Abstract
In the last decade, an unprecedented genetic diversity has been disclosed among Mycobacterium tuberculosis strains found worldwide. However, well-conserved genotypes seem to prevail in areas with high incidence of tuberculosis. As this may be related to selective advantages, such as advanced mechanisms to circumvent [M. bovis Bacille Calmette–Guerin (BCG)-induced] host defence mechanisms, we investigated the influence of strain diversity on the course of experimental disease. Twelve M. tuberculosis strains, representing four major genotype families found worldwide today, and the laboratory strain H37Rv were each used to infect BALB/c mice by direct intratracheal injection. Compared with H37Rv, infections with Beijng strains were characterized by extensive pneumonia, early but ephemeral tumour necrosis factor-alpha (TNF-α) and inducible isoform of nitric oxide synthetase (iNOS) expression, and significantly higher earlier mortality. Conversely, Canetti strains induced limited pneumonia, sustained TNF-α and iNOS expression in lungs, and almost 100% survival. Strains of the Somali and the Haarlem genotype families displayed less homogeneous, intermediate rates of survival. Previous BCG vaccination protected less effectively against infection with Beijing strains than against the H37Rv strain. In conclusion, genetically different M. tuberculosis strains evoked markedly different immunopathological events. Bacteria with the Beijing genotype, highly prevalent in Asia and the former USSR, elicited a non-protective immune response in mice and were the most virulent. Future immunological research, particularly on candidate vaccines, should include a broad spectrum of M. tuberculosis genotypes rather than a few laboratory strains.
INTRODUCTION
From the first exposure to Mycobacterium tuberculosis, a series of immune responses are triggered that define the course of the infection. However, this host defence response is not uniform in exposed people. In the vast majority of humans no disease develops at any time. Furthermore, a spectrum of possible clinical manifestations may occur at any stage of life in those patients who cannot control the infection [1]. The course of the infection and its epidemiological consequences depend upon a complex interplay of host, environmental and presumably bacterial factors. There is substantial information about the participation of environmental and host determinants [2, 3,4, 5, 6,7]. In contrast, the influence of the genetic diversity of the infecting organisms has not been examined in a systematic way.
The host immune response against mycobacterial infection has been explored. In mice and humans, infection is mainly controlled by macrophage activation induced through Th1 type cytokines. Interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) have a central role in this process by inducing macrophage activation and inducible isoform of nitric oxide synthetase (iNOS) expression. The NO produced in this process is essential − at least for mice − to kill intracellular mycobacteria [8,9]. This protective activity fails if there is a marked release of Th2 type cytokines [10,11]. The interplay of cytokines is depicted clearly in a BALB/c model of pulmonary tuberculosis following intratracheal infection [3,12–14]. In this model, an initial phase is dominated by high production of Th1 cell cytokines that, together with high levels of TNF-α and iNOS, temporarily control the infection. Granulomas develop in this phase. Three weeks after infection the expression of Th1 cell cytokines, TNF-α and iNOS start to decline. Gradually, pneumonic areas prevail over granulomas. Pneumonia, in co-existence with a high burden of bacteria, causes death.
The role of the genetic variability of M. tuberculosis in the outcome of the infection remains uncertain. In fact, until the early 1990s when DNA fingerprinting was introduced, it was believed that the M. tuberculosis complex constituted a genetically highly conserved group of bacteria with limited phenotypic differences influencing the pathogenesis. Therefore, most of the immunological research on tuberculosis was performed with laboratory strains, such as H37Rv and Erdman. However, recent epidemiological data suggest that differences in transmissibility and virulence among M. tuberculosis strains are related to the genetic background of the organisms [15,16]. Particular outbreak strains were found to elicit distinct immune paths and mortality rates in the course of experimental infection [17,18]. This issue has important implications for the control of tuberculosis and deserves further investigation in animal models [19]. Thus, in the light of the high degree of genetic diversity observed among M. tuberculosis isolates worldwide in the last decade, it seems worthwhile to investigate differences in pathogenicity and immunogenicity of a spectrum of bacterial genotypes.
In the present study we used the BALB/c mouse model of pulmonary tuberculosis described above to examine the course of infection in terms of survival, lung bacillary load, pathology and immune responses produced by different M. tuberculosis strains. These strains represent conspicuous families of genotypes of M. tuberculosis defined on the basis of insertion sequence (IS)6110 restriction fragment length polymorphism (RFLP) [20,21,22,23,24,25,26]. In addition, M. tuberculosis genotype-related differences in M. bovis Bacille Calmette–Guerin (BCG)-induced protection were also examined.
MATERIALS AND METHODS
M.tuberculosis strains
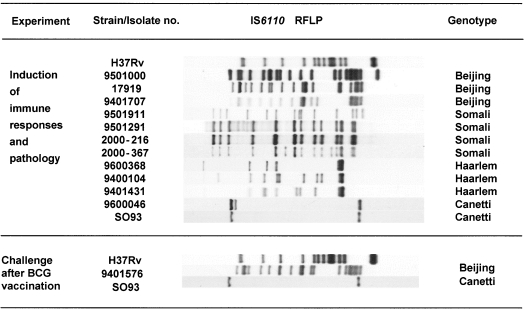
Figure 1 depicts the IS6110 RFLP patterns and genotype family of the strains used in each experiment. The IS6110 RFLP typing is the most widely applied and standardized DNA methodology for M. tuberculosis strain typing [23]. The establishment of a global database at the National Institute of Public Health and the Environment (RIVM) in the Netherlands facilitated the computer-assisted comparison of IS6110 RFLP patterns of 8000 M. tuberculosis isolates from a wide range of geographical origins. Genetically related strains according to IS6110 RFLP patterns were classified in major clades or genotype families [20,22]. The Beijing strains represent the predominant genotype family found in Asia [21,24]. The Somali strains are found frequently in East Africa and Asia and the Haarlem strains represent the most universal genotype found worldwide [22]. The Canetti genotype belong to the recently recognized new ssp. Canetti of the M. tuberculosis complex. The Canetti type strain is the original isolate obtained at the Pasteur Institute in 1969 and the SO93 strain was isolated in 1993 from a 2-year-old Somali refugee in the Netherlands [26]. The laboratory strain H37Rv was obtained from the American Type Culture Collection (Rockville, MD, USA). This strain was used as a control, because it was used in all previous immunopathological studies on this BALB/c mouse model [3,12–14].
Fig. 1.
Genotypes of the M. tuberculosis strains used in this study.
The organisms were grown in agitation at 37°C in Middlebrook 7H9 broth (Difco, Detroit, MI, USA), supplemented with albumin, catalase, and dextrose enrichment (Becton Dickinson, Cockysville, MD, USA). Cell suspensions were aliquoted and frozen at −70°C as soon as they reached the stationary phase as determined by densitometry. Reculture was kept to a minimum to avoid loss of virulence. M. bovis BCG (strain GL2, Pasteur Institute of Brussels) was grown as a surface pellicle on synthetic Sauton medium for 2 weeks and homogenized using a ball mill method [27].
Murine model of progressive pulmonary tuberculosis
The experimental model of pulmonary tuberculosis has been described in detail previously [3,12–14]. Pathogen-free male BALB/c mice were used at 6–8 weeks of age. Each animal was anaesthetized, the trachea was exposed via a small midline incision, and 2 × 105 viable cells were injected in 100 µl of phosphate buffer saline (PBS). After suturing the incision, the mouse was maintained in a vertical position until the effect of anaesthesia passed. Infected mice were kept in cages fitted with micro-isolators connected to negative pressure.
Thirteen groups of 68 mice were each infected with a different M. tuberculosis strain. The whole experiment was repeated and results of two experiments were pooled. Twenty mice in each group were left undisturbed and survival was recorded up to day 120. Eight mice (all survivors, if less than eight) were killed by exsanguination 3, 14, 21, 28, 60 and 120 days after infection. Lungs from four mice were prepared for histopathological studies. After eliminating hilar lymph nodes and thymic tissue, lungs of the remaining four animals were frozen in liquid nitrogen and kept at −70°C for microbiological and immunological studies.
Bacterial multiplication in the lung was investigated for the three Beijing members (9501000, 9401707 and 17919) and for two strains from the rest of the M. tuberculosis genotypes (Somali 2000216 and 2000367, Haarlem 9401431 and 9400104, Canetti 9600046 and SO93). Pathogenicity and immunogenicity was investigated only in mice infected with Canetti strain 9600046, Beijing 9501000 and H37Rv. These experiments were conducted at the National Institute of Medical Sciences and Nutrition, Mexico.
Colony-forming unit (CFU) counts
The right or left lung from four mice at each sacrifice time-point were used in two different experiments. Lungs were homogenized with a Polytron homogenizer (Kinematica, Luzern, Switzerland) in sterile tubes containing 3 ml of isotonic saline. Four dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H11 agar (Difco Laboratories, Detroit, MI, USA) enriched with oleic acid, albumin, catalase and dextrose. The number of colonies was counted 21 days postinoculation.
Histopathology and morphometry
Lungs were perfused with 10% formalin dissolved in PBS via the trachea, immersed for 24 h in the same fixative and embedded in paraffin. Five micrometres transverse sections, taken through the hilus, were stained with haematoxilin and eosin. The area in square micrometer occupied by the inflammatory infiltrate in the alveolar–capillary interstitium and blood vessel wall, as well as the percentage of lung surface affected by granulomas and pneumonia, were determined using an automated image analyser (Q Win Leica, Milton Keynes. UK) [28].
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of cytokines and iNOS in lung homogenates
Lung RNA was isolated with Trizol (Gibco BRL, Gaithersburgh, MD, USA) as described previously [29]. The cDNA was synthesized by using Maloney murine leukaemia virus reverse transcriptase (Gibco BRL) and oligo dT priming. The cDNA from four infected mice per sacrifice point was pooled. RT-PCR to detect the expression of IFN-γ, TNF-α and iNOs was performed as described previously [12–14]. PCR products were electrophoresed on 6% polyacrilamide gels. Cytokine mRNA was quantified in DNA nanograms with an image analysis densitometer coupled to a computer program (ID image analysis software, Kodak Digital Science, Rochester, NY, USA) using mass quantification DNA standards (Low DNA mass ladder, Gibco BRL). Expression of glyceraldehide 3 phophate dehidrogenase was used to check RNA integrity.
Challenge after BCG vaccination
Male BALB/c mice of 8–10 weeks of age were vaccinated intravenously with 0·5 mg (approximately 2 × 106 CFU) of freshly prepared BCG vaccine. Two months later, mice were challenged intravenously with 106 CFU of the strains H37Rv, Canetti or Beijing (Fig. 1). Control mice only received the intravenous M. tuberculosis challenge. Five weeks after challenge, mice were killed by cervical dislocation and CFU counts in spleen and lung were determined by plating on 7H11 Middlebrook agar supplemented with oleic acid, catalase, dextrose and thiophene as reported before [30]. These challenge experiments were conducted independently at the Pasteur Institute of Brussels.
Statistics
Chi-square test (Fisher's exact test when applicable) was employed to compare mice survival at each point time. A one-way anova and Student's t-test were used to compare morphometry and CFU counts.
RESULTS
Survival
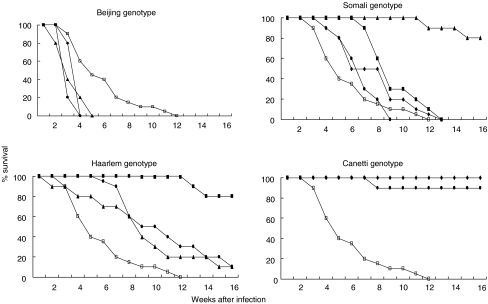
Death curves are presented in Fig. 2. Four weeks after infection all three Beijing genotype strains induced significantly higher mortality than H37Rv. Distinctively, this genotype killed all animals 4 or 5 weeks after infection. Conversely, virtually all mice infected with either strain of the Canetti genotype survived throughout the experiment. The strains belonging to other genotype families, including the control strain H37Rv, produced intermediate mortality rates.
Fig. 2.
Survival of BALB/c mice (20 mice/strain) infected by intratracheal injection (2 × 105 bacilli) with M. tuberculosis strains belonging to four different genotype families. Survival of mice infected with H37Rv is represented in all four graphics (together with strains of each genotype). Beijing genotype: •, 9501000; ⋄, 17919; ▴, 9401707; □, H37Rv. Somali genotype: •, 9501911; ⋄, 9501291; □, H3Rv; ▪, 2000367; ▴, 2000216. Haarlem genotype: •, 9600368; ▴, 9401431; ▪, 9400104; □, H37Rv. Canetti genotype: •, 9600046; □, H37Rv; ⋄, SO93.
Bacterial multiplication in the lungs
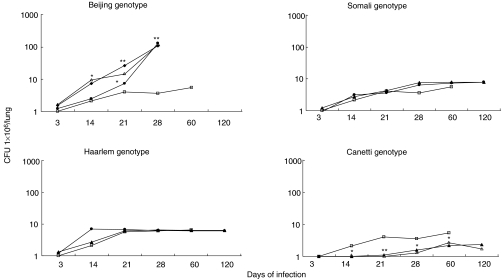
Figure 3 shows CFU in lungs of mice infected with strains belonging to each genotype family. Four weeks postinfection, mice infected with three different strains from the Beijing genotype showed a significant lung bacillary burden about two logs higher than that of control mice infected with strain H37Rv (P < 0·001). Mice infected with the Canetti genotype showed a significantly lower bacillary loads at all time points than did those infected with H37Rv (P < 0·05). CFU in the lungs of mice infected with the remaining strains did not differ statistically from each other or from counts obtained with H37Rv.
Fig. 3.
CFU in lung homogenates during the course of infection with M. tuberculosis strains belonging to four different genotype families. CFU from mice infected with H37Rv strain are represented in all four graphics (compared to H37Rv. *P < 0·05, **P < 0·001). Beijing genotype: •, 9501000; ▵, 9401707; ⋄, 17919; □, H37Rv. Somali genotype: •, 2000216; □, H37Rv; ▴, 2000367. Haarlem genotype: •, 9401431; □, H37Rv; ▴, 9400104. Canetti genotype: ▴, 9600046; □, H37Rv; ▵, SO93.
Pathological findings in the lungs
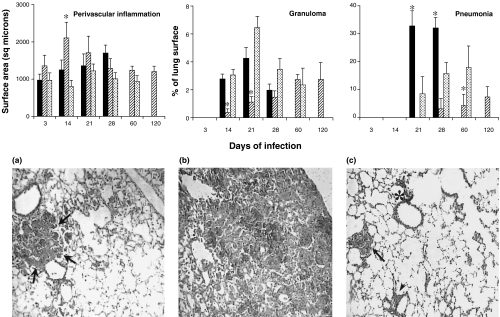
Results are presented in Fig. 4. The earliest histopathological event − a mononuclear inflammatory infiltrate in the alveolar–capillary interstitium and perivascular areas − was more evident in mice infected with the Canetti strain (9600046). Two and 3 weeks after infection, this strain produced larger perivascular infiltrates and significantly smaller areas of granuloma than the control strain H37Rv (P < 0·05). The area occupied by granulomas increased steadily throughout the experiment. Pneumonic patches induced by the Canetti strain appeared much later and were significantly smaller than those produced by H37Rv (P < 0·05). At the final time-point pneumonic areas occupied less than 10% of the lung surface and mice showed little clinical signs of disease.
Fig. 4.
The top panel shows the morphometric analysis of the inflammatory infiltrate produced in the perivenular area, and the percentage of lung surface affected by granuloma and pneumonia in mice infected by Beijing (▪), Canetti ( ) and H37Rv (
) and H37Rv ( )M. tuberculosis strains. Three random fields in each lung lobe were assessed. Data are means from eight mice in two different experiments. Asterisks represent statistical significance (P < 0·05) compared with H37Rv. Lower panel shows representative histopathological findings in the lungs 28 days after intratracheal instillation with different M. tuberculosis strains. (a) Typical small pneumonic patches (arrow) produced by H37Rv strain. (b) Massive pneumonia induced by the Beijing strain. (c) Granulomas (arrow), perivascular inflammation (arrow head) and peribronchial inflammation (asterisk) induced by Canetti strain (40× magnification, haematoxylin–eosin staining).
)M. tuberculosis strains. Three random fields in each lung lobe were assessed. Data are means from eight mice in two different experiments. Asterisks represent statistical significance (P < 0·05) compared with H37Rv. Lower panel shows representative histopathological findings in the lungs 28 days after intratracheal instillation with different M. tuberculosis strains. (a) Typical small pneumonic patches (arrow) produced by H37Rv strain. (b) Massive pneumonia induced by the Beijing strain. (c) Granulomas (arrow), perivascular inflammation (arrow head) and peribronchial inflammation (asterisk) induced by Canetti strain (40× magnification, haematoxylin–eosin staining).
Infection with Beijing bacteria (strain 9501000) started with a modest interstitial and perivascular inflammation that was oedematous rather than cellular. Granulomas peaked 3 weeks after infection and co-existed with pneumonic areas that were already significantly larger than those produced by H37Rv (P < 0·05). Four weeks postinfection, when mice showed clinical evidence of severe respiratory disease and the bacillary burden was at its peak, massive pneumonia prevailed over granulomas. Characteristic histopathological features four weeks after infection with the three strains are shown in Fig. 4.
Cytokine and iNOS gene expression in lung homogenates
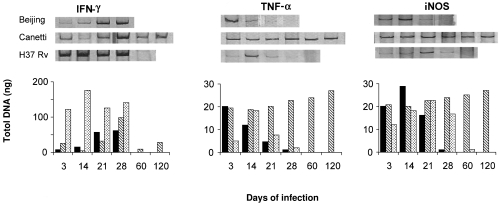
Results are presented in Fig. 5. H37Rv produced a strong IFN-γ expression that dropped abruptly to undetectable levels at day 60. In comparison with animals infected with H37Rv, those infected with Beijing bacteria (strain 9501000) showed a delayed and fainter expression of IFN-γ, and an earlier and augmented expression of TNFα and iNOS in their lungs. TNF-α and iNOS showed a pronounced drop before death. Mice infected with Canetti bacteria (9600046) showed an initially high expression of TNF-α and iNOS, which was reinforced towards the late stages of infection combined with a retarded and modest IFN-γ expression during the first and second week postinfection. Expression of IFN-γ was constant and moderate during the late stage.
Fig. 5.
Kinetics of cytokine and iNOS mRNA expression in lungs of BALB/c mice infected by intratracheal injection with Beijing (▪), Canetti ( ) and H37RV (
) and H37RV ( )M. tuberculosis strains. cDNA obtained by RT-PCR from four mice per time-point was pooled. Data are expressed as nanograms of cDNA.
)M. tuberculosis strains. cDNA obtained by RT-PCR from four mice per time-point was pooled. Data are expressed as nanograms of cDNA.
Efficacy of BCG vaccination against challenge with Beijing, Canetti and H37Rv strains
In contrast to the intratracheal infection, CFU counts following intravenous infection with Beijing and H37Rv genotypes did not differ 5 weeks after challenge, whereas multiplication of the Canetti strain was 10-fold lower. These findings are not comparable with those of the aerogenic model as the intravenous route results primarily in seeding to spleen and liver. Haematogenous spreading to the lungs is only subsequent to this event. Even though BCG conferred significant protection against the three strains, the CFU clearance was lower in Beijing infected organs compared to that of the H37Rv group, while the CFU values in the Canetti group were the lowest (Table 1).
Table 1.
CFU counts in organs of BCG vaccinated and non-vaccinated BALB/c mice, 5 weeks after challenge with the Beijing genotype, the M. canettii or the H37Rv strain of M. tuberculosis
| Spleen | Lung | |||||
|---|---|---|---|---|---|---|
| No. of mice | BCG Vaccination | CFUa | Δ | CFUa | Δb | |
| M. canettii | 4 | No | 4·75 ± 0·16 | – | 4·69 ± 0·08 | – |
| 4 | Yes | 3·76 ± 0·25 | 0·99** | 4·1 ± 0·1 | 0·59** | |
| H37Rv | 6 | No | 6·05 ± 0·31 | – | 5·94 ± 0·32 | – |
| 5 | Yes | 4·83 ± 0·15 | 1·22** | 4·56 ± 0·1 | 1·38** | |
| Beijing | 4 | No | 5·93 ± 0·12 | – | 5·76 ± 0·14 | – |
| 4 | Yes | 5·26 ± 0·15 | 0·67** | 5·2 ± 0·37 | 0·56* | |
Mean ± s.d. of CFU counts/organ expressed as log10 values;
difference in log10 compared to CFU counts/organ in unvaccinated mice.
P < 0·05;
P < 0·001.
DISCUSSION
To what extent the pathogenesis of tuberculosis depends on the infecting organism, and is independent of host factors, is an essential issue in tuberculosis research [19]. Our mouse model of progressive pulmonary tuberculosis is suitable to explore this question. First, it is based on aerogenic infection, which is the usual route in humans. Secondly, the rate of bacterial multiplication in the lungs correlates with the extent of tissue damage (pneumonia) and mortality. Thirdly, the infection is controlled successfully as long as a strong Th1 cell response is sustained [13, 14,28], which is endorsed by previous evidence on the protective role of Th1 cytokines against mycobacterial infection [31].
In the present study, we observed important differences in pathology and immune response after infection with M. tuberculosis strains of major genotype families found worldwide today. Beijing infection consistently induced accelerated bacterial multiplication, early and massive pneumonia and death. Conversely, Canetti infection induced a slowly progressive disease characterized by delayed bacterial multiplication, limited pneumonia, steadily increasing granulomas and virtually no mortality. In terms of virulence, various strains belonging to two other conspicuous genotype families − the so-called Haarlem and Somali clades − displayed rather intermediate and less homogeneous results.
The more severe pathology induced by the Beijing bacteria co-existed with a host immune response probably driven towards nonprotective mechanisms. The initially high, albeit transient, expression of TNF-α and iNOS suggests that macrophages infected with the Beijing strain were activated efficiently during the early phase of the infection. The faint expression of IFN-γ, however, seems to indicate that these macrophages were rapidly de-activated and did not stimulate Th1 cells efficiently enough to arrest bacillary multiplication, resulting in massive tissue damage and early mortality.
In contrast with the Beijing strain, Canetti bacteria elicited prompt and conspicuous inflammation. The high and sustained TNF-α and iNOS gene expression may have the arrested progression of disease, which was reflected by limited tissue damage and very low mortality. Perhaps this early and very efficient control of the infection by activated macrophages did not require high IFN-γ expression during the first and second weeks of the infection. Then, a higher and constant expression of IFN-γ was induced, which might have contributed to the very slow progression of disease.
The Beijing genotype family, which prevails in China, Mongolia, South Korea and Thailand, is the most pronounced example of large-scale genetic conservation among M. tuberculosis strains in one specific geographical area [21,24]. In Vietnam, Beijing strains were associated with young patients and, hence, with recent and active transmission of tuberculosis, suggesting their involvement in the emergence of tuberculosis in that country [32]. Furthermore, the Beijing genotype was significantly associated with resistance to antituberculosis drugs in a convincing number of studies in Asian areas, the former USSR and also the United States [33–36]. The first indication of a peculiar interaction between Beijing bacteria and the host defence system was observed in a recent study performed in Indonesia, where patients affected by Beijing strains developed early febrile responses to treatment twice as often as patients infected by other M. tuberculosis strains [37].
The first Canetti strain was isolated in France in 1969. Since then, this strain has been used extensively in the search of antigens for immunodiagnostic tests [38]. Later on, a strain with a similar genotype was isolated from a 2-year-old Somali patient, and Canetti bacteria were proposed as a new subspecies designation within M. tuberculosis[26]. In due time, other Canetti strains were isolated from human cases from Eastern Africa [39,40]. Although the epicentre of Canetti bacteria seems to be located in the Horn of Africa, their true incidence in that area remains to be determined.
Canetti bacteria produce smooth and glossy colonies, which is highly unusual for M. tuberculosis. Smooth colony morphology has been related to virulence of M. avium[41]. However, the original Canetti strain was deemed to be attenuated rather than virulent. Both, colony morphology and low virulence were attributed to preferential synthesis of phthioceranic acids over other phthienoic acids [38]. Our results clearly confirm the low virulence of Canetti bacteria in a mouse model.
It has been speculated that the long-term BCG mass vaccination may be one of the selective forces implicated in the successful spread of the Beijing strains [21]. In this study we found an indication that BCG vaccination confers poorer protection against Beijing, while the CFU values in the BCG vaccinated animals infected with Canetti bacteria were the lowest. This might have implications for future vaccine development.
In conclusion, using a well-characterized model of progressive pulmonary tuberculosis we demonstrated that genetically different M. tuberculosis strains elicit dissimilar immune responses in lung, which determines differences in pathology and mortality. In particular, the emerging Beijing genotype bacteria were the most virulent among the genotypes studied. Our results highlight the need for the elucidation of intraspecies differences in genome sequences and expression using new technologies, such as microarrays. This approach could contribute to the identification of M. tuberculosis genes associated with virulence and immunogenicity. The efficacy of new candidate tuberculosis vaccines should be tested in an animal model against a wide range of M. tuberculosis genotypes representative of the global tuberculosis epidemic.
Acknowledgments
B.L. was supported by a United Nations University fellowship during the execution of this project. R.H.P. holds grants from Wellcome Trust and Consejo Nacional de Ciencia y Tecnología, Mexico. V.R. holds grant PICT 5–9978 from SECYT, Argentina. K.H. holds grants from the FWO-Vlaanderen (G.0266·00), EEC (TB Vaccine Cluster QLK2-CT-1999–01093) and the Damiaanaktie Belgium. D. van S. holds a grant from the EEC (Molecular epidemiology of TB QLK2-2000–00630). The support of the EEC INCO-DEV Programme (ICA4-CT-2001–10087) is also acknowledged. We thank Prof G. A. W. Rook for reading the manuscript and making suggestions.
REFERENCES
- 1.Bloom BR, Murray CJ. Tuberculosis commentary on a reemergent killer. Science. 1992;257:1055–64. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 2.Nardell EA. Environmental control of tuberculosis. Med Clin North Am. 1993;77:1315–34. doi: 10.1016/s0025-7125(16)30196-1. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte. Infect Immun. 1997;65:3317–27. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AV. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy RC, Ruwende TC, Corrah TKP, McAdams KP, Whittle HC, Hill AV. Variation in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–4. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 6.Stead WW. Genetics and resistance to tuberculosis. Could resistance be enhanced by genetic engineering? Ann Intern Med. 1992;116:937–41. doi: 10.7326/0003-4819-116-11-937. [DOI] [PubMed] [Google Scholar]
- 7.Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Nat Acad Sci. 2000;97:8560–5. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan X, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 10.Seah GT, Scott GM, Rook GAW. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 11.Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous disease. J Immunol. 2001;166:3432–9. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Pando R, Orozco EH, Sampieri A, et al. Correlation between kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Pando R, Orozco EH, Arriaga AK, et al. Analysis of the local kinetics and localization of interleukin 1α, tumor necrosis factor α and transforming growth factor β during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez-Pando R, Schön T, Orozco EH, Serafín J, Estrada-Garcia I. Expression of nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis. Exp Toxicol Pathol. 2001;53:257–65. doi: 10.1078/0940-2993-00182. [DOI] [PubMed] [Google Scholar]
- 15.Valway SE, Sanchez MPC, Shinnick TF, et al. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–9. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 16.Caminero J, Pena MJ, Campos-Herrero MI, et al. Epidemiologic evidence for the spread of a Mycobacterium tuberculosis strain of the ‘Beijing’ genotype on Gran Canaria Island. Am J Respir Crit Care Med. 2001;164:1165–70. doi: 10.1164/ajrccm.164.7.2101031. [DOI] [PubMed] [Google Scholar]
- 17.Manca C, Tsenova L, Barry C, et al. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6. [PubMed] [Google Scholar]
- 18.Manca C, Tsenova L, Bergtold A, et al. Virulence of a Mycobacterium tuberculosis isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc Nac Acad Sci. 2001;98:5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishai W. Tuberculosis transmission − rogue pathogen or rogue patient? Am J Respir Crit Care Med. 2001;164:1104–5. doi: 10.1164/ajrccm.164.7.2108039c. [DOI] [PubMed] [Google Scholar]
- 20.Van Soolingen D. Utrecht University; 1996. Use of DNA fingerprinting in the epidemiology of tuberculosis. PhD thesis. [Google Scholar]
- 21.Van Soolingen D, Qian L, de Haas PEW, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–8. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremer K, van Soolingen D, Frothingham R, et al. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–18. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Embden JDA, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standarized methodology. J Clin Microbiol. 1993;31:406–9. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian L, van Embden JDA, van der Zanden AGM, Weltevreden EF, Duanmu H, Douglas JT. Retrospective analysis of the Beijing family of Mycobacterium tuberculosis in preserved lung tissues. J Clin Microbiol. 1999;37:471–4. doi: 10.1128/jcm.37.2.471-474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Soolingen D, de Haas PEW, Hermans PW, Groenen PM, van Embden JD. Comparison of various repetitive DNA elements as genetics markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:3234–8. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Soolingen D, Hoogenboezem T, de Haas PEW, et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997;47:1236–45. doi: 10.1099/00207713-47-4-1236. [DOI] [PubMed] [Google Scholar]
- 27.Huygen K, Abramowicz D, Vandenbussche P, et al. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–6. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Pando R, Pavon L, Orozco EH, Rangel J, Rook GAW. Interactions between hormone-mediated and vaccine mediated immunotherapy for pulmonary tuberculosis in BALB/c mice. Immunology. 2000;100:391–8. doi: 10.1046/j.1365-2567.2000.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chomzynski PA. Reagent for the single step simultaneous isolation of RNA, DNA and proteins from cells and tissue samples. Biotechniques. 1993;15:532–5. [PubMed] [Google Scholar]
- 30.Tanghe A, Lefèvre P, Denis O, et al. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–9. [PubMed] [Google Scholar]
- 31.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russel DG, Orme I. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anh DD, Van Borgdorff MWLN, Lan NTN, van Gorkom T, Kremer K, van Soolingen D. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg Infect Dis. 2000;6:302–5. doi: 10.3201/eid0603.000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marttila HJ, Soini H, Vyshnevskiy BI, et al. Rapid detection of rifampin-resistant Mycobacterium tuberculosis by sequencing and line probe assay. Scand J Infect Dis. 1998;30:129–32. doi: 10.1080/003655498750003492. [DOI] [PubMed] [Google Scholar]
- 34.Portaels F, Rigouts L, Bastian I. Addressing multi-drug resistant tuberculosis in penintentiary hospitals and in the general population of the former Soviet Union. Int J Tuberc Lung Dis. 1999;3:582–8. [PubMed] [Google Scholar]
- 35.Narvskaya OV, Mokrousov IV, Otten TF, Vyshnevskiy BI. Genetical marking of Mycobacterium tuberculosis polyresistant strains isolated in the North-West part of Russia. Probl Tuberk. 1999;3:39. [PubMed] [Google Scholar]
- 36.Bifani PJ, Plikaytis BB, Kapur V, et al. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA. 1996;275:452–7. [PubMed] [Google Scholar]
- 37.Van Crevel R, Nelwan RHH, de Lenne W, et al. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg Infect Dis. 2001;7:880–3. doi: 10.3201/eid0705.017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daffe M, McNeil M, Brennan P. Novel type specific lipooligosacharides from Mycobacterium tuberculosis. Biochemistry. 1991;30:378–88. doi: 10.1021/bi00216a011. [DOI] [PubMed] [Google Scholar]
- 39.Pfyffer GE, Auckenthaler R, van Embden JDA, van Soolingen D. Mycobacterium canetti, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa. Emerg Infect Dis. 1998;4:631–4. doi: 10.3201/eid0404.980414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerome P, Koeck JL, Bernatas T, Fabre M, Herve V, Vanerot A, Vincent V. Four new cases of lymph node tuberculosis due to M. tuberculosis subsp. canetti in Djibouti, Horn of Africa. Proceedings of the 21st Annual Congress of the European Society of Mycobacteriology, Vienna, Austria. 2000.
- 41.Schaefer WB, Davies CL, Cohn ML. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970;102:499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]