al. Leptin modulates the inflammatory response in acute pancreatitis. Digestion. 2002;65:149–60. doi: 10.1159/000064935. [DOI] [PubMed] [Google Scholar]
- 95.Arnalich F, Lopez J, Codoceo R, Jiménez M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survival in sepsis and septic shock. J Infect Dis. 1999;180:908–11. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- 96.Fernández-Real JM, Ricart W. Insulin resistance and inflammation in an evolutionay perspective: the contribution of cytokine genotype/phenotype to thriftiness. Diabetologia. 1999;42:1367–74. doi: 10.1007/s001250051451. [DOI] [PubMed] [Google Scholar]
- 97.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–9. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 98.Wick G, Schett G, Amberger A, Kleindiest R, Xu Q. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 99.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 100.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 101.Matarese G, La Cava A, Sanna V, et al. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol. 2002;23:182–7. doi: 10.1016/s1471-4906(02)02188-9. [DOI] [PubMed] [Google Scholar]