Abstract
Several studies have suggested an important role for the protein tyrosine kinase p56lck (Lck) in HIV infection; however, the exact nature of this role remains unclear. Using a series of well characterized Jurkat-derived cell lines having a wide range of Lck kinase activity, our results showed that, while the entry of HIV-1 into these cell lines was similar, the kinetics of virus production by these cells were very different. Cells expressing a kinase-inactive Lck showed accelerated viral replication, whereas, cells expressing Lck with normal or elevated enzymatic activity showed a delay in virus replication that was proportional to the initial level of endogenous Lck activity. The cell line having the highest initial Lck kinase activity showed the slowest rate of productive HIV-1 infection. Analysis of 2-LTR circles revealed that this inhibitory effect of Lck was not due to inhibition of reverse transcription of HIV-1 genome or migration of the proviral DNA into the nuclei. This affect of Lck was confirmed in additional studies that used either the S1T cell line lacking completely Lck or where the Lck activity was altered in Jurkat cells prior to infection. S1T cells showed a 3- to 12-fold increase in the level of infection compared to Jurkat cells despite similar CD4 and chemokine coreceptor expression and cell doubling times. Pretreatment of Jurkat with an antisense lck oligodeoxynucleotide inhibited the synthesis of functional Lck and facilitated the viral replication by the cells as did expressing a dominant-negative mutant Lck which increased the productive infection>3-fold. Conversely, whereas IL-16 had no affect on productive infection in S1T cells that lack Lck, IL-16 pretreatment of Jurkat cells resulted in an immediate (within 5 min) and sustained and gradual (over 5 h) increase in Lck activity that resulted in a reduction of HIV-1 replication that paralleled the increasing Lck kinase activity. These results show that the enzymatic activity of Lck kinase can affect viral replication, that a lack of, or decreased Lck activity facilitates viral replication. Conversely, Lck can mediate a delay in HIV-1 infection that is proportional to the initial endogenous Lck enzyme activity.
Keywords: HIV-1, AIDS, T lymphocytes, Lck, tyrosine kinases
INTRODUCTION
The cell surface glycoprotein, CD4, exhibits high-affinity for the human immunodeficiency virus (HIV) envelope protein gp120 and constitutes the principal component of the cellular receptor for HIV [1,2]. The src-family protein tyrosine kinase, p56lck(Lck), a proximal signalling molecule for activation of T lymphocytes through ligation of the T cell antigen receptor (TCR) complex [3,4] or the interleukin-2 (IL-2) receptor [5], is coupled to CD4 through interaction between cysteine residues located within the cytoplasmic tail of CD4 and the NH2-terminus of Lck [6,7]. In T cells that express CD4, 50–96% of the Lck is found bound to the cytoplasmic tails of this protein [7,8]. Oligomerization of the CD4 molecule on the surface of T cells results in a transient increase in the enzymatic activity of Lck [7,8] which participates in T cell activation by tyrosine phosphorylation of downstream proteins involved in the activation pathway [3,7].
Cells expressing both CD4 and CC or CXC chemokine receptors, the coreceptors for HIV, are primary targets of HIV infection [1,9–11]. Due to direct high-affinity binding to CD4 on T cells, HIV may act as a ‘pseudoligand’ and initialize agonistic or antagonistic biochemical signals similar to a natural ligand for CD4. Furthermore, studies from our laboratory [12–14] and others [15–17] have shown that HIV can affect intracellular signalling pathways resulting in increased tyrosine phosphorylation of a number of proteins, and specifically, an increase in the kinase activity of Lck and other src-family members.
The cytoplasmic tail of the CD4 molecule has been known to play important roles in modulation of HIV infection [18–21]. For example, it has been shown that cells expressing CD4 having point mutations at cysteines 20 and 23, so that Lck no longer associates with CD4 tails, results in greatly accelerated HIV replication [21]. While these findings implicate a potentially significant role for Lck in modulation of HIV infection, they do not distinguish between the requirements for the physical association of Lck with CD4 and/or the Lck kinase activity for the manifestation of this modulatory effect. Importantly, Lck itself has not been examined directly for a role in facilitation or inhibition of the initial stages of HIV infection. Therefore, the objective of this study was to clarify the role played by the Lck protein tyrosine kinase during the early stages of HIV-1 infection.
METHODS
Cells
The human leukaemic T cell line, Jurkat, was obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA. The Jurkat-derived mutant T cell lines J45·01 (J45), that has greatly depressed cell-surface CD45 expression [22], and J.CaM1·6 (JCaM), that has a kinase-inactive, truncated form of Lck [3], were obtained from ATCC. The J.CaM1·6 cell line transfected with an expression vector containing the full-length lck cDNA (JCaM-Lck) [3] was a kind gift from Dr A. Weiss (University of California, San Francisco, San Francisco, CA, USA). The S1T T cell line, which lacks both mRNA and protein for Lck [23], was provided by Dr G. Mills (MD Anderson Cancer Center, Houston, TX, USA). All cells prior to infection, with the exception of JCaM, were cultured in complete culture medium (RPMI containing 10% (v/v) fetal bovine serum (FBS), 2 mm l-glutamine, and gentamycin at 37°C in an atmosphere containing 5% CO2. Prior to infection, JCaM cells were starved of FBS for 24–48 h to increase surface expression of CD4.
Reagents
Antibodies used for these studies were FITC-conjugated anti-CD45 (Serotec), phycoerythrin (PE)-conjugated anti-CD4 (Serotec, Raleigh, NC, USA) and -CXCR4 (Pharmingen, San Diego, CA, USA), the 12G5 monoclonal anti-CXCR4 (fusin) (The AIDS Research and Reference Reagent Program, Rockville, MD, USA), FITC-conjugated goat anti-mouse immunoglobulin (BRL), a polyclonal anti-Lck (#974) made to a trpE-Lck fusion protein containing amino acids 2–148, kindly provided by Dr A. Veillette (McGill University, Montreal, Quebec, Canada), a polyclonal anti-Lck that recognizes amino acids 22–51 in the NH2-terminus was purchased from Upstate Biotechnology, Incorporated (UBI, Lake Placid, NY, USA), a monoclonal anti-Lck that recognizes amino acids 1–191 was purchased from Transduction Laboratories (Lexington, KY, USA) and 4G10 monoclonal antiphosphotyrosine (UBI). Interleukin-16 (IL-16) was produced as a recombinant protein from E.coli and was purchased from Research Diagnostics, Inc. (Flanders, NJ, USA). cDNA for the mutant dominant-negative Lck (K293R) [24] was a kind gift of Dr A. Veillette (McGill University).
Virus stock and in vitro infection
HIV-1IIIB was grown in Jurkat cells for 7 days prior to harvesting cell culture supernatants, titrated using gag p24 ELISA assays (Coulter, Miami FL), and aliquots stored at − 70°C. Multiplicity of infection (moi) was calculated as 1·5 virions/cell based on total p24gag levels or as 0·3 infectious virus particles/cell using MT-4 cells as previously described [21]. Aliquots were used immediately after thawing and cells were infected as described previously [13,25].
Fluorescence staining (FACS)
Each cell line used was assessed for CD4, CD45, and CXCR4 expression by standard immunofluorescent analysis. Briefly, 106 cells were suspended in PBS containing 0·2% (v/v) FBS and incubated for 30 min at 4°C with anti-CD4-PE and anti-CD45-FITC, or anti-CXCR4-PE, or anti-CXCR4 followed with goat anti-mouse immunoglobulin-FITC. Cells were then washed, resuspended in washing buffer, and analysed immediately using a FACScan cell analyser (Becton-Dickinson & Co. Mountain View, CA, USA).
Antisense oligodeoxynucleotides and transfections
15 mer phosphorothioate oligodeoxynucleodies (S-oligos) were purchased from the DNA Synthesis Laboratory, University of Calgary, Calgary, Alberta, Canada. These were: antisense sequence complementary to the AUG start codon of human lck (TGCAGCCACAGCCAT). The sense human lck sequence (ATGGGCTGTGGCTGC) and a scrambled S-oligo sequence (TCTTTACCCTTAGGC) were used as controls. Jurkat (106 cells) were incubated with S-oligos overnight prior to infection. After 2 h of infection with HIV-1IIIB (moi, 0·3 or 1·5), the cells were washed and then cultured in the presence of fresh S-oligos which were replenished daily until sample collection. Transfection of K293R kinase-dead Lck was performed with 8 µg DNA of plasmid pcDNA3 containing the full length K293R insert and 5 × 106 Jurkat cells by electroporation [25]. Transfectants were selected in the presence of 1 mg/ml Geniticin (G418, Gibco/Invitrogen Canada Inc., Burlington, ON, Canada) and tested by Western immunoblot for stable-expression of mutant Lck protein.
Metabolic labelling
Metabolic labelling of newly synthesized proteins was performed essentially as previously described (26). Briefly, after overnight incubation with S-oligos, 5 × 106 Jurkat cells were washed and resuspended in serum-, methionine- and cysteine-free medium (RPMI-1640 selectamine kit, Gibco/Invitrogen) containing 100 µCi/ml of each[35S]-methionine (ICN Biomedicals;>600 Ci/mmol) and[35S]-cysteine (ICN Biomedicals;>1000 Ci/mmol) and incubated for 3 h at 37°C in 5% CO2. The cells were washed and lysed in radioimmuno-precipitation assay (RIPA) buffer (1% Nonident NP-40, 0·1% SDS, 0·1% sodium deoxycholate, 50 mmol/l HEPES, pH 7·3, 150 mmol/l NaCl, 2 mmol/l sodium orthovanadate, 50 µmol/l ZnCl2, 2 mmol/l EDTA, 2 mmol/l PMSF) and the Lck protein immunoprecipitated with a polyclonal anti-Lck antibody. After separation of metabolically labelled Lck on SDS-PAGE, the gel was fixed for 15 min in 7% acetic acid and then soaked in an enhancement solution (Amplify, Amersham Bioscience Inc., Baie d’Urfe, PQ, Canada). After drying, the protein bands were identified by autofluorography.
Immunoprecipitation and immunoblotting
Cells were lysed in RIPA buffer as previously described [26]. After 10 min incubation on ice, damaged nuclei, any unlysed cells and debri were removed by centrifugation at 4°C for 10 min at 14 000× g, and the total protein of each lysate measured by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Total cell lysates were boiled in reduced SDS-sample buffer for 10 min or immunprecipitated prior to SDS-PAGE. Immunoprecipitation was done using previously prepared immune-complexes of polyclonal anti-Lck and Protein A-Sepharose beads (Amersham Bioscience Inc.) as previously described [26]. Proteins were separated by denaturing SDS-PAGE and transferred to nitrocellulose membranes. Western immunoblots were performed as described previously [26]. For Lck protein detection, membranes were blocked overnight in 5% (w/v) nonfat skimmed milk. For phosphotyrosine (ptyr)-containing proteins, membranes were blocked in 5% (w/v) bovine serum albumin (Boehringer Mannheim). After blocking, the membranes were incubated with monoclonal anti-Lck or -ptyr followed with the appropriate secondary antibody and subjected to enhanced chemiluminescence (ECL, Amersham Bioscience Inc.) or, first incubated with rabbit anti-mouse immunoglobulin (from Dr Rob Sutherland, The Toronto Hospital, Toronto, Ontario, Canada) followed by incubation with 125I-Protein A (Amersham Bioscience Inc.) and detection by autoradiography.
In vitro kinase assay
In vitro immune-complex kinase assays were performed as previously described [27]. Briefly, equal cell numbers were centrifuged, pellets lysed in RIPA lysis buffer, and immunoprecipitated with polyclonal anti-Lck. Immunoprecipitates were washed free of unbound protein using kinase buffer (50 mmol/. HEPES, pH 7·23, 150 mmol/l NaCl, 1 mmol/l MgCl2, 1 mmol/l MnCl2 and 0·5% NP-40) and incubated with 10 µCi γ32P-ATP (specific activity, 4500 Ci/mmol; ICN Biomedicals, Irvine, CA, USA) in kinase buffer. Acid-treated rabbit muscle enolase (Boerhringer Mannheim) was added (2 µg/test) as an exogenous substrate as described previously [28]. Proteins were separated by denaturing SDS-PAGE and transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA, USA) which were then analysed after autoradiography.
Viral entry
Viral entry levels were determined by semiquantitative measurement of gag p24 of entry virions as described previously [21]. Briefly, 106 cells were incubated on ice with HIV-1IIIB for 30 min, then incubated at 37°C for 90 min, washed twice and incubated for 2 min on ice in 200 µl of complete culture medium at pH 3·0 to remove virions that had not entered cells. Cells were washed twice with cold PBS and suspended in 200 µl fresh complete medium and a gag p24 antigen ELISA assay performed according to the manufacturer's directions. The incubation period at 37°C was omitted for controls.
2-LTR circle analysis
The 2-LTR circles were assayed as previously described using a nested PCR [25]. Two million cells were infected with HIV-1IIIB for 2 h, washed, and cultured for 8 and 24 h. The cells were pelleted and suspended in 50 µl of solution A (10 mm Tris-HCl, pH 8·3 and 100 mm KCl) and lysed by addition of 50 µl of solution B (10 mm Tris-HCl, pH 8·3, 1% Tween 20, and 1% Nonidet P-40) containing 25 µg of proteinase K, followed by 60 min incubation at 60°C. The lysates were then boiled for 30 min, and 2 µl amplified in 25 µl reaction mixture containing 10 mm Tris-HCl, pH 8·3, 50 mm KCl, 2·5 mm MgCl2, 0·2 mm each dATP, dCTP, dGTP, dTTP, 1 µm each of forward (M667) and reverse (U32) primers [29], and 2·5 units Taq polymerase. After an initial 5 min at 95°C in a thermocycler, the target sequence was amplified by 20 cycles of 94°C for 60 s, 65°C− 45°C (with 1°C drop per cycle) for 45 s, and 72°C for 60 s; 10 cycles of 94°C for 60 s, 45°C for 45 s, and 72°C for 60 s; and final 7 min at 72°C. Four µl PCR products were further amplified under the same amplification conditions except that dTTP was replaced by 0·19 mm dTTP plus 0·01 mm 11-digoxygenin-dUTP, the two primers replaced by 1 µm each of U5–2LTR and U3–2LTR [29], and the previous amplification cycles replaced by 25 cycles of 94°C for 60 s, 55°C for 45 s and 72°C for 60 s. A portion of the PCR product was analysed on a 2% agarose gel containing ethidium bromide, and photographed. To estimate the 2-LTR-derived product, a 0·5-µl aliquot of PCR product was hybridized with a 5′-biotinylated AA55 probe [30], then captured in microwells and assayed colourimetrically after reaction with enzyme linked-antidigoxygenin antibody followed by enzyme substrate, according to the manufacturer's (Boerhinger-Mannheim) directions. The amount of 2-LTR-derived PCR product, up to an optical density of 0·7, was linearly proportional to the input volume of a 2-LTR containing template preparations (r > 0·95). To normalize 2-LTR levels with HIV-1 entry into cells, the amount of total HIV was determined by amplification of a region of gag using SK38/SK39 primer pairs (30) under conditions described above for the second PCR (the nested PCR), except that amplification was achieved by 40 cycles of 94°C for 60 s and 45°C for 60 s. A portion of the PCR product was analysed on a 2% agarose gel and gag-specific product quantified by hybridization with 5′-biotinylated SK19 [31] probe, as described above. A parallel assay using 8E5 cells that contain one HIV provirus per cell [32], showed a linear proportionality between the copies of HIV provirus template and the amount of gag-specific PCR product (r > 0·95).
RESULTS
Differential activity of Lck kinase in Jurkat-derived cell lines and its modulation during HIV-1 infection
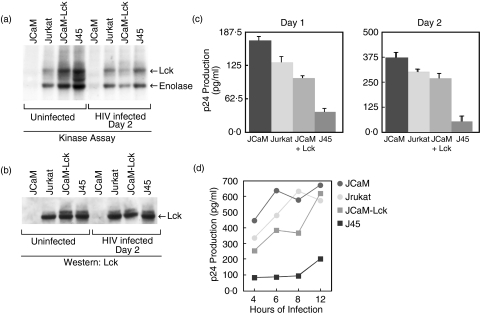
To define the role of Lck kinase in HIV infection, a human T cell line, and mutants of this cell line, that differ in their enzymatic activity of Lck protein were selected. Jurkat cells express a functionally active wild-type Lck protein and are considered a model cell line for the study of T cell signalling pathways [3]. The Jurkat-derived mutant cell line JCaM has an approximately 150 bp deletion within exon 7 of the lck kinase domain. This deletion leads to mRNA that translates to give a truncated, approximately 51 kDa, form of Lck that is kinase inactive [3]. JCaM-Lck cells are JCaM cells that are transfected with wild-type Lck cDNA and express levels of Lck kinase activity similar to that of the parent Jurkat cells [3]. The Jurkat-derived mutant J45 has greatly depressed expression of the cell surface CD45 protein tyrosine phosphatase [22]. Reduced CD45 expression has been shown to result in hyperphosphorylation [33] and increased kinase activity [34–38] of endogenous Lck compared to parent cells. Figure 1a,b shows, respectively, Lck kinase activities and Lck protein levels present in these cell lines. As previously reported [3], there was no detectable Lck kinase activity, or 56 kDa Lck protein, in uninfected JCaM cells. Slightly higher Lck kinase activity was evident in JCaM-Lck cells compared to parental Jurkat cells. As expected [33], the J45 cell line showed the highest Lck kinase activity. After infection with HIV-1, the kinase activity of Lck in both JcaM-Lck and J45 cells was substantially reduced without a change in protein levels.
Fig. 1.
Lck kinase activity inversely correlates with HIV replication. Jurkat T cells or Jurkat-derived JCaM, J45, or JCaM transfected with wild-type lck cDNA (JCaM-LCK) were infected with HIV-1IIIB (moi 1·5) and cell cultures assayed for p24 antigen. (a) The level of kinase activity of the various Jurkat-derived cell lines using an in vitro kinase assay of uninfected cells and HIV infected cells after 2 days. Equal amounts of cell equivalents were immunoprecipitated from cellular lysates using anti-Lck. The immunocomplexes were incubated with [γ32P]ATP, the proteins fractionated by SDS-PAGE, electrotransferred to Immobilon-P membranes, and the radiolabelled proteins were visualized by autoradiography. Results show both autophosphorylation of Lck and phosphorylation of an exogenous substrate, rabbit muscle enolase. These results are representative of three independent experiments. (b) The Lck protein level of the various Jurkat-derived cell lines, uninfected and after HIV infection for 2 days, determined from total cellular lysates from (a), using Western immunoblotting with anti-Lck and ECL. Equal loading of total protein was based on equal cell equivalents for each lysate. These results are representative of three independent experiments. (c) The relative level of viral p24 antigen determined by ELISA for each Jurkat-derived cell line infected with HIV-1IIIB after 1 or 2 days of infection. Cells (106/ml) were infected with HIV-1IIIB (moi 1·5) for 2 h, washed, and cultured for the indicated days prior to gag p24 measurement. Results represent the mean p24 antigen level ± SEM of triplicate cultures. These results are representative of at least five independent experiments. (d) p24 antigen production from the various Jurkat-derived cell lines after varying the length of time of initial HIV-1IIIB infection, from 4 to 12 h. Results represent the average p24 antigen level of duplicate cultures. The results shown are for 2 days after initial HIV-1 infection.
Lck kinase activity inversely correlates with viral replication
To examine the affect of Lck kinase activity on HIV infection, we determined the level of viral replication in the various Jurkat-derived cell lines following infection with HIV-1IIIB. As shown in Fig. 1c, the level of virus production inversely correlated with increasing Lck kinase activity of the cell lines (see Fig. 1a). Thus, the highest p24 antigen levels were found for JCaM cells and the lowest p24 antigen production found for J45 cells. While the differences in virus production between JCaM and J45 cell lines remained largely unchanged, the differences between JCaM, Jurkat and JCaM-Lck cell lines were slightly more pronounced on day 1 of infection than on the second day of infection. The latter may be related to the fact that, after 2 days of infection, the differences in Lck kinase activity are much less evident among the various cell lines (see Fig. 1a). The apparent continued lack of productive infection of J45 cells at day 2 even though Lck kinase activity is similar to Jurkat and JCaM-Lck may be related to its substantially higher initial Lck activity compared to the other cell lines or, possibly, it is due to other factors. However, by day 3 or day 4 of culture, p24 antigen levels were similar for all cell lines, including J45 (data not shown). This indicates that although the early stages of HIV infection are affected by the initial activity of Lck kinase, the kinase activity and, thus, any inhibitory effect of Lck, is reduced or eliminated over time of infection. This hypothesis is supported by a substantial reduction in the kinase activity observed at day two of infection (Fig. 1a) without a reduction in the amount of Lck protein (Fig. 1b)
As shown in Fig. 1d, the inverse correlation of Lck kinase activity to viral replication was independent of the time of initial exposure to the HIV although, with prolonged exposure to virus, J45 cells showed a slightly higher productive infection but continued to be significantly delayed compared to the other cell lines after 2 days of infection.
Taken together, these data suggest that the initial level of endogenous Lck kinase activity may serve to inhibit some aspect of the HIV-1 life cycle, and that, eventually, the virus, or a protein encoded by its genome, such as Nef [39,40], may target Lck to diminish its kinase activity in order to facilitate the infection.
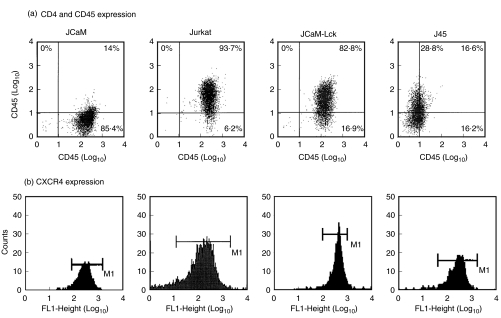
A difference in viral replication is not due to differences in HIV receptor expression
Because infection with the T cell-tropic HIV-1IIIB requires both CD4 and CXCR4 on the surface of T cells [9,11], we examined the expression of these receptors in the various Jurkat-derived T cell lines. As shown in Fig. 2, while Jurkat, JCaM-Lck, and J45 T cell lines expressed both CD4 and CXCR4 at similar levels, the JCaM cell line expressed very low levels of CD4 but similar levels of CXCR4 as compared to the other Jurkat-derived cells. It is therefore, exceptional that despite having a very low level of cell-surface expression of CD4, this did not impede viral entry (see below) and, in fact, JCaM cells were infected to the highest level.
Fig. 2.
FACS analysis of CD45 and HIV cell surface receptors. (a) Two colour FACS analysis for CD45 and CD4 cell surface antigens of the various Jurkat-derived cell lines. (b) FACS analysis for CXCR4 α-chemokine HIV coreceptor of the various Jurkat-derived cell lines.
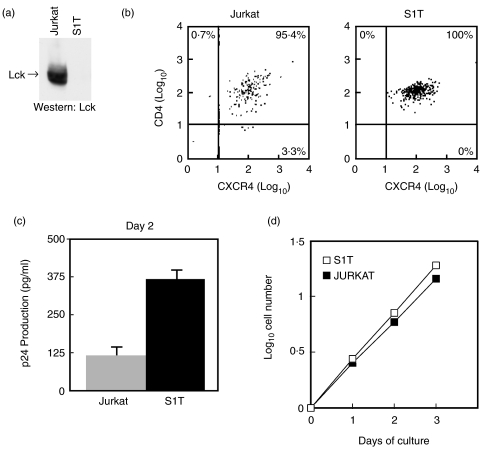
Absence of Lck kinase facilitates HIV-1 infection
To further confirm that low or absent Lck kinase is responsible for the increased rate of infection, we used another T cell line, S1T, that lacks both lck mRNA and protein [23] (Fig. 3a) but expresses both CD4 and CXCR4 at levels similar to Jurkat T cells (Fig. 3b). As shown in Fig. 3c, after 2 days of infection with HIV-1, S1T cells produced an approximate 3-fold higher level of viral replication than did Jurkat cells. However, this relative difference in virus production, unlike that shown in Fig. 1c, was maintained or even increased over consecutive days of infection [data not shown and Fig. 8]. S1T cells are derived from a patient infected with HTLV-I [23,41], however, production of tax protein that may enhance HIV infection cannot explain the higher viral replication in these cells as this cell line does not produce the HTLV-I tax protein [41]. Moreover, the increased infection of S1T compared to Jurkat was not accounted for by differences in the rate of proliferation of these cells over the short culture time used to monitor infection since their population doubling times, calculated from log-phase growing cultures as previously described [42] (Fig. 3d), were 19·7 h for Jurkat cells and 17·3 h for S1T cells.
Fig. 3.
Lack of Lck kinase facilitates HIV productive infection. (a) The S1T human T cell line lacks Lck protein compared with Jurkat T cells. 2 × 107 Jurkat and S1T T cells were lysed, immunoprecipitated with anti-Lck, the proteins separated by SDS-PAGE and electrotransferred to nitrocellulose membranes. The membrane was blocked with 5% skim-milk and Lck protein identified by Western immunoblot with anti-Lck using ECL readout. (b) Two-colour FACS analysis of cell surface CD4 and CXCR4 using Jurkat and S1T human T cell lines. (c) Relative viral p24 antigen production from Jurkat and S1T cells determined after 2 days following infection with HIV-1IIIB (moi, 1·5). The amount of entry virus was determined to be 157 and 151 pg of p24gag, respectively (Table 1). Results represent the mean p24 antigen level ± SEM of triplicate cultures. These results are representative of two independent experiments. (d) Exponential increase in cell number by Jurkat and S1T T cell lines over time in culture. Cells were seeded at 2 × 105 cells/ml and aliquots withdrawn and cells counted over the indicated days of culture. Population doubling times were calculated from the log-phase growing cultures.
Fig. 8.
Inhibition of HIV-1 infection by IL-16 requires Lck kinase. (a) S1T cells (5 × 105) were untreated or pretreated with 5 × 10−9M human recombinant IL-16 for 4 h prior to infection with HIV-1IIIB (moi, 0·3). HIV-1 replication was measured by gag p24 ELISA after 2 days of culture. Results represent the mean p24 antigen level ± SEM of triplicate cultures. P= n.s. (b) In parallel with S1T cells above, Jurkat cells (5 × 105) were untreated or pretreated with 5 × 10−9 M human recombinant IL-16 for 4 h prior to infection with HIV-1IIIB (moi, 0·3). HIV-1 replication was measured by gag p24 ELISA after 2 days of culture. Results represent the mean p24 antigen level ± SEM of triplicate cultures. P-value was determined using a paired Student's t-test.
Lck kinase activity does not modulate virus entry
We determined the amount of entry virions by measuring intracellular p24 antigen levels after 30 min exposure of cells to HIV-1IIIB on ice followed by 90 min incubation at 37°C [21]. Although there was great variation in the constitutive activity of Lck and the ability of these cell lines to become productively infected (Fig. 1a,c), this did not correlate with the ability of the virus to enter the cell. Results in Table 1 show that the levels of p24 antigen detected within the different cell lines after initial exposure to virions were comparable with the exception of JCaM. Indeed, JCaM cells showed the lowest viral entry, presumably due to their low CD4 expression; however, in spite of the lowest levels of viral entry, these cells exhibited the highest levels of HIV replication (Fig. 1c,d).
Table 1.
Determination of initial viral entry using measurement of p24 antigen*
| Cell line | p24 antigen level (pg/ml)† |
|---|---|
| Jurkat | 157 |
| JCaM | 97 |
| J45 | 138 |
| S1T | 151 |
See Methods[21].
Results were similar in two experiments and represent the value obtained at 37°C minus the control result.
Lck kinase activity inhibits neither reverse transcription of HIV-1 nor 2-LTR circles
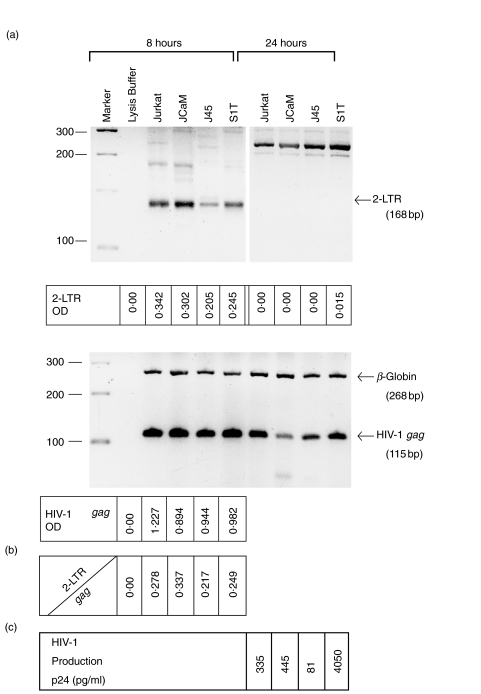
After the completion of reverse transcription of the HIV genome in the cytoplasm, the newly synthesized linear proviral DNA migrates to the nucleus and, only inside the nucleus, its two terminal ends are joined to give rise to 2-LTR circles [29,43,44]. The appearance of 2-LTR circles is therefore a clear evidence for completion of the proviral DNA synthesis and its nuclear migration. These circles are transient in nature but accumulate at notably greater levels in cells with inhibition of proviral integration than in cells without any such inhibition [29,43]. We therefore quantified 2-LTR circles and the total proviral DNA in cells that were assayed for viral entry (in Table 1), after 8 and 24 h of infection with HIV-1, and the values of 2-LTR circles were normalized to the total proviral DNA in the cells. Results in Fig. 4a show that all cell lines contained appreciable evels of 2-LTR circles at 8, but not 24, hours after the initial infection, and that their total proviral DNA contents at 8 h were consistent with results of viral entry shown in Table 1. The ratios of 2-LTR circles to total proviral DNA in the cell lines ranged from 0·217 to 0·337 and showed no correlation with their Lck kinase activity or productive HIV infection (Fig. 4b,c). Again, it can be seen that viral replication in S1T cells lacking Lck is increased (>12-fold) compared to Jurkat cells (Fig. 4c).
Fig. 4.
PCR analysis of 2-LTR circles and viral gag DNA. (a) The indicated cell lines (2 × 106 cells) were infected with HIV-1IIIB (moi 1·5). After 8 or 24 h of infection, total cellular DNA was prepared from cells by a quick lysis procedure [29]. The DNA was amplified by PCR using specific primers as described in the Methods and 2-LTR circles and gag DNA determined and quantified. The upper panel are the results from 2-LTR circle determinations. The position of the 2-LTR products (168 bp) are indicated by an arrow. The lower panel are the results from the gag PCR. The position of the HIV-1 gag (115 bp) and the beta-globin (268 bp) control are indicated by arrows. The 2-LTR and HIV-1 gag optical density (OD) were quantified using a biotinylated AA55 probe [31] and enzyme-linked antidigoxygenin (see Methods). (b) The ratio of 2-LTR circles to HIV-1 gag optical density for each cell line. (c) The level of HIV-1 replication after 2 days of infection for each cell line as determined by measurement of gag p24 antigen production using ELISA.
Blocking the synthesis of Lck in Jurkat cells causes accelerated viral replication
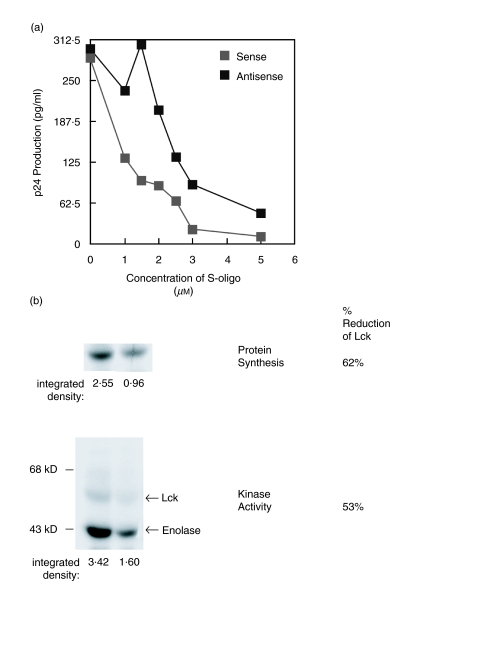
To further examine the inhibitory effect of Lck on productive HIV infection, we inhibited the synthesis of Lck protein in cells by treatment with an antisense oligodeoxynucleotide specific to Lck and monitored its effect on HIV replication. As shown in Fig. 5a, Jurkat T cells pretreated with antisense lck phosphorothioate oligodeoxynucleotides (S-oligos) showed an increase in viral replication compared to a sense S-oligo control sequence over a wide concentration range (1–5 µm). A scrambled S-oligo sequence that was base-matched to the antisense sequence also failed to increase the viral replication compared to the antisense S-oligo [data not shown]. The increase in viral replication observed with antisense lck S-oligo is even more remarkable considering that phosphorothioate oligodeoxynucleotides themselves are known to inhibit HIV infection [45,46], presumably due to inhibition of the viral reverse transcriptase [45]. This may explain why we did not observe more dramatic differences and why the optimal window for the antisense affect is narrow, over 1–2 µm; at higher concentrations, the S-oligos both inhibit nonspecifically. Indeed, at 1·5 µm of the antisense lck S-oligo, the increase in viral replication appears maximal, approximately 5-fold higher compared to control (Fig. 5a).
Fig. 5.
Blocking Lck protein synthesis and kinase activity results in accelerated viral replication. (a) Jurkat T cells (106/ml) were untreated or incubated with 1–5 µm phosphorothioate oligodeoxynucleotide 15mer antisense-lck or sense-lck sequences 12–16 h prior to infection with HIV-1IIIB (moi 1·5). Viral gag p24 antigen production was then measured by ELISA after 2 days of culture. Results represent the average of duplicate cultures. These results are representative of at least three independent experiments. (b) The level of newly synthesized Lck protein and Lck kinase activity following treatment with antisense-lck or sense-lck 15mer phosphorothioate oligodeoxynucleotide sequences as in A, above. Newly synthesized Lck was assessed by specific immunoprecipitation using anti-Lck after metabolic labelling with [35S]-labelled amino acids. The [35S]-labelled Lck was isolated by SDS-PAGE and the protein visualized by autofluorography. The kinase activity of total cellular Lck was assessed by in vitro kinase assay using rabbit muscle enolase as an exogenous substrate (see). The percentage reduction in newly synthesized Lck protein and total cellular Lck kinase activity of the antisense compared to the sense treated cells was determined after densitometry analysis of the autofluorograph and autoradiograph, respectively.
The treatment of Jurkat cells with 1·5 µm antisense lck S-oligo led to only partial inhibition of both the synthesis of Lck protein (by 62%) and the Lck kinase activity (by 53%) relative to the control cells treated with sense lck S-oligo (Fig. 5b). These data, while supporting an inhibitory effect of the Lck kinase activity on HIV replication, show that even partial inhibition of the kinase activity is sufficient for a noticeable increase in viral replication.
Expression of a dominant-negative Lck results in markedly increased viral replication
To further support our antisense DNA experiments, we expressed a dominant-negative Lck in Jurkat cells by transfecting into these cells a mouse Lck that had been point mutated within its ATP binding site resulting in an Lck that is kinase-inactive and has a dominant-negative affect [24,47]. Transfected Jurkat cells were shown to over-express this dominant-negative form of Lck (Fig. 6a) and these cells were infected by HIV-1 to a> 3-fold higher level than were untransfected Jurkat cells or Jurkat transfected to contain only the empty-vector (Fig. 6b).
Fig. 6.
Jurkat cells transfected to express a dominant-negative Lck show facilitation of HIV-1 infection. (a) Jurkat cells (106/ml) transfected with empty pcDNA3 vector (E) or vector containing Lck mutated at lysine 273 to arginine (K273R) were shown to over-express K273R mutant by Western immunoblot. (b) Cells (5 × 105, Jurkat (untransfected), or transfected with empty vector (E) or mutant (K273R)) were infected with HIV-1IIIB (moi, 0·3) and HIV replication measured by gag p24 ELISA after 2 days of culture. Results represent the mean p24 antigen level ± SEM of triplicate cultures. P-value was determined using a paired Student's t-test.
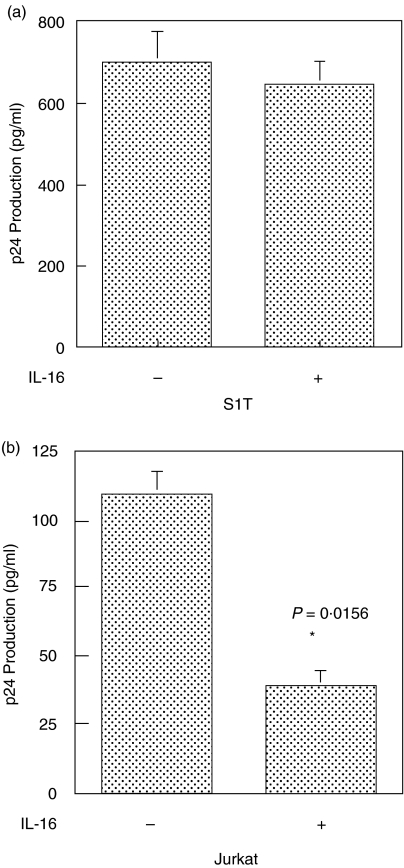
IL-16 stimulation of Jurkat T cells results in an immediate and sustained increase in Lck kinase activity that correlates with a delay of HIV infection
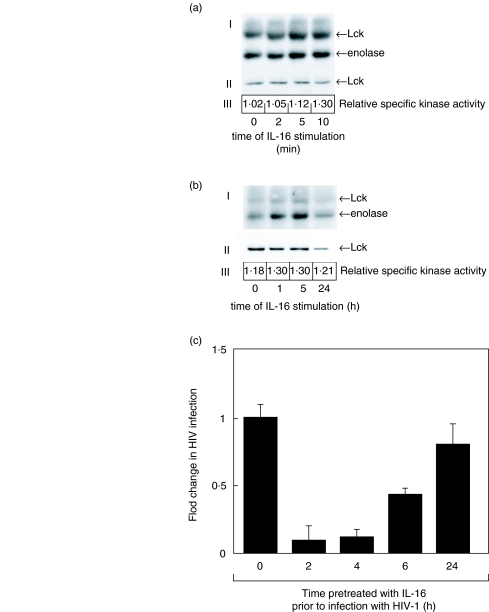
Since decreasing the Lck kinase activity in Jurkat T cells results in increased viral replication (Figs 5 and 6), it predicts that an increase in the Lck kinase activity would have a negative affect on viral replication. To test this, we stimulated the Jurkat T cells with IL-16, a cytokine previously shown to associate with CD4 and increase the kinase activity of Lck [48,49]. As shown in Fig. 7a, incubation of Jurkat T cells with 5 × 10−9M recombinant IL-16 results in an immediate (within 5 min) increase in Lck kinase activity as indicated by the increased autophosphorylation of Lck as well as the increased tyrosine phosphorylation of an exogenous Lck substrate, rabbit muscle enolase (Fig. 7a,b). A single treatment with the IL-16 also led to a slow but continual increase of the Lck kinase activity over a period of at least 5 h (Fig. 7b). To test whether IL-16 inhibition of HIV infection may, at least in part, be mediated through its effect on Lck kinase activity, we next treated the Jurkat cells with IL-16 for various times prior to infection with HIV-1IIIB and monitored the virus production. Our results (Fig. 7c) show that IL-16 can inhibit HIV-1 production in Jurkat T cells up to 90% but only when the cells are exposed to the cytokine 2–4 h prior to initiating the infection. After 6 h of pretreatment with IL-16, the virus production is inhibited by 60%; however, if Jurkat cells were treated with IL-16 for 24 h prior to infection, the virus replication was inhibited by only 20%. These results suggest that the IL-16 inhibition of HIV infection is dependent on the length of IL-16 stimulation of cells prior to infection, and parallels the IL-16-mediated increase in the Lck kinase activity in the cell. These data also confirm our previous findings using a loss of function approach (Figs 5 and 6), that relatively small changes in Lck kinase activity can have significant affects on viral replication.
Fig. 7.
IL-16 stimulation results in a sustained increase in Lck kinase activity that correlates with inhibition of HIV-1 infection. (a) In vitro kinase assay (I) and Western immunoblot (II) of Lck protein following stimulation of Jurkat T cells (106/ml) with 5 × 10−9m human recombinant IL-16 for 2–10 min Arrows indicate location of Lck and enolase. The relative specific kinase activity of Lck (III) was determined as previously described [14] as the ratio of the optical density of the enolase band measured from the kinase experiment to the optical density of the Lck protein band measured from the Western immunoblot. (b) In vitro kinase assay (I) and Western immunoblot (II) of Lck protein following stimulation of Jurkat T cells (106/ml) with 5 × 10−9M human recombinant IL-16 for 1–24 h. Arrows indicate the position of Lck and enolase. The relative specific kinase activity (III) of Lck was determined as described above. These results are representative of at least three independent experiments. C. HIV-1 viral replication as measured by gag p24 ELISA following IL-16 treatment of Jurkat T cells. Jurkat cells (106/ml) were untreated or pretreated with 5 × 10−9M human recombinant IL-16 for the indicated times prior to infection with HIV-1IIIB (moi 0·3). Results represent the mean p24 antigen level ± SEM of triplicate cultures. These results are representative of at least three independent experiments.
To insure that the IL-16 affect is on the Lck kinase, we used S1T cells that do not express Lck (Fig. 3a). Figure 8 shows that while HIV-1 infection of Jurkat cells expressing Lck can be significantly inhibited with IL-16 after a 4-h pretreatment, infection of S1T cells is unaffected. IL-16 also had no affect on the infection of JCaM cells (data not shown). These results are entirely consistent with an inverse relationship between the Lck kinase activity and HIV replication in the cell and confirm previous work that has shown IL-16 to inhibit HIV infection [50,51] in a manner that requires CD4-mediated signalling [52].
DISCUSSION
It was suggested in previous studies that Lck can influence certain outcomes of HIV-1 infection of T cells, including apoptosis [20], syncytium formation [53], and productive infection [18,21]. However, these studies [18,20,21] did not examine the Lck enzyme but, rather, utilized studies of mutant CD4 molecules that could not associate with Lck to conclude their findings. To address the issue of the role that Lck itself may play in the initial stages of HIV-1 infection, we used different cell lines, including a series derived from the same Jurkat parent, that express varying levels of Lck kinase activity. In addition, we utilized strategies to inhibit or activate Lck within the same cell line. This approach has allowed for a direct assessment of the potential role of Lck in productive HIV-1 infection.
We determined that the initial, constitutive level of Lck activity correlated with an ability to resist productive HIV-1 infection. Indeed, the higher the enzyme activity of Lck, the greater the affect on viral replication. This inverse affect on HIV infection depending on the initial constitutive activity of Lck appears to only delay productive infection. However, as viral replication increased, this correlated with diminishing Lck kinase activity (Fig. 1a), without any marked change in Lck protein levels (Fig. 1b), suggesting that diminished Lck activity may be important for optimal viral replication supporting the notion of Lck kinase activity providing some resistance to viral replication [54].
It is known that when T cells are activated, their Lck kinase activity increases [21] and that in order for primary cells to be productively infected with HIV in vitro, there must be mitogenic activation [55]. Based on the results reported herein, this would predict some resistance to HIV infection; however, mitogenic activated human primary T cells easily replicate virus [55]. To reconcile this, previously our laboratory has reported that primary T cells that are activated with PHA and IL-2 and express high Lck kinase activity when treated with either gp120 or a gp120 analogue, Peptide T, down-regulate Lck kinase activity to almost undetectable levels while up-regulating the kinase activity of other tyrosine kinases [12]. This is consistent with the idea that the virus requires a low or absent level of Lck kinase activity to optimize its infection. Indeed, the HIV-1 Nef protein has been shown to be a critical protein required for viral replication and data has suggested that the association of Nef and Lck is central to the enhancement of viral replication [56,57]. Nef binds Lck through specific protein–protein interaction motifs and has been shown to decrease its kinase activity causing impairment of both proximal and distal Lck-mediated signalling events [39,40,57]. Thus, the production of Nef protein may be a response by the virus to insure that Lck kinase activity is down-regulated and remains low [56,57]. This is the most intriguing explanation for the global decrease in Lck kinase activity without a change in the level of protein seen in our studies after 2 days of infection (Fig. 1a,b). Our results may also apply to HIV infection in other physiological situations. Indeed, it is well known that CD4-negative cells that lack Lck can be infected by HIV [58,59]. This may indicate some natural selection process resulting in the virus able to utilize cell receptors other than CD4, such as the recently reported VPAC1 [25], to avoid activation of Lck.
Our studies support the previous conclusions of Tremblay et al. [21] who showed that CD4 constructs either lacking a cytoplasmic domain or mutated to not allow association of Lck to the cytoplasmic domain resulted in facilitation of viral replication. However, in contrast, Benikrane et al. [18], using a similar approach, showed the opposite result on viral replication. These two opposing studies are difficult to reconcile, however, Tremblay et al. showed similar results with two different virus strains, HIV-1IIIB and HIV-1SF, using two different cell line targets (A2·01 and HSB-2); whereas, Benikrane et al. [18] used only the A2·01 cell line and a single virus strain, HIV-1LAI. Our studies that support the work of Tremblay et al. [21] used the same IIIB virus but different cells (Jurkat). It is possible that the LAI virus envelope is substantially different from IIIB and SF and that it would induce different signalling in the target cells. For example, perhaps the LAI virus activates other src-family tyrosine kinases, or signalling pathways, that are redundant for the Lck signal.
In our studies, the initial level of kinase activity of Lck appears to be the single factor responsible for differences in the early stages of viral replication. This is most evident when using Jurkat cells where the constitutive activity of Lck is modulated up or down resulting in, respectively, decreased or increased viral replication. Differences in viral replication was independent of viral entry and was not attributed to a differential HIV reverse transcription or migration of proviral DNA into nuclei (Table 1 and Fig. 4). The loss of 2-LTR circles after 24 h of infection and subsequent virus production indicates successful proviral integration in each cell line. Furthermore, the lowest ratio of 2-LTR circles to proviral DNA observed in J45 cells, a dividing cell line showing the highest Lck kinase activity, is indicative of a lack of inhibition of proviral integration relative to the other cell lines. This suggests that the lowest viral replication found in these cells could not be due to reduced proviral integration. Taken together, these data indicate that Lck kinase activity delays HIV viral replication by affecting the virus life cycle at a postintegration step. This is also a proposed mechanism to explain the IL-16 effect on HIV replication [52].
We used IL-16 to increase the Lck kinase activity [48,49] because we wanted to sustain an increase in enzymatic activity as opposed to a rapid, but transient, increase in Lck such as occurs with normal T cell activation [7,8] or crosslinking of the CD4 [21]. Indeed, our results with J45 cells and the IL-16 experiments may indicate that the Lck kinase activity need be raised and sustained for a period of time prior to infection for maximal delay in HIV infection. This suggests that some downstream event tied to the Lck kinase activation [60–62] or the induction of another cellular factor, previously suggested [50] for the mechanism of the IL-16 affect on HIV-1 infection, is responsible for the inhibitory effects on viral replication. Preliminary results in our laboratory indicate that IL-16 may affect the association between Lck and the SHP-1 protein tyrosine phosphatase (data not shown). Results obtained from experiments in the motheaten mouse that lacks functional SHP-1 showed a sustained increase in Lck kinase activity and that one function of SHP-1 is to negatively regulate the activity of Lck [62]]. Our unpublished results show that following IL-16 treatment, we observe a dissociation of SHP-1 from Lck and this loss of negative regulation by SHP-1 may be the mechanism by which IL-16 results in its sustained increase in Lck kinase activity.
Identification of host genetic factors that may provide resistance to infection with HIV is critically important [63] to defining potentially novel targets for therapeutic intervention that may influence the infectivity of this virus. In the work reported herein, we have identified the tyrosine kinase, p56lck (Lck), as a potentially important cellular protein that can provide some resistance to productive HIV-1 infection. A high initial Lck kinase activity prior to infection with HIV-1, or a sustained elevation of its kinase activity, was found to inversely correlate with productive HIV-1 infection. Thus, our work demonstrates that Lck kinase plays an important role in regulating productive infection with HIV-1, as previously suggested by other workers [18–21]. Our results also support the previous conclusions of Tremblay et al. [21] of a facilitating effect on HIV replication when Lck is decreased or absent.
Acknowledgments
The authors thank Drs G.B. Mills and A. Veillette for cells and reagents and Drs H-U. Simon and D.J. Phipps for critical review of the manuscript. Dr S. Yousefi is a recipient of a Natural Sciences and Engineering Research Council (NSERC) of Canada Fellowship award. This work was supported in part by grants from the National Health Research and Development Program (NHRDP) – Health Canada, the Canadian Foundation for AIDS Research (CANFAR), and the Ontario Ministry of Health, AIDS Bureau.
REFERENCES
- 1.Pinching AJ, Nye KE. Defective signal transduction – a common pathway for cellular dysfunction in HIV infection? Immunol Today. 1990;11:256–9. doi: 10.1016/0167-5699(90)90100-n. [DOI] [PubMed] [Google Scholar]
- 2.Broder CC, Berger EA. CD4 molecules with a diversity of mutations encompassing the CDR3 region efficiently support human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion. J Virol. 1993;67:913–26. doi: 10.1128/jvi.67.2.913-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–93. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 4.Glaichenhaus N, Shastri N, Littman DR, Turner JM. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991;64:511–20. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- 5.Horak ID, Gress RE, Lucas PJ, Horak EM, Waldmann TA, Bolen JB. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci USA. 1991;88:1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–65. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 7.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 8.Mustelin T, Burn P. Regulation of src family tyrosine kinases in lymphocytes. Trends Biochem Sci. 1993;18:215–20. doi: 10.1016/0968-0004(93)90192-p. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor. functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 10.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 11.Unutmaz D, Littman DR. Expression pattern of HIV-1 coreceptors on T-cells: implications for viral transmission and lymphocyte homing. Proc Natl Acad Sci USA. 1997;94:1615–8. doi: 10.1073/pnas.94.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipps DJ, Reed-Doob P, Macfadden DK, Piovesan JP, Mills GB, Branch DR. An octapeptide analogue of HIV gp120 modulates protein tyrosine kinase activity in activated peripheral blood T lymphocytes. Clin Exp Immunol. 1995;100:412–8. doi: 10.1111/j.1365-2249.1995.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phipps DJ, Read SE, Piovesan JP, Mills GB, Branch DR. HIV infection in vitro enhances the activity of src-family protein tyrosine kinases. AIDS. 1996;10:1191–8. doi: 10.1097/00002030-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Phipps DJ, Yousefi S, Branch DR. Increased enzymatic activity of the T-cell antigen receptor-associated fyn protein tyrosine kinase in asymptomatic patients infected with the human immunodeficiency virus. Blood. 1997;90:3603–12. [PubMed] [Google Scholar]
- 15.Cohen DI, Tani Y, Tian H, Boone E, Samelson LE, Lane HC. Participation of tyrosine phosphorylation in the cytopathic effect of human immunodeficiency virus-1. Science. 1992;256:542–5. doi: 10.1126/science.1570514. [DOI] [PubMed] [Google Scholar]
- 16.Hivroz C, Mazerolles F, Soula M, Fagard R, Graton S, Meloche S, Sekaly RP, Fischer A. Human immunodeficiency virus gp120 and derived peptides activate protein tyrosine kinase p56lck in human CD4 T lymphocytes. Eur J Immunol. 1993;23:600–7. doi: 10.1002/eji.1830230303. [DOI] [PubMed] [Google Scholar]
- 17.Stefanova I, Saville MW, Peters C, et al. HIV infection-induced posttranslational modification of T cell signaling molecules associated with disease progression. J Clin Invest. 1996;98:1290–7. doi: 10.1172/JCI118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benkirane M, Jeang KT, Devaux C. The cytoplasmic domain of CD4 plays a critical role during the early stages of HIV infection in T-cells. EMBO J. 1994;13:5559–69. doi: 10.1002/j.1460-2075.1994.tb06893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benkirane M, Schmid-Antomarchi H, Littman DR, Hirn M, Rossi B, Devaux C. The cytoplasmic tail of CD4 is required for inhibition of human immunodeficiency virus type 1 replication by antibodies that bind to the immunoglobulin CDR3-like region in domain 1 of CD4. J Virol. 1995;69:6904–10. doi: 10.1128/jvi.69.11.6904-6910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbeil J, Tremblay M, Richman DD. HIV-induced apoptosis requires the CD4 receptor cytoplasmic tail and is accelerated by interaction of CD4 with p56lck. J Exp Med. 1996;183:39–48. doi: 10.1084/jem.183.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay M, Meloche S, Gratton S, Wainberg MA, Sekaly RP. Association of p56lck with the cytoplasmic domain of CD4 modulates HIV-1 expression. EMBO J. 1994;13:774–83. doi: 10.1002/j.1460-2075.1994.tb06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88:2037–41. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills GB, Arima N, May C, Hill M, Schmandt R, Li J, Miyamoto NG, Greene WC. Neither the LCK nor the FYN kinases are obligatory for IL-2-mediated signal transduction in HTLV-I-infected human T cells. Int Immunol. 1992;4:1233–43. doi: 10.1093/intimm/4.11.1233. [DOI] [PubMed] [Google Scholar]
- 24.Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991;350:62–6. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 25.Branch DR, Valenta LJE, Yousefi S, Sakac D, Singla R, Bali M, Sahai BM, Ma XZ. VPAC1 is a cellular neuroendocrine receptor expressed on T cells that actively facilitates productive HIV-1 infection. AIDS. 2002;16:309–19. doi: 10.1097/00002030-200202150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Branch DR, Guilbert LJ. Differential expression of tumor necrosis factor-alpha isoforms from lipopolysaccharide- and cytokine-stimulated mouse macrophages. Int J Biochem Cell Biol. 1996;28:949–55. doi: 10.1016/1357-2725(96)00061-1. [DOI] [PubMed] [Google Scholar]
- 27.Branch DR, Mills GB. pp60c–src expression is induced by activation of normal human T lymphocytes. J Immunol. 1995;154:3678–85. [PubMed] [Google Scholar]
- 28.Cooper JA, Hunter T. Identification and characterization of cellular targets for tyrosine protein kinases. J Biol Chem. 1983;258:1108–15. [PubMed] [Google Scholar]
- 29.Levy-Mintz P, Duan L, Zhang H, et al. Intracellular expression of single-chain variable fragments to inhibit early stages of the viral life cycle by targeting human immunodeficiency virus type 1 integrase. J Virol. 1996;70:8821–32. doi: 10.1128/jvi.70.12.8821-8832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 30.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen ISY. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–22. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 31.Abbott MA, Poiesz BJ, Byrne BC, Kwok S, Sninsky JJ, Ehrlich GD. Enzymatic gene amplification. qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988;158:1158–69. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- 32.Gratzl S, Moroni C, Hirsch HH. Quantification of HIV-1 viral RNA and proviral DNA by isotopic competitive PCR. J Virol Meth. 1997;66:269–82. doi: 10.1016/s0166-0934(97)00064-5. [DOI] [PubMed] [Google Scholar]
- 33.Hurley TR, Hyman R, Sefton BM. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993;13:1651–6. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns CM, Sakaguchi K, Appella E, Ashwell JD. CD45 regulation of tyrosine phosphorylation and enzyme activity of src family kinases. J Biol Chem. 1994;269:13594–600. [PubMed] [Google Scholar]
- 35.Duplay P, Alcover A, Fargeas C, Sekaly RP, Branton PE. An activated epidermal growth factor receptor/Lck chimera restores early T cell receptor-mediated calcium response in a CD45-deficient T cell line. J Biol Chem. 1996;271:17896–902. doi: 10.1074/jbc.271.30.17896. [DOI] [PubMed] [Google Scholar]
- 36.D'Oro U, Sakaguchi K, Appella E, Ashwell JD. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Oro U, Ashwell JD. Cutting edge. the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–83. [PubMed] [Google Scholar]
- 38.Ashwell JD, D'Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–6. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 39.Greenway A, Azad A, Mills J, McPhee D. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J Virol. 1996;70:6701–8. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collette Y, Dutartre H, Benziane A. Ramos-Morales, Benarous R, Harris M, Olive D. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. J Biol Chem. 1996;271:6333–41. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 41.Sakaki Y, Terashi K, Yamaguchi A, et al. Human T-cell lymphotropic virus type I Tax activates lung resistance-related protein expression in leukemic clones established from an adult T-cell leukemia patient. Exp Hematol. 2002;30:340–5. doi: 10.1016/s0301-472x(02)00775-0. [DOI] [PubMed] [Google Scholar]
- 42.Guilbert LJ, Winkler-Lowen B, Smith A, Branch DR, Garcia-Lloret M. Analysis of the synergistic stimulation of mouse macrophage proliferation by macrophage colony-stimulating factor (CSF-1) and tumor necrosis factor alpha (TNF-alpha) J Leuko Biol. 1993;54:65–72. doi: 10.1002/jlb.54.1.65. [DOI] [PubMed] [Google Scholar]
- 43.Bukrinsky M, Sharova N, Stevenson M. Human immunodeficiency virus type 1 2-LTR circles reside in a nucleoprotein complex which is different from the preintegration complex. J Virol. 1993;67:6863–5. doi: 10.1128/jvi.67.11.6863-6865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelman A, Englund G, Orenstein JM, Martin MA, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995;69:2729–36. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsukura M, Shinozuka K, Zon G, Mitsuya H, Reitz M, Cohen JS, Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci USA. 1987;84:7706–10. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein CA, Neckers LM, Nair BC, Mumbauer S, Hoke G, Pal R. Phosphorothioate oligodeoxycytidine interferes with binding of HIV-1 gp120 to CD4. J Acq Immune Def Synd. 1991;4:686–93. [PubMed] [Google Scholar]
- 47.Yang WC, Olive D. Tec kinase is involved in transcriptional regulation of IL-2 and IL-4 in the CD28 pathway. Eur J Immunol. 1999;29:1842–9. doi: 10.1002/(SICI)1521-4141(199906)29:06<1842::AID-IMMU1842>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Center DM, Berman JS, Kornfeld H, Theodore AC, Cruikshank WW. The lymphocyte chemoattractant factor. J Laboratory Clin Med. 1995;125:167–72. [PubMed] [Google Scholar]
- 49.Ryan TC, Cruikshank WW, Kornfeld H, Collins TL, Center DM. The CD4-associated tyrosine kinase p56lck is required for lymphocyte chemoattractant factor-induced T lymphocyte migration. J Biol Chem. 1995;270:17081–6. doi: 10.1074/jbc.270.29.17081. [DOI] [PubMed] [Google Scholar]
- 50.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 51.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins AL. Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med. 1997;3:659–64. doi: 10.1038/nm0697-659. [DOI] [PubMed] [Google Scholar]
- 52.Maciaszek JW, Parada NA, Cruikshank WW, Center DM, Kornfeld H, Viglianti GA. IL-16 represses HIV-1 promoter activity. J Immunol. 1997;158:5–8. [PubMed] [Google Scholar]
- 53.Briand G, Barbeau B, Corbeil J, Tremblay M. Enhancement of HIV-1-induced syncytium formation in T cells by the tyrosyl kinase p56lck. Virology. 1997;231:10–9. doi: 10.1006/viro.1997.8518. [DOI] [PubMed] [Google Scholar]
- 54.Collette Y, Olive D. Non-receptor protein tyrosine kinases as immune targets of viruses. Immunol Today. 1997;18:393–400. doi: 10.1016/s0167-5699(97)01104-3. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson M, Haggerty S, Lamonica CA, Meir CM, Welch SK, Wasiak AJ. HIV-1 replication is controlled at the level of T cell activation and proviral integration. J Virol. 1990;64:2421–5. doi: 10.1128/jvi.64.5.2421-2425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng H, Hoxie JP, Parks WP. The conserved core of human immunodeficiency virus type 1 Nef is essential for association with Lck and for enhanced viral replication in T-lymphocytes. Virology. 1999;264:5–15. doi: 10.1006/viro.1999.9937. [DOI] [PubMed] [Google Scholar]
- 57.Guntermann C, Dye J, Nye KE. Human immunodeficiency virus infection abolishes CD4-dependent activation of ZAP-70 by inhibition of p56lck. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:201–12. doi: 10.1097/00042560-199703010-00002. [DOI] [PubMed] [Google Scholar]
- 58.Harouse JM, Kunsch C, Harle HT, et al. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989;63:2527–33. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fantini J, Cook DG, Nathanson N, et al. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus (HIV-1) is associated with cell surface expression of glactosyl ceramide, a potential alternative gp 120 receptor. Proc Natl Acad Sci USA. 1993;90:2700–4. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briand G, Barbeau B, Tremlay M. Binding of HIV-1 to its receptor induces tyrosine phosphorylation of several CD4-associated proteins, including the phosphatidylinositol 3-kinase. Virology. 1997;228:171–9. doi: 10.1006/viro.1996.8399. [DOI] [PubMed] [Google Scholar]
- 61.Popik W, Pitha PM. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–41. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenz U, Ravichandran KS, Burakoff SJ, Neel BG. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperreponsiveness. Proc Natl Acad Sci USA. 1996;93:9624–9. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill CM, Littman DR. Natural resistance to HIV? Nature. 1996;382:668–9. doi: 10.1038/382668a0. [DOI] [PubMed] [Google Scholar]