Abstract
The intestinal flora play an important role in experimental colitis and inflammatory bowel disease (IBD). Using colonic explant cultures from 132 IBD and control subjects, we examined tumour necrosis factor-alpha (TNF-α), interleukin (IL)-1 and interleukin-1 receptor antagonist (IL-1RA) production in vitro in response to bacterial activators. Unstimulated TNF-α release was increased significantly in rectal biopsies from involved IBD tissue, correlating with inflammation severity. Whereas lipopolysaccharide (LPS) only moderately stimulated TNF-α production from inflamed tissue, pokeweed mitogen (PWM) induced its release in all groups, with a stronger response in involved IBD tissue. Superantigen staphylococcal enterotoxin A (SEA) had a similar, but weaker effect. SEB was observed to be the strongest inducer of TNF-α for all groups, again with a more marked response in inflamed tissue. Stimulated release of IL-1 was considerably less than for TNF-α. The superantigens’ superior potency over LPS was not as marked for IL-1 as it was for TNF-α. In addition to IL-1, IL-1RA release was also triggered by the bacterial products. The net effect of activation on the IL-1RA/IL-1 ratio was relatively modest. Release of the proinflammatory cytokines TNF-α and IL-1, as well as that of the anti-inflammatory cytokine IL-1RA was increased by incubation of colonic tissue with bacterial factors. TNF-α production and release was increased significantly in involved colonic explants from IBD. SEB was even capable of inducing TNF-α release from uninvolved colonic tissue.
Keywords: Crohn's disease, cytokines, intestinal mucosa, organ culture, ulcerative colitis
INTRODUCTION
Inflammatory bowel diseases (IBD) are chronic immune-mediated disorders of yet unknown aetiology. An increased number of activated leucocytes are present in IBD intestinal mucosa, resulting in heightened production of proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-12, IFN-γ and chemokines [1–4]. Among these, TNF-α is of particular importance in view of recent trials that demonstrated impressive clinical improvement following TNF-α neutralization using monoclonal antibodies [5–7]. Overproduction of TNF-α also characterizes experimental models of chronic intestinal inflammation [8]. TNF-α plays a pivotal role in orchestrating the inflammatory response and is implicated in the activation of macrophages, co-stimulation of T cells, up-regulation of adhesion molecules, release of proinflammatory cytokines from immune cells, as well as chemokines from epithelial cells [9]. IL-1 also appears to be an important mediator of inflammation in IBD, with increased levels in intestinal tissue from IBD patients [10]. The activity of IL-1 is determined in part by the levels of the IL-1 receptor antagonist (IL-1RA), which competes with IL-1 for receptor binding. We and others have provided evidence that IL-1 and IL-1RA are imbalanced in IBD mucosa [10,11]
Despite intensive efforts, the precise triggering events and the mechanisms that perpetuate the inflammation and cause IBD relapses remain poorly understood. The hypothesis held most widely is that there is an abnormal and uncontrolled immune response to normally occurring gut intraluminal constituents [12]. Bacterial products are prime suspects, because Crohn's disease (CD) recurrence is typically restricted to intestinal segments exposed to luminal flora [13]. IBD patients have been shown to have aberrant intestinal immune tolerance to commensal bacterial antigens [14,15]. Furthermore, the development of colitis in several transgenic and gene knock-out animal models is contingent on colonization by conventional gut bacteria [12,16,17].
Lipopolysaccharide (LPS) is a Gram-negative bacterial cell wall component that activates peripheral blood mononuclear cells (PBMC), but not lamina propria mononuclear cells (LPMC) from normal intestinal mucosa [18,19]. Bacterial superantigens such as staphylococcal enterotoxins A (SEA) and B (SEB) induce T cell proliferation and cytokine secretion [20]. Alterations in the lymphocyte T cell receptor (TCR) Vβ repertoire have been observed in autoimmune disorders, implicating such superantigens [21]. Specific and persistent oligoclonal expansion of circulating and gut T cells in IBD has been taken as an indication that they have responded to specific bacterial antigens [22]. Furthermore, SEB was shown recently to induce a self-limited enteropathy, characterized by altered villus-crypt in BALB/c and T cell-reconstitued SCID mice [23]. Pokeweed mitogen (PWM) stimulates helper T cells and B cells and is a strong inducer of cytokine release by PBMC [24]. Activation of T cells by PWM is monocyte-dependent [25].
Ex vivo colonic organ culture has been reported by us and others to be a useful in vitro model to study cytokine production in intestinal disorders [10,26,27]. The goal of this study was to compare intestinal TNF-α, IL-1 and IL-1RA release to the aforementioned bacterial products, using colonic explant cultures from IBD and control patients.
PATIENTS AND METHODS
Materials
Dulbecco's phosphate buffered saline (PBS), CMRL 1066 and penicillin–streptomycin were purchased from Gibco BRL (Life Technologies, Burlington, Ontario, Canada). Fetal calf serum, TRIS, Escherichia coli LPS serotype 026:B6, PWM, SEA and SEB were from Sigma (St Louis, MO, USA). Anti-proteases were obtained from ICN (Mississauga, Ontario, Canada).
Organ cultures
Colonic specimens were obtained from 132 consenting patients at the time of colonoscopy for evaluation of gastrointestinal symptoms [Table 1]. Six biopsies were taken from macroscopically diseased areas of the rectal mucosa. If no macroscopic lesions were visible, six rectal specimens were taken randomly. To determine mucosal disease severity, one representative sample was fixed in formalin for routine histopathology. An experienced pathologist classified patients blindly into one of the four groups, ranging form normal histology (score 0) to severe inflammation (score 3), as we have described previously [10].
Table 1.
Clinical characteristics and basal cytokine release from colonic explants
| N-IC | CD− | IC | CD+ | UC+ | |
|---|---|---|---|---|---|
| n | 26 | 30 | 20 | 45 | 21 |
| Sex (M/F) | 12/14 | 16/14 | 9/11 | 21/24 | 11/10 |
| Age (years) | 13 | 15 | 14 | 14 | 12 |
| Histological score | |||||
| 0 | 26 | 30 | – | – | – |
| 1 | – | – | 14 | 25 | 1 |
| 2 | – | – | 6 | 17 | 14 |
| 3 | – | – | – | 3 | 6 |
| Treated | – | 13 | – | 16 | 8 |
| TNF-α (pg/100 µg) | 4·0 | 4·4 | 6·0 | 15·5* | 45·8* |
| (2·1–5·0) | (2·4–6·1) | (2·8–8·8) | (6·0–25·5) | (19·6–61·1) | |
| IL-1β (pg/100 µg) | 1·9 | 2·3 | 6·0* | 12·1* | 27·5* |
| (1·1–3·1) | (1·7–3·4) | (2·7–11·1) | (5·5–24·0) | (14·8–44·1) | |
| IL-1RA (pg/100 µg) | 218 | 248 | 445 | 391 | 930* |
| (145–378) | (133–430) | (202–690) | (242–645) | (385–2980) | |
| IL-1RA/IL-1β | 104 | 118 | 50 | 33* | 29* |
| (60–195) | (83–191) | (39–93) | (25–57) | (20–65) | |
Age and cytokine values are expressed as median (interquartile range).
P < 0·05 versus N-IC. Groups: N-IC: non-inflammatory controls; CD−: Crohn's disease with rectal tissue non-involved; IC: inflammatory controls; CD+: Crohn's disease with rectal involvement; UC+: ulcerative colitis with rectal involvement.
Colonic biopsies were transported immediately to the laboratory in CMRL medium on ice and weighed. The tissue was either homogenized in 1 ml of ice-cold PBS (pH 7·4) containing an antiprotease cocktail [AEBSF (2·5 mg/ml), leupeptin, aprotinin, antipain and pepstatin (0·5 mg/ml)], or placed immediately surface-up on a sterilized steel grid in an organ culture dish (Falcon). The central well was filled with 1 ml of medium, consisting of CMRL 1066 supplemented with 10% heat inactivated fetal calf serum, TRIS 20 mm, 100 U penicillin–streptomycin, 50 µg gentamicin, 0·25 µg amphotericin B and 1 µg β-retinyl acetate. Explants were maintained for either 4 (short-term culture) or 18 (prolonged culture) h, as reported previously [10]. Briefly, the cultures were incubated at 37°C in an humidified 95% air/5% CO2 atmosphere, and rocked gently at 20 cycles/min. At the end of each culture period, the supernatants were collected, aliquoted and stored at − 70°C until assayed. Biopsies were cultured in media or in the presence of PWM (50 µg/ml), LPS (100 µg/ml), SEB (50 ng/ml) or SEA (10 ng/ml). These concentrations provide a half-maximal response. The study protocol and consent forms were approved by the Ethics Review Committee of Ste Justine Hospital.
Patient populations
Characteristics of the patient groups are provided in Table 1. Among the 30 CD patients with uninvolved rectal mucosa (CD−), 13 were treated and 15 had at least one other site of colonic involvement. Mean disease duration was 12 months (1·5 months−6 years). Of the 45 CD patients, 25 had mildly inflamed tissue while 20 had moderate to severe inflammation. Among the CD group with tissue involvement, 16 were on treatment. Their mean disease duration was 9·5 months (1 month−7 years). All the 21 ulcerative colitis (UC) patients had moderate to severe rectal inflammation. Eight were treated at the time of their biopsies. The mean UC disease duration was 6 months (2 months−15 years). The inflammatory control (IC) group was comprised of 20 patients with acute self-limited colitis. The non-inflammatory control (N-IC) group consisted of 26 children with normal colonic biopsies.
Cytokine determination
TNF-α, IL-1β and IL-1 RA assays were performed using commercially available ELISA kits, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). Cytokine release corresponded to the amount measured in supernatants. All values were ajusted for the weight of the biopsy specimens.
To take into account differences in basal amounts of cytokines found in the supernatants from the different groups, net colonic release was calculated. Paired biopsies were cultured for 18 h with and without the bacterial factors and cytokines were measured in supernatants. The value measured in the supernatant from the explants cultured without the antigen was substracted from that of the paired explants incubated with the antigen.
Statistical analysis
Non-normally distributed data were expressed as medians. The variation between group medians was then tested using the Kruskal–Wallis test for non-parametric data. Thereafter, the differences between groups were assessed with the two-tailed Mann–Whitney U-test. The effect of clinical variables on cytokine excretion within each group was tested using the one-tailed Mann–Whitney U-test. A P-value < 0·05 was considered statistically significant.
RESULTS
Explant basal cytokine release
All colonic explants released measurable amounts of TNF-α, IL-1 and IL-1RA during the culture period (Table 1). TNF-α levels in supernatants from involved CD and UC explants were significantly higher than those from the N-IC. Moderately to severely inflamed tissue from CD patients (score 2 and 3) released significantly more TNF-α than mildly inflamed (score 1) mucosa (23·3 versus 9·2 pg/100 µg, P < 0·05). IL-1 levels from IC and involved IBD tissues were significantly higher than those from N-IC explants. More severely inflamed CD tissue released greater amounts of IL-1 than mildly inflamed tissue (12·3 versus 6·6 pg/100 µg, P < 0·05). IL-1RA release was significantly higher in explants with involved UC. CD explants with higher histological scores released more IL-1RA than those with mild inflammation (399 versus 339 pg/100 µg, P < 0·05). IL-1RA/IL-1 ratios were significantly lower in supernatants from involved CD and UC.
Modulation of colonic TNF-α release by bacterial activators
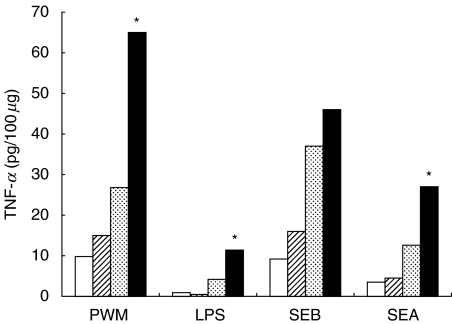
Involved IBD and IC explants stimulated with LPS, SEB and SEA released significantly higher amounts of TNF-α than the N-IC tissue (Table 2). When TNF-α concentrations released by explants cultured for 18 h in the presence of PWM were compared, only supernatants from CD and UC biopsies had significantly higher amounts than N-IC. Similar data were obtained with a shorter incubation period (4 h), although the values were lower (data not shown). Taken together, our data reveal that even when stimulated with different bacterial activators, non-involved IBD explants did not release as much TNF-α as involved tissue.
Table 2.
TNF-α in colonic explant supernatants cultured for 18 h in the presence of bacterial products
| TNF-α (pg/100 μg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | n | PWM | n | LPS | n | SEB | n | SEA |
| N-IC | 9 | 14·3 (7·0–30·5) | 9 | 3·0 (1·6–6·0) | 12 | 14·6 (10·7–16·6) | 10 | 5·9 (5·6–10·4) |
| CD− | 10 | 16·6 (7·5–36·2) | 10 | 4·6 (1·9–10·0) | 15 | 22·4 (12·8–46·0) | 8 | 11·3 (7·6–32·8) |
| IC | 6 | 23·1 (10·7–31·4) | 12 | 8·0 (4·1–19·0)* | 9 | 47·0 (17·4–107·0)* | 4 | 69·3 (45·1–72·8)* |
| CD+ | 13 | 40·2 (13·0–52·1)* | 15 | 14·1 (8·5–27·5)* | 15 | 55·2 (43·9–65·0)* | 13 | 49·8 (22·2–57·2)* |
| UC+ | 7 | 110·0 (69·6–126·0)* | 7 | 43·8 (22·2–83·1)* | 6 | 61·0 (35·2–106·5)* | 6 | 84·1 (45·8–120·4)* |
Results are expressed as median (IQR)
P < 0·05 versus N-IC explants stimulated with the same activator. Groups: N-IC: non-inflammatory controls; CD−: Crohn's disease with rectal tissue noninvolved; IC: inflammatory controls; CD+: Crohn's disease with rectal involvement; UC+: ulcerative colitis with rectal involvement.
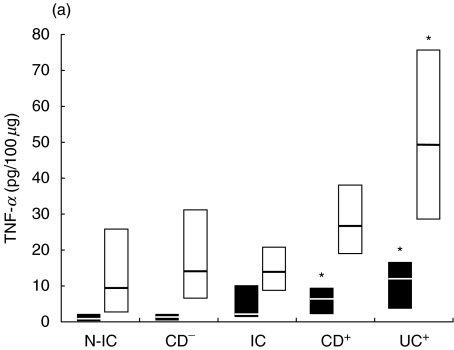
Because basal TNF-α release was higher in involved explants, the net release was calculated for each stimulated explant (Fig. 1). Stimulation with PWM had a moderate effect on TNF-α release from non-involved biopsies, while involved UC tissue consistently released high amounts of TNF-α. LPS weakly stimulated TNF-α release, but the response by involved explants was significantly stronger. SEB induced high levels of TNF-α release from the explants in all groups, particularly in explants from involved IBD as well as in IC patients. SEA proved to be a weaker stimulus than SEB, and its effect was more marked in involved IC and UC biopsies. As response magnitude was related to the degree of inflammation, the biopsies were classified according to their inflammatory score and the release of TNF-α was examined. More severely inflamed CD biopsies released higher net amounts of TNF-α after 18 h than did mildly involved tissue in response to PWM, LPS, SEB, and SEA (Fig. 2).
Fig. 1.
Net colonic TNF-α release in supernatants of colonic explants cultured for 18 h in the presence of (a) LPS (▪) or PWM (□) and (b) SEA (▪) or SEB (□). The box and whisker plots show the median values (horizontal lines) and first to third quartiles in boxes. Net release was calculated as described in Material and methods. *P < 0·05 versus N-IC explants stimulated with the same activator. Groups: N-IC: non-inflammatory controls; CD−: Crohn's disease with rectal tissue non-involved; IC: inflammatory controls; CD+: Crohn's disease with rectal involvement; UC+: ulcerative colitis with rectal involvement.
Fig. 2.
Net colonic TNF-α release in supernatants of colonic explants from Crohn's disease patients according to inflammation severity score. Biopsies were cultured in the absence and the presence of LPS, PWM, SEA and SEB. N-IC: non-inflammatory controls, CD+: mildly inflamed CD tissue, CD2+: moderately and severely inflamed CD tissue. *P < 0·05 versus other groups with the same bacterial activator. □, N-IC; ( ), CD−; (
), CD−; ( ), CD+; ▪, CD2+.
), CD+; ▪, CD2+.
Modulation of IL-1 release by immune activators
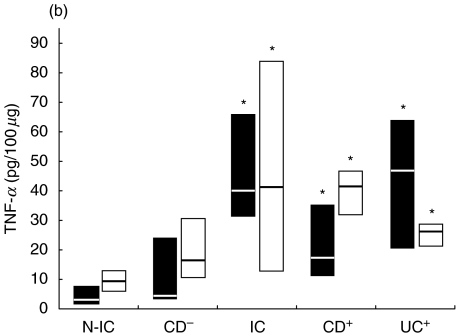
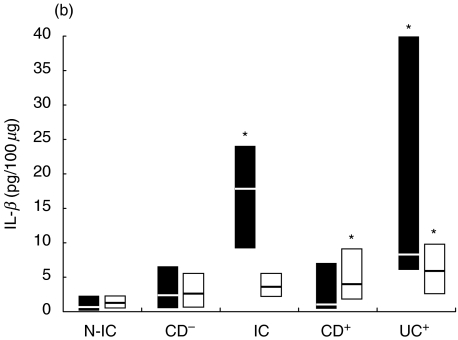
As observed for TNF-α, IL-1 concentrations after stimulation were significantly higher in supernatants from inflamed tissue (6·8–26·5, 8·8–17·8 and 20·0–51·0 pg/100 µg for IC, CD and UC groups, respectively), compared to values from N-IC explants (1·6–3·8 pg/100 µg). IL-1 concentration in supernatants were generally lower than those of TNF-α, as was IL-1 release induced by the bacterial activators (Fig. 3). PWM induced higher IL-1 release from UC tissue, while the other activators induced higher IL-1 release from involved tissue compared to biopsies from N-IC patients. Release from inflamed tissue was triggered most potently by PWM, followed by SEB and SEA. When uninvolved tissue was considered, PWM and SEB were the more effective stimulants. SEA also induced slightly higher IL-1 from uninvolved CD tissue. Moderately to severely inflamed tissue released higher amounts of IL-1 compared to mildly involved tissue when stimulated with LPS, SEB and SEA (7·2 versus 1·9, 10·2 versus 3·0, 4·1 versus 1·0 pg/100 µg). In response to LPS, the difference in cytokine release by involved and uninvolved tissue was more marked for IL-1 (Fig. 3) than for TNF-α (Fig. 1).
Fig. 3.
Net colonic IL-1 release in supernatants of colonic explants cultured for 18 h in the presence of (a) LPS (▪) or PWM (□) and (b) SEA (▪) or SEB (□). The box and whisker plots show the median values (horizontal lines) and first to third quartiles in boxes. Net release was calculated as described in Material and methods. *P < 0·05 versus N-IC explants stimulated with the same activator. Groups: N-IC: non-inflammatory controls; CD−: Crohn's disease with rectal tissue non-involved; IC: inflammatory controls; CD+: Crohn's disease with rectal involvement; UC+: ulcerative colitis with rectal involvement.
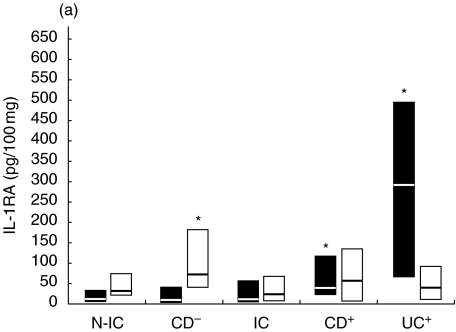
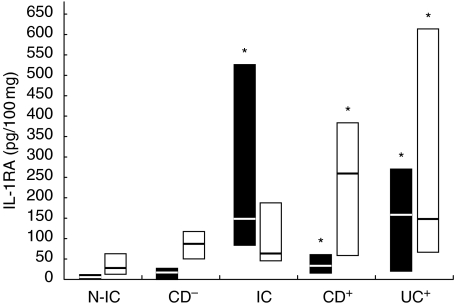
Modulation of IL-1RA release by immune activators
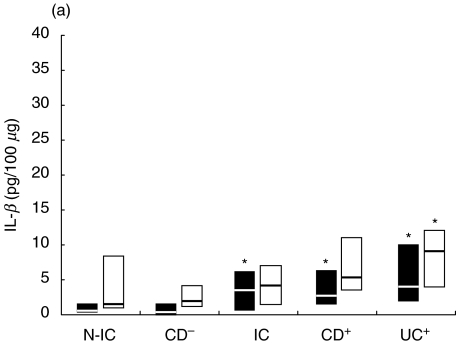
IL-1RA concentrations were elevated in stimulated inflamed tissue (294–815, 340–600 and 820–1700 pg/100 µg for IC, CD and UC, respectively) compared to N-IC (187–300 pg/100 µg). IL-1RA concentrations in supernatants from IC tissue were not significantly more induced than those from N-IC biopsies following stimulation with LPS, PWM and SEB. As for IL-1, SEA was a potent stimulator for IC tissue with acute inflammatory infiltrates (Fig. 4). IL-1RA release from involved tissue was triggered more efficiently by SEB and SEA, although a positive response was also observed in UC explants following LPS stimulation. SEB and PWM were the most efficient to evoke IL-1RA release from uninvolved tissue. The effect of stimulation on IL-1RA/IL-1 ratios was not as dramatic as expected. If the bacterial factors triggered IL-1 release, they also stimulated the release of its receptor antagonist. Overall, the ratio was decreased by about 20–40%. PWM induced the most important decline in IL-IRA/IL1 ratios. Supernatants of involved CD explants had lower ratios that were further decreased after incubation with PWM and LPS (38–26 and 41–36, respectively). SEB only minimally affected the IL-1RA/IL-1 ratio, while the ratios from IC and UC culture (66–26 and 30–14, respectively) were decreased after SEA stimulation. With uninvolved tissue, the IL-1RA/IL-1 ratio was not changed after incubation with LPS, while it was moderately decreased by SEB and SEA. PWM was again the more potent stimulatory agent, as the IRA/IL-1 ratio from N-IC decreased from 163 to 85.
Fig. 4.
Net colonic Il-1RA release in supernatants of colonic explants cultured for 18 h in the presence of (a) LPS (▪) or PWM (□) and (b) SEA (▪) or SEB (□). The box and whisker plots show the median values (horizontal lines) and first to third quartiles in boxes. Net release was calculated as described in Material and methods. *P < 0·05 versus N-IC explants stimulated with the same activator. Groups: N-IC: non-inflammatory controls; CD−: Crohn's disease with rectal tissue non-involved; IC: inflammatory controls; CD+: Crohn's disease with rectal involvement; UC+: ulcerative colitis with rectal involvement.
DISCUSSION
The major goal of this study was to determine the effect of bacterial antigens on colonic mucosal proinflammatory cytokine production in IBD. Given the critical importance of the intestinal microflora to the development of several experimental models of colitis [12,16,17], as well as the clinical utility of antibiotic therapy in Crohn's disease, we focused our investigation on gut flora-derived products. Each of the microbial products tested increased colonic TNF-α release in all patient groups (Fig. 1). The relatively weak stimulation induced by LPS is in accordance with previous studies using isolated LPMC [18,19]. Low TNF-α and IL-1 release in response to LPS by non-inflamed specimens probably reflects the small number of CD14+ cells present in normal mucosa [28]. On the other hand, involved IBD mucosa is the site of dense accumulation of recently immigrated CD14+ macrophages, secreting large amounts of proinflammatory cytokines [28,29]. These macrophages also express several Toll-like receptors that mediated cellular activation by bacterial components [30]. PWM was more potent than LPS in eliciting TNF-α release and, as with LPS, the response was greater in inflamed biopsies. This is in accordance with Rugtveit et al. [29], who found that PWM-stimulated LPMC from involved IBD tissue released more TNF-α than from cells isolated from controls. The important role of newly recruited macrophages is supported by the finding that PWM and LPS induced TNF-α release was threefold higher from more severely inflamed CD biopsies than that from mildly involved tissue.
Superantigens induced greater TNF-α release in inflamed tissue and were the most powerful activators for the IC group's tissue. More severely inflamed CD tissue released higher amounts than mildly involved explants upon SEA or SEB stimulation, although the difference was less marked than with LPS or PWM. This could be due to increased total T cell numbers, increased subpopulations of T cells resulting either from gut homing or from modifications dependent upon the mucosal environment, MHC expression on APC or co-stimulatory molecules, such as ICAM, B7-1/B7-2 or LFA-3 [31]. TNF-α release from uninvolved mucosa upon SEA or SEB stimulation probably reflects the proportion of T cells bearing appropriate Vβ elements in the resident T cell population.
In a previous study, we observed that TNF-α release from unstimulated biopsies was higher in patients with colonic inflammation in an area other than the rectum than in those with a totally spared colon [32]. An alternative explanation is that TNF-α may have been released by cellular sources others than T cells, including macrophages, fibroblasts, eosinophils, mast cells, epithelial and Paneth cells [8,33]. Mast cells have been observed to release TNF-α upon contact with bacteria, causing neutrophil influx into the lung and peritoneum [34], while bacterial invasion of epithelial cells induced the release of TNF-α and chemokines [35]. Sequential secretion of TNF-α and chemokines, followed by leucocyte accumulation, occurred upon SEA injection into air pouches [36]. Neutralizing antibodies against TNF-α inhibited leucocyte recruitment by 75%. Interestingly, T cells were not required to induce this inflammatory response [36]. Recent evidence also pointed to an important role for TNF-α originating from cells others than T lymphocytes. In the CD4CD45RBhi transfer model, recipient non-T cell TNF-α is essential and sufficient for the induction of colitis [8].
The bacterial products used in this study also induced IL-1 release of comparable amplitude. We observed that LPS was almost as potent as the superantigens in eliciting IL-1 release from inflamed mucosal tissue, while the latter were far more potent than LPS to induce TNF-α release. These data may be explained in part by the greater contribution of CD14+ macrophages to IL-1 release than for TNF-α[29]. Our data suggest that the release of TNF-α is regulated more tightly than that of IL-1. IL-1 plays an important role in at least one model, the TCR-α−/− mouse, which shares several homologies with UC [17]. IL-1RA release was also elicited by the bacterial factors. Interestingly, UC tissue released more IL-1RA than CD explants.
A key role for TH1-type CD4+ T cells has emerged in both CD and animal models of colitis [16,17, 37]. Activation of CD4+ T cells may occur secondary to loss of tolerance to commensal bacteria [37,38]. TNF-α was shown recently to act as a co-factor for TH1 cell development, and its neutralization by the cA2 antibody down-regulated IFN-γ production in CD patients [39]. In several models, increased TNF-α preceded the appearance of T cells, the TH1 cytokine profile and the development of colitis [8,40].
Taken together, the data herein provide further evidence for an important role of proinflammatory cytokines in the pathogenesis of IBD. Our findings support the concept that bacterial luminal products amplify the immune response in IBD tissue in part by inducing TNF-α and IL-1 release. The data substantiate further the role of bacterial products in IBD relapse or initiation. Monocyte/macrophage activation by normal bacteria and their products has been shown recently to alter physiological epithelial ion transport and transepithelial electrical resistance [41]. Clinically, these changes may explain, in part, the chronic diarrhoea in such patients. The immune activation elicited by SEB may allow triggering of abnormal immunological responses silenced normally by the tolerogenic mucosal milieu. SEB administration has been shown to induce an experimental enteropathy mediated by CD4+ T cells, accompanied by increased MHC II expression [23]. The fact that SEB potently increased TNF-α release from histologically normal colonic mucosa suggests that it could be an important mediator in the initiation of the inflammatory cascade.
Acknowledgments
This work was supported by a Research Initiative Award from the Medical Research Council of Canada/Astra Canada (SD, EGS) and by a grant from the Crohn's and Colitis Foundation of Canada (CCFC). The investigators also wish to acknowledge technical support from the Ste Justine Hospital Research Foundation (SL, EGS) and the Lecavalier IBD Fund (CD, EGS). Salary support was obtained from Research Scholarship Awards from the FRSQ (SL, EGS) and by a Research Chair Award from the CCFC and the Canadian Institutes of Health Research (EGS).
Glossary
Abbreviations
- CD
Crohn's disease
- IL-1
interleukin 1
- IL-1RA
interleukin 1 receptor antagonist
- LPMC
lamina propria mononuclear cellslamina propria mononuclear cells
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cell
- PWM
pokeweed mitogen
- SEA
staphylococcal enterotoxin A
- SEB
staphylococcal enterotoxin B
- TNF
tumour necrosis factor
- UC
ulcerative colitis
REFERENCES
- 1.Brandzaeg P, Haraldsen G, Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin Immunopathol. 1997;18:555–89. doi: 10.1007/BF00824058. [DOI] [PubMed] [Google Scholar]
- 2.Beck PL, Wallace JL. Cytokines in inflammatory bowel disease. Med Inflam. 1997;6:95–103. doi: 10.1080/09629359791785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadakis KA, Targan SR. Current theories on the causes of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:283–96. doi: 10.1016/s0889-8553(05)70057-1. [DOI] [PubMed] [Google Scholar]
- 4.Dionne S, Ruemmele FR, Seidman EG. Immunopathogenesis of inflammatory bowel disease: role of cytokines and immune cell–enterocyte interactions Inflammatory Bowel Diseases. Nestle Nutrition Workshop Series. In: Bistrian BR, Walker-Smith JA, editors. Vol. 2. Switzerland: Karger; 1999. pp. 41–57. [DOI] [PubMed] [Google Scholar]
- 5.Stack WA, Mann SD, Roy AJ, et al. Randomized controlled trial of CDP571 antibody to tumor necrosis factor-α in Crohn's disease. Lancet. 1997;349:521–4. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 6.Targan SR, Hanauer SB, Van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 7.Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117:761–9. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 8.Corrazza N, Eichenberger S, Eugster HP, Mueller C. Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene (RAG) 2−/− mice upon transfer of CD4+CD45RBhi T cells. J Exp Med. 1999;190:1479–91. doi: 10.1084/jem.190.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999;169:175–94. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 10.Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1 and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:435–42. doi: 10.1046/j.1365-2249.1998.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andus T, Daig R, Vogl D, et al. Imbalance of the interleukin 1 system in colonic mucosa − association with intestinal inflammation and interleukin 1 receptor agonist genotype 2. Gut. 1997;41:651–7. doi: 10.1136/gut.41.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartor RB. Postoperative recurrence of Crohn's disease: the enemy is within the fecal stream. Gastroenterology. 1998;114:398–400. doi: 10.1016/s0016-5085(98)70492-5. [DOI] [PubMed] [Google Scholar]
- 13.D'Haens GR, Geboes K, Peeters M, Baert F, Pennincks F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–7. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 14.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer Zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–75. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Ann Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 17.Bhan AK, Mizoguchi E, Smith RN, Mizoguchi A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol Rev. 1999;169:195–207. doi: 10.1111/j.1600-065x.1999.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 18.Reinecker HC, Steffen M, Witthoef TT, et al. Enhanced secretion of tumor necrosis factor-α, IL-6 and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–6. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 20.Uchiyama T, Yan XJ, Imanishi K, Yagi J. Bacterial superantigens − mechanisms of T-cell activation by the superantigens and their role in the pathogenesis of infectious diseases. Microbiol Immunol. 1994;38:245–56. doi: 10.1111/j.1348-0421.1994.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 21.Renno T, Acha-Orbea H. Superantigens in autoimmune disease: still more shades of gray. Immunol Rev. 1996;154:175–90. doi: 10.1111/j.1600-065x.1996.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 22.Probert CS, Chott A, Turner JR, et al. Persistent clonal expansions of peripheral blood Vβ4+ lymphocytes in chronic inflammatory bowel disease. J Immunol. 1996;157:3183–91. [PubMed] [Google Scholar]
- 23.Benjamin MA, Lu J, Donnely G, Dureja P, McKay DM. Changes in murine jejunal morphology evoked by the bacterial superantigen Staphylococcus aureus enterotoxin B are mediated by CD4+ T cells. Infect Immun. 1998;66:2193–9. doi: 10.1128/iai.66.5.2193-2199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katial RK, Sachanandani D, Pinney C, Lieberman MM. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin Diagn Lab Immunol. 1998;5:78–81. doi: 10.1128/cdli.5.1.78-81.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallart T, Angel de la Fuente M, Josep Barceló J, Alberola-Ila J, Lozano F. Desialylation of T lymphocytes overcomes the monocyte dependency of pokeweed mitogen-induced T-cell activation. Immunology. 1997;90:52–6. doi: 10.1046/j.1365-2567.1997.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noguchi M, Hiwatashi N, Liu Z, Toyota T. Secretion imbalance between tumor necrosis factor alpha and its inhibitor in inflammatory bowel disease. Gut. 1998;43:203–9. doi: 10.1136/gut.43.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen EM, Jahnsen FL, Lundin KEA, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 28.Mahida YR. The key role of macrophages on the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rugtveit J, Nilsen EM, Bakka A, Carlsen H, Branstzaeg P, Scott H. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493–505. doi: 10.1016/s0016-5085(97)70030-1. [DOI] [PubMed] [Google Scholar]
- 30.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 31.Parra E, Wingren AG, Healula G, Kalland T, Dohlsten M. The role of B7-1 and LFA-3 in costimulation of CD8+ T-cells. J Immunol. 1997;158:637–42. [PubMed] [Google Scholar]
- 32.Dionne S, Ruemmele F, Laberge S, Seidman EG. The effect of inflammation severity and of treatment on the production and release of TNFα by colonic explants in inflammatory bowel disease. Alim Pharmacol Ther. 2000;14:1435–42. doi: 10.1046/j.1365-2036.2000.00851.x. [DOI] [PubMed] [Google Scholar]
- 33.Beil WJ, Weller PF, Peppercorn MA, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of subcellular sites of TNF-α in colonic Crohn's disease. J Leukoc Biol. 1995;58:284–98. doi: 10.1002/jlb.58.3.284. [DOI] [PubMed] [Google Scholar]
- 34.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–9. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 35.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tessier PA, Naccache PH, Diener KR, et al. Induction of acute inflammation in vivo by Staphylococcal superantigens. II. Critical role for chemokines, ICAM-1, and TNF-α. J Immunol. 1998;161:1204–11. [PubMed] [Google Scholar]
- 37.Strober W, Ludviksson BR, Fuss IJ. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann Intern Med. 1998;128:848–56. doi: 10.7326/0003-4819-128-10-199805150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Cong BY, Brandweih SL, McCabe RP, et al. CD4+ cells reactive to enteric bacterial antigens in spontaneously colitis C3H/HejBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–64. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plevy SE, Landers CJ, Prehn J, et al. A role for TNF-α and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997;159:6276–82. [PubMed] [Google Scholar]
- 40.McDonald SA, Palmen MJHJ, Van Rees EP, MacDonald TT. Characterization of the mucosal cell-mediated immune response in IL-2 knockout mice before and after the onset of colitis. Immunology. 1997;91:73–80. doi: 10.1046/j.1365-2567.1997.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zareie M, Singh PK, Irvine EJ, Sherman PM, McKay DM, Perdue MH. Monocyte/macrophage activation by normal bacteria and bacterial products. Implications for altered epithelial function in Crohn's disease. Am J Pathol. 2001;158:1101–9. doi: 10.1016/S0002-9440(10)64057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]