Abstract
Cell-mediated T-helper type-1 (Th1) responses play a vital role in the immunopathogenesis of genital infections caused by herpes simplex virus 2 (HSV-2). We investigated the role of Th responses in HSV-2 infection at different disease stages by analysing the production of Th cytokines in HSV-stimulated peripheral blood mononuclear cells (PBMCs). IFN-γ production decreased over time following a recurrence, whereas levels of IL-10, and to a lesser extent IL-2, remained elevated during this period. In addition, PBMCs from asymptomatic seropositive individuals produced high levels of IFN-γ and low levels of IL-10, in contrast to individuals with a history of genital ulcers. Following a recurrence, virus copy number in the genital lesions decreased progressively over time, in a manner similar to IFN-γ production by HSV-2-stimulated PBMCs. Enhanced production of IFN-γ may modulate HSV replication and B7 expression on monocytic cells of HSV-infected individuals. In contrast to seronegative controls, IFN-γ failed to enhance B7 expression on monocytic cells of HSV-infected individuals. In addition, monocytic cells from HSV-2-infected individuals with recurrent disease supported greater HSV replication than did those of HSV-infected asymptomatic individuals or seronegative controls. Furthermore, addition of IFN-γ resulted in enhanced HSV replication in monocytic cells of HSV-infected individuals with recurrent disease, in contrast to the inhibition observed in HSV-seropositive asymptomatic individuals and seronegative controls. Taken together, our results suggest that dysregulated production of IFN-γ at different disease stages and the impaired ability of monocytic cells to respond to IFN-γ may play a role in the pathogenesis of recurrent genital herpes disease.
Keywords: HSV, IFN-γ, IL-10, B7
INTRODUCTION
Genital infection by herpes simplex virus type 2 (HSV-2) is one of the most common sexually transmitted diseases. It can also cause potentially fatal encephalitis, and severe systemic disease in immunocompromised hosts [1]. The pathogenesis of HSV infection involves acute infection, latency in neuronal cells, intermittent viral reactivation, and recurrent disease [1]. Genital HSV infections are characterized by symptomatic recurrences that may occur 4–15 times per year, with lesions lasting for periods of 3–12 days [2,3]. The development of HSV disease is likely to be influenced by the effectiveness of the host immune response [4]. Innate and cell mediated responses have been suggested to play a vital role in the control of HSV infections [4–6]. Depletion and adoptive transfer experiments in mice suggest a protective role for natural killer (NK) cells, macrophages, and CD4+ and CD8+ T cells in controlling HSV infection [4–8]. It has been suggested that T helper type-1 (Th-1) responses may curtail viral replication during primary infection, whereas cytotoxic T lymphocytes may prevent neuronal spread and the establishment of viral latency [4,5,7,8].
Immune responses are determined by T helper (Th) cytokines in the microenvironment [9–13] and by specific stimulatory interactions between CD28 molecules expressed on T cells and B7 molecules present on antigen presenting cells (APC) [14]. Differential roles for the B7 isoforms B7-1 and B7-2 in the development of distinct Th-1 and Th-2 responses have been postulated [15–17]. Although some studies have provided conflicting results with respect to the establishment of distinct Th responses by B7-1 and B7-2, it is likely that alterations in the balance of B7 isoform expression on antigen presenting cells may modulate Th responses [15,16].
The role of Th cytokines in HSV disease has been investigated in experimental animals and in human subjects [18–20]. IFN-γ was found to be the predominant cytokine produced by HSV-stimulated peripheral blood mononuclear cells (PBMC) [20]. However, the specific aspects of the host immune response such as the production of Th cytokines and the expression of costimulatory molecules on APC, with respect to disease reactivation, are poorly understood. Recent reports suggest that patients expressing certain HLA types have been shown to exhibit relatively frequent HSV recurrences [21]. Therefore, it is likely that the immune system may in part determine the frequency and the severity of genital HSV recurrences. Specifically, impaired regulation of Th cytokines and/or costimulatory molecules, such as B7 receptor expression on antigen presenting cells, in HSV infection may be associated with the ability of monocytes/macrophages to permit viral replication. Therefore, a critical balance in the regulation of Th cytokines may be necessary during viral reactivation to establish specific Th responses and maintain a symptom-free remission period.
To address these issues, we analysed the production of Th1 and Th2 cytokines by HSV-2 stimulated PBMC from HSV-2 infected individuals in the following disease stages:
active disease patients with a viral outbreak and clinically detectable lesions in the 2 weeks prior to specimen collection (AD);
nonactive disease patients who had a history of genital lesions but who were in a recurrence-free phase of at least 2 weeks duration (NAD);
asymptomatic seropositive individuals who had no history of genital ulcers (AS);
healthy seronegative controls (NEG).
Our results demonstrate that during the recurrence-free phase IFN-γ production by HSV-2-stimulated PBMC decreases over time, whereas IL-10 production remains elevated. In addition, we observed impaired IFN-γ-mediated regulation of B7 isoforms on monocytes of HSV-2-infected individuals. Since IFN-γ has been shown to inhibit HSV replication in vitro in a variety of cell types [22–24], we investigated whether the enhanced IFN-γ production observed in HSV-infected individuals could modulate HSV replication in monocytes/macrophages from HSV-infected and uninfected individuals. Our results show that HSV did not replicate in macrophages from HSV seronegative individuals and IFN-γ did not have any effect on virus replication in these cells. In contrast, HSV replicated in vitro in monocytes from asymptomatic seropositive individuals and patients with recurrent disease, and IFN-γ enhanced viral replication in the monocytes/macrophages from these subjects. Taken together, our results suggest that dysregulated production of IFN-γ and impaired IFN-γ-mediated responses with respect to the regulation of B7 receptor expression and viral replication in monocytic cells may play a role in the pathogenesis of recurrent genital herpes disease.
SUBJECTS AND METHODS
Patients and collection of samples
Thirty-three individuals with recurrent genital herpes, seven asymptomatic HSV-2 seropositive individuals and six HSV-seronegative healthy controls participated in the study. All the HSV-2-infected individuals recruited for this study were otherwise healthy. The study was approved by the Research Ethics Board of the Ottawa Hospital, University of Ottawa, Ottawa, Ontario, Canada. The age, distribution and sex of the patients are detailed in Table 1. All the HSV-2 infected individuals recruited for this study were previously tested for AIDS and other sexually transmitted diseases. The selection of individuals for this study was based on their symptoms, clinical history, and absence of HIV and any other sexually transmitted diseases. Furthermore, none of the patients were diagnosed for any autoimmune disease or were immuno-compromised. None of the patients received either antiproliferative or anti-inflammatory medication. However, all patients with active disease and recurrent genital herpes were on episodic treatment with acyclovir. For asymptomatic seropositive individuals, the spouse or sexual partners of patients with genital HSV disease were examined and blood drawn for analysis.
Table 1.
Demographics of participants of HSV-2 study
| Gender | ||||||
|---|---|---|---|---|---|---|
| Disease stage | Total number (samples/patients) | Age† (Years) | (F) | (M) | Time since last episode | Number of recurrences/year |
| Active disease (AD) | 26/21 | 33 ± 7 | 13 | 8 | 1 day to 2 weeks | 4–12 |
| Non-active disease (NAD) | 28/12 | 42 ± 16 | 5 | 7 | 3 weeks to 7 months | 3–12 |
| Asymptomatic seropositive (AS) | 7/7 | NA | NA | NA | Nil | |
| Seronegative (NEG) | 7/6 | 33 ± 12 | 3 | 3 | Nil | Nil |
Mean ± Standard deviation; NA, data not available.
At the first patient visit, informed consent was obtained, clinical information was recorded, a blood and a genital swab sample were taken, and patients agreed to report all genital HSV-2 outbreaks to the clinic staff. More than one blood sample was drawn from some patients who were seen several times at different disease stages to study the secretion of Th cytokines and proliferative responses to HSV antigens at various times following disease recurrence. All swabs were placed immediately in 1 ml of Kult-Sure™ viral collection and transport medium (NCS Diagnostics Inc., Etobicoke, Ontario, Canada). The system is intended for collection and transportation of specimens suspected to contain viruses and Chlamydia. The participants were divided in four groups:
the active disease patients (AD) who had clinically detectable lesions within the 2 weeks preceding examination, and their genital swabs yielded HSV-DNA as detected by PCR;
the patients with nonactive disease (NAD) who had a history of 6 or more recurrences of genital lesions but who were in a recurrence-free period and had no clinically detectable lesions in the preceding 2 weeks;
the asymptomatic seropositive patients (AS) who had no clinical history of genital lesions but HSV-2 antibodies were detected by Western blot analysis;
asymptomatic seronegative subjects (NEG) as healthy adult controls.
Isolation and culture of peripheral blood mononuclear cells (PBMC)
Blood samples were collected in polyethylene tubes containing heparin as an anticoagulant and PBMC were isolated by density gradient centrifugation in Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) as described [25,26]. The cell layer consisting mainly of mononuclear cells was collected, washed three times in PBS and resuspended at a concentration of 2 × 106 cells/ml in Iscove's Modified Dulbecco's Medium (IMDM; Sigma, Saint Louis, MO, USA), containing 10% fetal calf serum (FCS; Gibco, Grand Island, NY, USA), 100 U/ml penicillin, and 100 µg/ml gentamicin.
Preparation of HSV type specific and control antigens
HSV-1 and HSV-2 antigens were prepared as described earlier [3]. Briefly, confluent monolayers of human fetal lung cell line (HFL# 62; ATCC) were infected with HSV-1 or HSV-2 (kindly provided by Dr K. Rosenthal, McMaster University, Hamilton, Canada). When the majority of cells exhibited a cytopathic effect, the culture supernates were removed and centrifuged at 600 × g for 15 min The supernates were incubated at 56°C for 2 h to inactivate the virus, aliquotted and stored at − 80°C. Viral inactivation was confirmed by the failure of the antigen preparation to produce a cytopathic effect when used to inoculate HFL cell monolayers. The control antigen was prepared similarly from uninfected cells.
Cell proliferation assays
PBMC (1 × 106 cells/ml) were stimulated with anti-CD3 antibodies (OKT-3, American type culture collection (ATCC), Rockville, MD, 1 : 200 final dilution) for 48 h. Cells were also stimulated separately with either HSV-1, HSV-2 or control antigen (1 : 10 final dilution) for 5 days. Stimulation experiments were done in triplicate in a final volume of 200 µl in 96 well plates (Falcon Labware, Oxnard, CA, USA). The cells were then pulse-labelled with 0·5 µCi[3H] thymidine (Amersham, Arlington Heights, IL, USA) and cultured for a further 16 h. [3H] thymidine incorporation was measured by liquid scintillation counting (1450 Microbeta Wallac, Turku, Finland). The stimulation indices (S.I.) calculated were the ratios of [3H] thymidine incorporation (cpm) by PBMC cultured in the presence of antigen/mitogen to incorporation by either PBMC cultured in the absence of antigen/mitogen or by PBMC cultured in the presence of control antigens. A stimulation index of more than three was considered a positive response.
Collection of supernatants for measurement of cytokine production by ELISA
Cells (1 × 106/ml) were cultured in the presence of either HSV-1, HSV-2 or control antigen (1 : 10 final dilution) in 24 well tissue culture plates (Falcon Labware). Based on a preliminary kinetic experiment showing that PBMC exhibit maximal[3H] thymidine incorporation 5 days after stimulation with HSV-1 or HSV-2 antigens (data not shown), the supernatants were harvested after 5 days and stored at − 70°C.
IL-2 and IL-4
IL-2 and IL-4 were measured by sandwich enzyme-linked immunoabsorbentassay (EIA) using the human IL-2 and IL-4 Quantikine™ Kit (R & D Systems Inc., Minneapolis, MN), as described by the manufacturer. Recombinant human IL-2 and IL-4 provided with the kit were used as standards. The sensitivity of both assays was 8 pg/ml.
IL-10 and IFN-γ
IL-10 and IFN-γ produced by PBMC were also measured by sandwich ELISA using two different monoclonal antibodies (MAb) to distinct epitopes, as described previously [25]. Briefly, the primary human and viral IL-10 antibody (clone JES3–9D7, PharMingen, Mississauga, Ontario, Canada) and the human IFN-γ-antibody (clone 25718·11, R & D Systems) were used at concentrations of 5 and 4 µg/ml, respectively. Recombinant IL-10 (PharMingen) and IFN-γ (Endogen, Woburn, MA, USA) were used as standards. IL-10 biotinylated antibody (clone JES3–12G8, PharMingen) and IFN-γ biotinylated antibody (#BAF285, R & D Systems) were used as detection anti-bodies at concentrations of 4 µg/ml and 200 ng/ml, respec-tively. Streptavidin-peroxidase (Jackson Immuno-Research, West Grove, PA, USA) was used at a final dilution of 1 : 1000. The colour reaction was developed using o-phenylenediamine dihydrochloride (OPD; Sigma) and hydrogen peroxide and was measured at 450 nm. The sensitivity of both assays was 8 pg/ml.
Detection of viral DNA by polymerase chain reaction (PCR) analysis
Viral DNA was isolated using a monophase solution containing guanidine thiocyanate and phenol (TRI Reagent solution, Molecular Research Center Inc., Cincinnati, OH, USA) as described [27]. HSV in the DNA isolated from clinical samples was detected by PCR analysis using HSV-1 and HSV-2 primers, as previously described [3]. The amplified PCR products were detected and quantified using the colourimetric Digene Sharp Signal System as described by the manufacturer (Advanced Biotechnologies Inc., Columbia, MD, USA). Briefly, 10 µl of DNA isolated from each sample was added into 90 µl of PCR reaction mixture. To prepare standard curves for quantification, 10 µl of serial 10-fold dilutions of the standard HSV-1 or HSV-2 DNA (1 × 107 copies/µl) provided by the manufacturer were each added to 90 µl of PCR reaction mixture. HSV-1 and HSV-2 types were distinguished via distinct 3′ primers, DNAp3–1 and DNAp3–2, which are specific for HSV-1 and HSV-2, respectively [28–30]. The DNAp5 HSV primer recognized the common sequences for both HSV-1 and HSV-2. For quantification of HSV copies, the 5′ end HSV primers were biotinylated (Digene Diagnostics Inc.). PCR was carried out for 30 cycles in a programmable DNA thermal cycler (480; Perkin-Elmer), as follows: DNA denaturation at 94°C for 60 s, primer annealing at 65°C for 1 min 30 s, and primer extension at 72°C for 2 min For the last cycle, the DNA molecules were allowed to extend for 10 min at 72°C. The amplified PCR products were detected by electrophoresis on a 1·5% agarose gel and were stained with ethidium bromide. The sequence of DNAp3–1, DNAp3–2 and DNAp5 HSV primers (Accession # AY038367) were as follows: Primer DNAp3–1, sense (n-2026) 5′-CCT CGC GTT CGT CCT CGT CCT CC-3′ (n-2004); primer DNAp3–2, sense (n-1951) 5′-CCT CCT TGT CGA GGC CCC GAA AC-3′ (n-1929) and primer DNAp5, sense (n-1564) 5′-ATC GTG AAC ATC GAC ATG TAC GG-3′ (n-1583). By using these primers, we were able to distinguish between the presence of HSV-1 and HSV-2 on the basis of the PCR product size, 469 and 391 bp, respectively [28–30]. To determine that the DNA isolated from the clinical samples was not degraded, PCR for the β-actin gene was performed as described earlier [26]. The primers for β-actin were as follows: sense (n-1038) 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′ (n-1067), and antisense (n-1876) 5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3′ (n-1905). Clinical samples from patients with genital lesions but who were negative by PCR were spiked with HSV-2 DNA to rule out the presence of PCR inhibitors.
The amplified PCR products were transferred on to nylon filters (Amersham) and were hybridized with the oligonucleotide probe HSV-P3 [28–30]. The sequence for HSV-P3 is as follows: sense (nt 1561) 5′-GGC GTA GTA GGC GGG GAT GTC GCG-3′ (nt 1583). This oligonucleotide probe was labelled with alkaline phosphatase using the E-Link™ and E-Link Plus™ oligonucleotide labelling kit according to the manufacturer's recommendations (Genosys, The Woodlands, TX, USA). The filters were prehybridized with 10 ml of prehybridization buffer for 4 h at 42°C. Hybridization was performed at 42°C overnight with 10 ml of prehybridization buffer containing 20 µl of the alkaline phosphatase labelled oligonucleotide probe. After successive washes with 2× SSC plus 0·1% SDS, 0·5x SSC plus 0·1% SDS, and buffers A (100 mm Tris, pH 7·4, 150 mm NaCl), buffer B (2% blocking buffer in A), and buffer C (100 mm Tris pH 9·5, 100 m NaCl and 50 mm MgCl2), the damp filters were sprayed two or three times with LumiPhos 530 (Boehringer Mannheim) and were exposed to X-ray film (X-Omat; Kodak) at 37°C for between 30 min and 2 h.
Quantification of PCR products by ELISA
Quantification of the amplified PCR products obtained as above was performed according to the recommendations of the manufacturer (Digene Sharp Signal System, Advanced Biotechnologies Inc.). Briefly, 25 µl of PCR reaction mixture containing 5′-biotinylated products was hybridized at 65°C for 30 min with 25 µl (500 pmol/l) of specific single-stranded RNA probe complementary to the biotinylated strand of the PCR. The resultant RNA:DNA hybrids were captured onto the surface of streptavidin-coated microwells. The hybridized contents were transferred onto the streptavidin coated microwells and incubated at 25°C for 30 min on a rotary shaker (1200 r.p.m./min). The detection reagent, RNA-DNA antibody conjugated to alkaline phosphatase, was then added, with further agitation at 25°C for 30 min After washing of the wells, a colourimetric substrate (para-nitrophenylphenol, PNPP) was added and the plates incubated at 37°C for 2 h. The plates were read at 405 nm using a microplate ELISA reader. Ten-fold dilutions of the PCR controls were utilized to calculate the amount of HSV DNA copies contained in the clinical specimens.
Plaque reduction assay
PBMCs (106 cells/ml) were seeded in 12 well tissue culture plates for 24 h. Cells were then infected with HSV at a concentration of 0·01 PFU per cell for another 48 h in the presence or absence of 200 U/ml of IFN-γ. Cells were homogenized, subjected to three freeze thaw cycles, and the suspension was sonicated and centrifuged at 6000 r.p.m. for 10 min The titre of the infectious virus was determined in a standard viral plaque assay on Vero cell monolayers. The results were expressed as the number of PFU per 106 cells.
Flow cytometric analysis
Unstimulated PBMC (3 × 106/ml) were cultured in the presence of either IL-10 (1 ng/ml), IFN-γ (1 ng/ml), HSV-1 or HSV-2 antigens. The monocytes were analysed for B7-1 and B7-2 expression after 24 and 48 h of stimulation as previously described [25,31]. Briefly, cells were washed once with PBS/0·05% sodium azide and distributed into flow cytometry tubes (Sarstedt, Numbrecht, Germany). Cells were stained with 5 µl of either PE labelled anti-B7-1 (Becton-Dickinson, Lincoln Park, NJ, USA) or PE labelled anti-B7-2 (Pharmingen) monoclonal antibodies and 2·5 µl of FITC labelled anti-CD14 monoclonal antibody (Becton-Dickinson). Autofluorescence and isotype controls (IgG1 for B7-1 and IgG2b for B7-2; both Becton-Dickinson) were included (data not shown). For PBMC, two distinct populations (lymphocytes and monocytes) were visualized by light scatter properties. For analysis of B7 expression on monocytes in PBMC, cells were gated in the monocytic gate. The gates were set in accordance with the gates obtained with the isotype matched control antibodies. B7-1 and B7-2 expressing CD14 + monocytes were observed in clusters distinct from lymphocytes and mean channel fluorescence (MCF) was obtained. Data were acquired on a Becton-Dickinson FACScan flow cytometer by counting 5000 events. Validity of comparisons in the expression levels of B7-1 and B7-2 isoforms between different patient populations was ensured through the use of Calibrite™ Beads (Becton Dickinson). Data were analysed using the WinMDI software package (J. Trotter, Scripps Institute, San Diego, CA, USA).
Statistical analysis
Means were compared by the two-tailed Student's t-test. The results are expressed as mean ± standard error (SEM). Linear regression analysis was performed using the Statistical Analysis function of Microsoft Excel.
RESULTS
Proliferative responses of PBMCs to HSV antigens
The proliferative responses of PBMC from the 4 study groups to HSV-1, HSV-2 antigens and anti-CD3 antibodies are shown in Table 2. PBMCs were classified as responders (R) and nonresponders (NR) based on their proliferative response to HSV-2 stimulation. PBMCs showing a stimulation index (SI) of more than 3 were designated as responders, and those with an SI of less than 3 were classified as nonresponders. Four PBMC samples from four patients showing symptoms of active disease (AD) did not proliferate in response to HSV antigens and were designated as nonresponders. In contrast, 22 PBMC samples collected from 17 AD patients at different time points proliferated in response to HSV antigens and were characterized as responders. In the category of nonactive disease (NAD), six PBMC samples from two patients collected at different time points were found to be non responders whereas 22 PBMC samples collected from 10 patients at different time points were characterized as responders. It may be pointed out that none of the responders from either AD or NAD patients converted into the nonresponder category or vice versa. Only one blood sample was drawn from asymptomatic seropositive (AS) and seronegative patients. Of the 7 AS patients, four were found to be nonresponders and three responders. Similarly, among the six seronegative individuals, six were found to be nonresponders (Table 2). All the samples responded to anti-CD3 Ab. Samples from patients with recurrent genital herpes demonstrated a trend towards higher SI in response to anti-CD3 Ab compared to the asymptomatic seronegative controls, although the difference was not statistically significant (P = 0·089; Table 2).
Table 2.
Proliferation of PBMC from HSV-patients at different disease stages in response to HSV antigens
| Disease stage | No. samples/no. patients S/P | Responders S/P | Non-Responders S/P | SI toαCD3 Ab* | SI to HSV-1* | SI to HSV-2* | |||
|---|---|---|---|---|---|---|---|---|---|
| Active disease | 26/21 | 22/17 | 4/4 | R: | 122·9 ± 22·2 | R: | 6·4 ± 0·9 | R: | 7·7 ± 1·3 |
| NR: | 77·3 ± 32·7 | NR: | 1·3 ± 0·4 | NR: | 1·2 ± 0·1 | ||||
| Non-active disease | 28/12 | 22/10 | 6/2 | R: | 76·4 ± 13·7 | R: | 21·2 ± 6·1 | R: | 10·0 ± 1·9 |
| NR: | 157·0 ± 64·3 | NR: | 3·3 ± 0·6 | NR: | 1·2 ± 0·2 | ||||
| Asymptomatic | |||||||||
| Seropositive | 7/7 | 3/3 | 4/4 | ND | ND | R: | 75·6 ± 32·2 | ||
| NR: | 2·3 ± 0·6 | ||||||||
| Seronegative | 7/6 | 0 | 7/6 | NR: | 45·6 ± 5·9 | NR: | 1·3 ± 0·2 | NR: | 1·0 ± 0·2 |
PBMC (1 × 106/ml) were stimulated with αCD3 antibodies (1:200 final dilution of culture supernatant of αCD3 monoclonal antibody hybridoma), HSV-1 or HSV-2 antigens. PBMC were classified as responders (R) and nonresponders (NR) on the basis of proliferative responses to HSV-2 antigen: R, Stimulation Index (SI) > 3; NR, SI < 3.
Mean ± SEM. ND, not done
Production of Th cytokines by HSV-2 stimulated PBMC
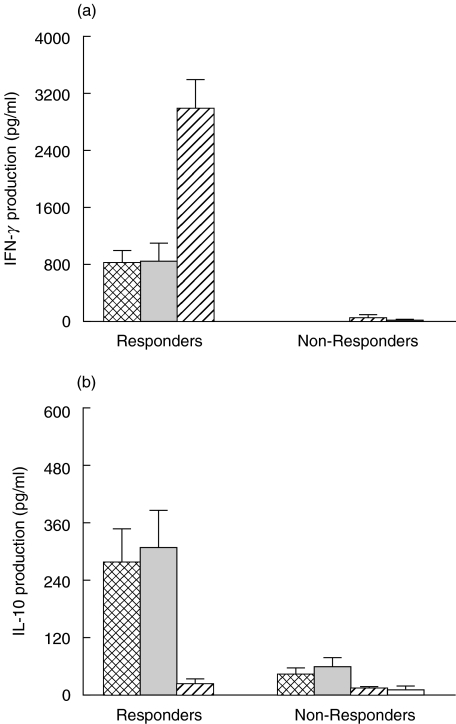
PBMCs from HSV-2-infected responder and nonresponder patients may secrete distinct patterns of Th cytokines. In order to study Th responses in HSV-2 disease, we analysed production of Th1 (IFN-γ) and Th2 (IL-4, IL-10) cytokines in the supernatants of PBMCs harvested five days after stimulation with HSV-2 antigens. Th1 and Th2 cytokine secretion by responders and nonresponders were compared in AD, NAD, AS patients and HSV seronegative controls (NEG). PBMC from AD and NAD responders secreted significantly higher levels of IFN-γ than did those from nonresponders (P = 0·018) and HSV seronegative controls (P = 0·034). However, there was no difference in the levels of IFN-γ production among responders from the 2 disease groups (AD and NAD) (Fig. 1a). PBMCs from AS responders (n = 3) secreted significantly higher levels of IFN-γ than did PBMC from responders in the AD and NAD groups (P < 0·001; Fig. 1a).
Fig. 1.
Production of (a) IFN-γ and (b) IL-10 by PBMC from HSV-infected individuals at different stages of disease and HSV-seronegative controls. PBMC (1 × 106/ml) obtained from patients with AD ( ), n = 21), NAD (
), n = 21), NAD ( ), n = 12) and asymptomatic HSV-seropositive (AS,(
), n = 12) and asymptomatic HSV-seropositive (AS,( ), n = 7) and HSV-seronegative controls (NEG, □, n = 6) were stimulated with HSV-2 antigen for 5 days and the supernatants analysed for IL-10 and IFN-γ production by ELISA as described in the Subjects and Methods section. Patients were classified as responders and nonresponders based on the ability of PBMC to proliferate in response to HSV-2 antigens as described in Subjects and Methods section. The results indicated are mean ± SEM.
), n = 7) and HSV-seronegative controls (NEG, □, n = 6) were stimulated with HSV-2 antigen for 5 days and the supernatants analysed for IL-10 and IFN-γ production by ELISA as described in the Subjects and Methods section. Patients were classified as responders and nonresponders based on the ability of PBMC to proliferate in response to HSV-2 antigens as described in Subjects and Methods section. The results indicated are mean ± SEM.
Similarly, PBMC from AD and NAD responders secreted significantly higher levels of IL-10 than did those from nonresponders (P = 0·032), aymptomatic seropositive (P = 0·025) and HSV seronegative controls (P = 0·038). There was no difference in the levels of IL-10 production between responders in the AD and NAD groups (Fig. 1b). In contrast, levels of IL-10 produced by PBMCs from the responders and the nonresponders in the AS group did not differ significantly when compared to the levels produced by the AD and NAD individuals in the nonresponders category. Furthermore, PBMC from nonresponders in the AD and NAD groups did not secrete significantly higher levels of IL-10 than did nonresponders in the AS and seronegative groups. HSV-stimulated PBMCs from HSV patients produced very low levels of IL-4 that were not significantly different in AD, NAD and AS groups of patients (data not shown).
Th1 and Th 2 cytokine secretion by PBMC from patients at different disease stages
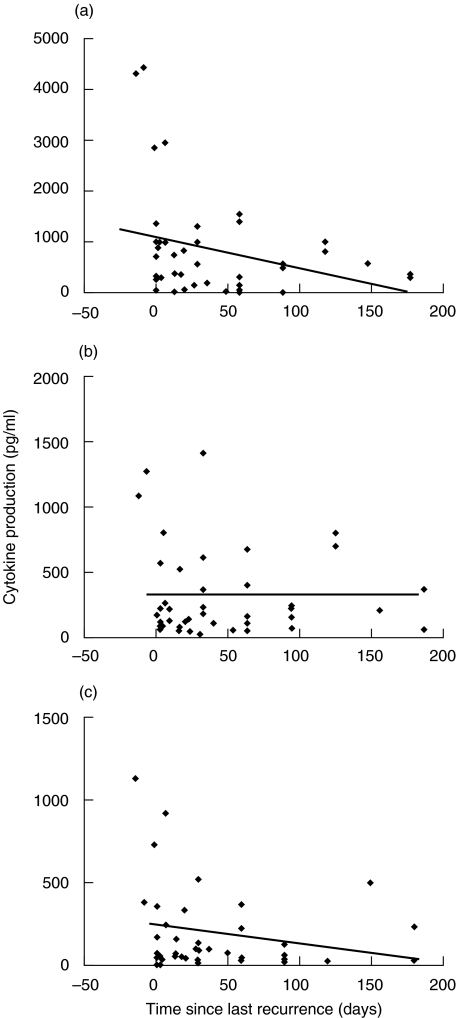
The levels of Th cytokines produced at different times following infection may also have an impact on the disease process. The above results, showing an elevation in the IFN-γ response in AS, AD and NAD patients as compared with that in healthy controls, suggest that Th1/Th2 cytokine levels may vary at different disease stages. We analysed the production of IFN-γ, IL-10 and IL-2 by HSV-2 stimulated PBMCs from patients at different time points from their last HSV-2 genital outbreak (as per the patient's clinical chart). Peak levels of IFN-γ, IL-10 and IL-2 production were noted around the time of recurrence of active disease (Fig. 2). IFN-γ levels decreased progressively during the recurrence-free phase (r = 0·48; P= 0·046). However, the high levels of IL-10 produced during a recurrence persisted throughout the study period and, unlike IFN-γ, a progressive decrease was not observed (Fig. 2). Levels of IL-2 production decreased over time but the association was not found to be significant (P = 0·24) (Fig. 2).
Fig. 2.
Kinetics of (a) IFN-γ, (b) IL-10 and (c) IL-2 production by PBMC from HSV-infected individuals in AD and NAD categories at various times before and after the onset of a genital HSV recurrence (day 0). PBMC (1 × 106/ml) obtained from patients with AD (n = 17) and NAD (n = 10) were stimulated with HSV-2 antigen for 5 days and the supernatants analysed for IL-2, IL-10 and IFN-γ production by ELISA as described in the legend to Fig. 1 and in Subjects and Methods section. The coefficient of linear regression (P-values) was calculated for each cytokine using the statistical analysis function of Microsoft Excel. (a) P= 0.046; (b) P= 0.71;
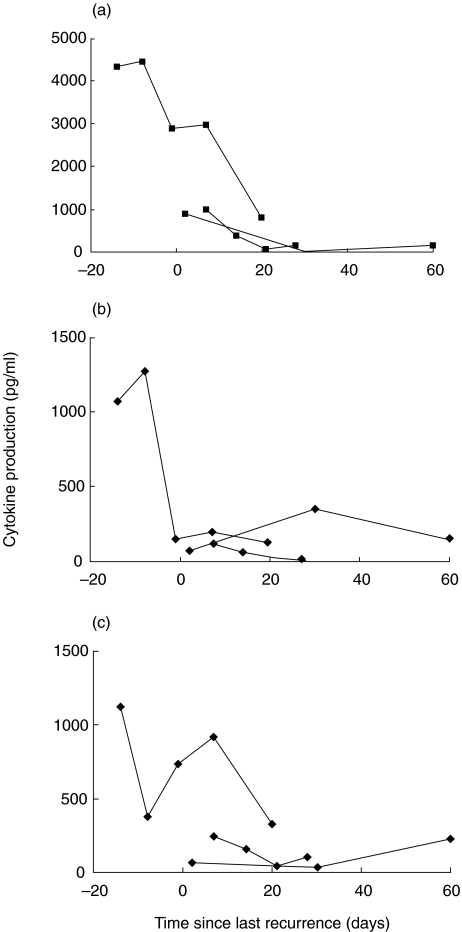
To confirm the above observations, we performed a longitudinal analysis of cytokine production by HSV stimulated PBMCs from three HSV-infected individuals in the responder AD category over a period of 2 months (Fig. 3). The highest levels of IFN-γ were found immediately before or at the time of disease, following which levels decreased consistently in all patients over a maximum of 60 days. We did not observe any pattern in IL-10 and IL-2 levels over the same period (Fig. 3).
Fig. 3.
Longitudinal analysis of (a) IFN-γ, (b) IL-10 and (c) IL-2 production by PBMC from three HSV infected individuals immediately after recurrence. Blood from three representative HSV-infected individuals were obtained at different times during recurrence and after regression of the genital symptoms. PBMC from these patients were analysed for the production of IL-2, IL-10 and IFN-γ as described in the legend to Fig. 1.
Quantification of viral copy numbers in genital swabs during active disease and the recurrence-free phase
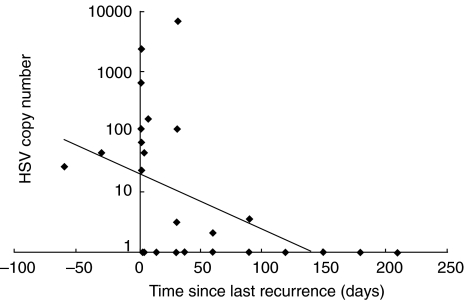
Several in vitro studies and animal models have suggested that IFN-γ inhibits HSV replication [22–24]. To investigate whether an association exists between Th cytokine production by HSV-stimulated PBMC and the presence of virus in genital lesions, quantitative HSV PCR was performed on extracts from 34 genital swabs from 20 patients with recurrent genital herpes (AD and NAD). HSV DNA was detected in 15 swabs (44%) from 13 patients; in the remaining 7 patients, HSV DNA was not detected. As expected, high numbers of viral DNA copies were detected at the time of recurrence. These numbers declined sharply in 4–6 months thereafter (Fig. 4), as did IFN-γ levels (Figs 2 and 3). Comparison of IFN-γ production by HSV-2 stimulated PBMCs with HSV copy number present in the genital swabs revealed a significant correlation (r = 0·44; P= 0·048).
Fig. 4.
Analysis of HSV copy number in the genital swabs obtained from HSV-infected individuals in AD and NAD categories. HSV was quantified by HSV-type specific PCR in genital swabs (n = 34) from AD and NAD individuals. The HSV was detected in the DNA isolated from clinical samples by PCR analysis using the Digene Sharp Signal System with HSV-1 and HSV-2 primers, as described in Subjects and Methods section.
Regulation of B7 expression by IFN-γ and IL-10 on monocytes in HSV infected individuals
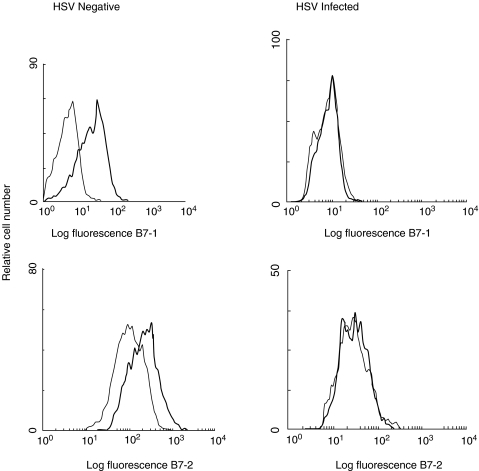
We and others have previously shown that IFN-γ up-regulates expression of both B7-1 (CD80) and B7-2 (CD86), whereas IL-10 down regulates the expression of B7.2 on monocytes [26,31,32]. The above results showing that IFN-γ and IL-10 are produced by HSV-2-stimulated PBMC suggest that regulation of B7 expression on monocytes in these patients may be altered. Therefore, PBMC from 11 patients with recurrent infection and from four HSV seronegative healthy controls were stimulated with either IL-10 or IFN-γ. B7 expression on monocytes was examined by flow cytometry and representative histograms from HSV-infected individuals are shown in Fig. 5. Prior to IL-10 and IFN-γ treatment, monocytes of HSV-infected patients and seronegative controls expressed the same levels of B7-1 or B7-2 (Fig. 5). IL-10 moderately enhanced the expression of B7-1 and down regulated the expression of B7-2 on monocytes from both HSV-negative and HSV-infected individuals (data not shown). In agreement with our previous observations [31], IFN-γ consistently up-regulated the expression of both B7-1 and B7-2 on monocytes from HSV-negative individuals (Fig. 5). In contrast, IFN-γ did not alter the expression of either B7-1 or B7-2 on monocytes from 7 of 9 HSV-infected individuals examined. These results suggest that monocytes of HSV-infected individuals exhibit impaired IFN-γ-mediated regulation of B7-1 and B7-2 expression.
Fig. 5.
Effect of IFN-γ on B7-1 and B7-2 expression on CD14 + monocytes from HSV-infected and HSV-seronegative controls. PBMC from nine HSV-infected individuals with both AD and NAD stages and six HSV-seronegative controls were cultured in the presence (——) or absence (——) of IFN-γ for 24 h. PBMC were stained with either PE-labelled anti-B7-1 or PE-labelled anti-B7-2 antibodies and counterstained with FITC-labelled anti-CD14 antibodies for monocytes as described in Subjects and Methods section. The cells in the monocytic gate were analysed for the expression of B7-1 and B7-2. The comparability in the expression levels of B7-1 and B7-2 isoforms between different patient populations was ensured through the use of Standard Brite Flow Cytometric Fluorescence Intensity Standardization beads (Coulter). Histograms from one representative HSV-infected and one HSV-seronegative control is shown.
Virus growth in PBMC cultures from patients and controls
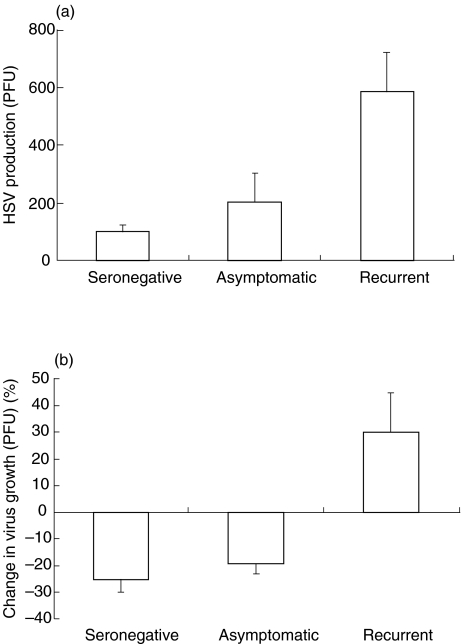
Since IFN-γ has been shown to inhibit HSV replication in vitro in a variety of cell types [22–24], and in view of the above observations suggesting impaired IFN-γ-mediated B7 regulation, we hypothesized that IFN-γ may also affect HSV replication in monocytes/macrophages of HSV-infected individuals. Freshly isolated PBMCs are unable to support replication of HSV-2. However, when PBMCs are cultured for 24 h and then infected with HSV, the monocytes/macrophages become positive for HSV-2 late antigens as observed by immunofluorescence (data not shown). We investigated the ability of HSV-2 to replicate in PBMCs obtained from HSV-seronegative controls and HSV-infected individuals in the presence or the absence of IFN-γ. Freshly isolated PBMCs were cultured overnight (∼20 h) in the presence or absence of 200 U/ml of IFN-γ followed by infection with HSV-2 at a MOI of 0·01 PFU/cell (Fig. 6). We did not observe any pfu in untreated cells obtained from HSV seropositive patients and patients with recurrent disease suggesting the absence of endogenous virus in these cultures. Low levels of viral replication were observed in untreated PBMCs from seronegative controls following infection with HSV (Fig. 6a). However, PBMCs isolated from both groups of HSV-seropositive individuals (asymptomatic and patients with recurrent disease) exhibited two fold and six fold increases in HSV replication, respectively (Fig. 6a). Addition of IFN-γ to the PBMCs obtained from HSV-seronegative and HSV-seropositive asymptomatic individuals resulted in decreased virus growth of approximately 20%. In contrast, addition of IFN-γ to PBMCs obtained from patients with recurrent disease resulted in increased HSV-2 replication (Fig. 6b).
Fig. 6.
Effect of IFN-γ on HSV replication in PBMCs of HSV-infected and HSV-seronegative individuals. (a) PBMCs (1 × 106/ml) obtained from HSV-seronegative, HSV-seropositive asymptomatic and HSV-infected individuals with recurrent disease were seeded into 12 well plates and cultured for 24 h. Cells were then infected with 0·01 PFU of HSV-1 for 48 h. (b) Cells were pretreated with 200 U/ml of IFN-γ prior to infection. Cell supernatants obtained following three cycles of freeze thawing were analysed for HSV replication by standard plaque forming assay using vero cell monolayers.
DISCUSSION
In this study, we have addressed the role of Th responses in HSV genital infection by analysing the production of Th cytokines at various disease stages in HSV-2-stimulated PBMCs. We observed high levels of IFN-γ production in asymptomatic seropositive individuals and in patients immediately after the onset of disease, with the levels consistently decreasing during the recurrence-free phase. In contrast, the high levels of IL-10 produced by HSV-2-stimulated PBMC during and after recurrent episodes of genital infection remained elevated for up to three months. In addition, monocytes from HSV-infected individuals exhibited impairments in IFN-γ-mediated responses with respect to HSV replication and regulation of B7 expression. These results suggest that dysregulated production of IFN-γ and IL-10 at different disease stages, and the impaired ability of monocytic cells to respond to IFN-γ, may play a role in the pathogenesis of recurrent genital herpes disease.
IFN-γ has been shown to mediate protective effects in HSV infection both in vitro and in vivo[22–24]. IFN-γ has also been shown to inhibit HSV replication in mouse macrophages and can induce lysis of HSV-infected cells [33–36]. Mice depleted of IFN-γ have been found to be highly susceptible to HSV infection [24,34,37]. In HSV-infected neonates and postpartum women (populations which are known to have increased susceptibility to severe HSV disease), PBMCs showed diminished HSV-stimulated proliferation which was associated with decreased IFN-γ production [38,39]. In this study, we show that HSV antigen-stimulated PBMCs from AS, AD and NAD patients secreted significantly higher levels of IFN-γ compared to the HSV-seronegative controls. Peak levels of IFN-γ production were noted at the time of a recurrence of active disease and IFN-γ levels decreased significantly over a period of 4–6 weeks during the recurrence-free phase. We also observed a similar pattern by longitudinal analysis of IFN-γ production in 3 individuals in the AD category. The highest levels of IFN-γ were found immediately before or at the time of recurrence, following which levels decreased consistently in all patients over time. Our results, showing a correlation between IFN-γ production and HSV copy number in the genital lesions, suggests that IFN-γ production may be driven by HSV-2 replication and IFN-γ in turn may inhibit HSV replication in HSV-infected epithelial cells in the genital lesions. The precise role of IFN-γ in HSV recurrence is not clear, however, the results of this study, suggest its role in HSV pathogenesis.
Clearance of HSV infection by NK cells [5] and CD4+ and CD8+ T cells [4,5,40], has been shown to be critically dependent on IFN-γ. IFN-γ is also known to enhance antiviral macrophage function by inducing nitric oxide and TNF-α production [22,41], products which specifically inhibit HSV replication [22,41]. Bearing in mind the anti-HSV properties of IFN-γ, our observations of high levels of IFN-γ production by HSV-stimulated PBMC, especially in patients with recurrent disease, raise the question whether IFN-γ exhibits antiviral effects under in vivo conditions in HSV infection. Therefore, we determined whether IFN-γ is able to exert its antiviral effects on macrophages of HSV-infected individuals. We found that monocytic cells from HSV-seronegative individuals cultured for 24 h can support low levels of viral replication. However, a six fold increase in viral growth was observed when monocytic cells from HSV-infected individuals with recurrent disease were infected with HSV-2. In addition, IFN-γ enhanced viral growth in monocytic cells from HSV-infected individuals with recurrent disease, in contrast to the inhibitory effect observed in monocytic cells of asymptomatic HSV-seropositive and HSV seronegative individuals. The clinical significance of the impaired IFN-γ mediated effects on viral replication is not clear at present, but it is likely that failure of IFN-γ to inhibit virus replication and rather to enhance replication in patients with recurrent disease may have a role in the pathogenesis of HSV genital infections.
To further determine whether IFN-γ-mediated responses are impaired in HSV-infected individuals, we analysed the effects of IFN-γ on B7-1 and B7-2 expression on monocytic cells of HSV-infected individuals. We did not observe any changes in the levels of expression of B7-1 and B7-2 on monocytic cells of HSV-infected individuals as compared to HSV-seronegative individuals. However, in contrast to HSV-seronegative individuals, IFN-γ failed to enhance either B7-1 or B7-2 expression on monocytic cells of HSV-infected individuals. Given that the IL-10-mediated effects on B7 expression remained unaltered, our results suggest a defect in the IFN-γ-mediated signalling pathway in monocytes of HSV-infected individuals. The immunopathological consequences of this defective pathway are not clear at present, but they may include alterations in the IFN-γ-mediated anti-HSV effects, such as inhibition of HSV replication [22,41,42]. Further studies are necessary to understand the molecular mechanisms responsible for the observed impairment in IFN-γ-mediated responses. It is possible that high concentrations of IFN-γ in the microenvironment may desensitize the monocytes, or that HSV antigens may specifically disrupt IFN-γ-mediated regulation of B7 expression. Our results also show that HSV antigens up-regulated the expression of both B7-1 and B7-2 receptors, indicating that B7 induction may be mediated by factors released following interaction of HSV antigen with immune cells.
Our results are in agreement with previous studies which suggest that HSV-stimulated PBMC do not produce the Th2-inducing cytokine IL-4 [11,13]. However, HSV-stimulated PBMC did produce IL-10, another cytokine associated with the development of Th2 responses [13]. Our finding that the elevated levels of IL-10 production by HSV-stimulated PBMC persisted for prolonged periods after a recurrence, while levels of IFN-γ diminished during this period, suggests that the balance of these cytokines at different stages of disease may play a role in initiating or inhibiting recurrence of infection and in HSV immunopathogenesis. IL-10 is produced by Th2, Th0, CD8+ T cells and monocytes [43,44]. The fact that in HSV infection IL-10 is produced in an antigen-dependent manner and IL-4 production is minimal indicates that the IL-10 production we observed may not have originated from typical Th2 type cells.
The disease status of the nonresponding HSV-2-infected individuals is not clear at present. Non-responders, who constituted 10–20% of patients with recurrent infections (AD and NAD) were characterized by an inability of the PBMC's to proliferate and produce Th cytokines following stimulation with HSV antigens. There was, however, no clinical distinction between these individuals and the responders. The impact of this immunological unresponsiveness on virus reactivation and development of recurrent infections needs to be investigated.
Taken together, our results show consistently high levels of IFN-γ and IL-10 at the time of recurrence of HSV-2 disease. Our observation of a progressive decrease of IFN-γ production associated with persistently elevated levels of IL-10 in response to stimulation with HSV antigens suggests that these two cytokines may play a role in virus reactivation and development of recurrent infections. Furthermore, impaired IFN-γ-mediated responses with respect to B7 regulation and HSV replication observed in PBMCs from HSV + individuals with recurrent disease suggests impairment of the IFN-γ receptor mediated signalling pathway. The results of these studies may contribute to an understanding of the immunopathogenesis of recurrent genital ulcers, and also to the development of therapies to control them.
Acknowledgments
We would like to thank Dr K. Rosenthal (McMaster University, Hamilton, Ontario) for providing us with HSV-1 and HSV-2 strains, and Sheila Leudoux, Chantal Bergeron and Susan Blakney for assisting with sample and clinical data collection. We would also like to thank Dr Andrew Badley for critically reading the manuscript. This work was supported in part by grants from the Ministry of Health AIDS Bureau (F.D.M. and A.K.).
REFERENCES
- 1.Stanberry LR. Pathogenesis of herpes simplex virus infection and animal models for its study. Curr Top Microbiol Immunol. 1992;179:15–30. doi: 10.1007/978-3-642-77247-4_2. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–54. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Mitoma F, Ruben M, Sacks S, MacPherson P, Caissie G. Detection of viral DNA to evaluate outcome of antiviral treatment of patients with recurrent genital herpes. J Clin Microbiol. 1996;34:657–63. doi: 10.1128/jcm.34.3.657-663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid DS, Rouse BT. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski JF, Welsh RM. The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J Immunol. 1986;136:3481–5. [PubMed] [Google Scholar]
- 6.Kohl S. Role of antibody-dependent cellular cytotoxicity in defense against herpes simplex virus infections. Rev Infect Dis. 1991;13:108–14. doi: 10.1093/clinids/13.1.108. [DOI] [PubMed] [Google Scholar]
- 7.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–100. [PubMed] [Google Scholar]
- 8.Sethi KK, Omata Y, Schneweis KE. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983;64:443–7. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15:121–9. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria- induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 11.Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–7. [PubMed] [Google Scholar]
- 12.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179:1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–9. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson CB. Distinct roles for the costimulatory ligands B7–1 and B7–2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 15.Lenschow DJ, Ho SC, Sattar H, et al. Differential effects of anti-B7–1 and anti-B7–2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuchroo VK, Das MP, Brown JA, et al. B7–1 and B7–2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 17.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7–1 and B7–2 do not deliver identical costimulatory signals, since B7–2 but not B7–1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 18.Shimeld C, Easty DL, Hill TJ. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and cytokines. J Virol. 1999;73:1767–73. doi: 10.1128/jvi.73.3.1767-1773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikloska Z, Danis VA, Adams S, Lloyd AR, Adrian DL, Cunningham AL. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions – do herpes simplex virus-infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–38. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 20.Carmack MA, Yasukawa LL, Chang SY, et al. T cell recognition and cytokine production elicited by common and type-specific glycoproteins of herpes simplex virus type 1 and type 2. J Infect Dis. 1996;174:899–906. doi: 10.1093/infdis/174.5.899. [DOI] [PubMed] [Google Scholar]
- 21.Lekstrom-Himes JA, Hohman P, Warren T, et al. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J Infect Dis. 1999;179:1077–85. doi: 10.1086/314729. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu T, Bi Z, Reiss CS. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–8. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 23.Feduchi E, Alonso MA, Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989;63:1354–9. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–71. [PubMed] [Google Scholar]
- 25.Kumar A, Angel JB, Aucoin S, et al. Dysregulation of B7.2 (CD86) expression on monocytes of HIV-infected individuals is associated with altered production of IL-2. Clin Exp Immunol. 1999;117:84–91. doi: 10.1046/j.1365-2249.1999.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim W, Ma W, Gee K, et al. Distinct role of p38 and c-Jun N-terminal kinases in IL-10-dependent and IL-10-independent regulation of the costimulatory molecule B7.2 in lipopolysaccharide-stimulated human monocytic cells. J Immunol. 2002;168:1759–69. doi: 10.4049/jimmunol.168.4.1759. [DOI] [PubMed] [Google Scholar]
- 27.Kryworuchko M, Gee K, Diaz-Mitoma F, Kumar A. Regulation of CD44–hyaluronan interactions in Burkitt's lymphoma and epstein-barr virus-transformed lymphoblastoid B cells by PMA and interleukin-4. Cell Immunol. 1999;194:54–66. doi: 10.1006/cimm.1999.1491. [DOI] [PubMed] [Google Scholar]
- 28.Kimura H, Shibata M, Kuzushima K, Nishikawa K, Nishiyama Y, Morishima T. Detection and direct typing of herpes simplex virus by polymerase chain reaction. Med Immunol (Berl. 1990;179:177–84. doi: 10.1007/BF00195248. [DOI] [PubMed] [Google Scholar]
- 29.Tsurumi T, Maeno K, Nishiyama Y. Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 2 and comparison with the type 1 counterpart. Gene. 1987;52:129–37. doi: 10.1016/0378-1119(87)90039-4. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs JS, Chiou HC, Hall JD, et al. Sequence and mapping analyses of the herpes simplex virus DNA polymerase gene predict a C-terminal substrate binding domain. Proc Natl Acad Sci USA. 1985;82:7969–73. doi: 10.1073/pnas.82.23.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creery WD, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7–1 and B7–2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–7. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 32.Buelens C, Willems F, Delvaux A, et al. Interleukin-10 differentially regulates B7–1 (CD80) and B7–2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–72. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 33.Heise MT, Virgin HW. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–9. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendrzak JA, Morahan PS. The role of macrophages and macrophage cytokines in host resistance to herpes simplex virus. Immunol Series. 1994;60:601–17. [PubMed] [Google Scholar]
- 35.Klotzbucher A, Mittnacht S, Kirchner H, Jacobsen H. Different effects of IFN gamma and IFN alpha/beta on ‘immediate early’ gene expression of HSV-1. Virology. 1990;179:487–91. doi: 10.1016/0042-6822(90)90322-i. [DOI] [PubMed] [Google Scholar]
- 36.Kohl S, Loo LS, Drath DB, Cox P. Interleukin-2 protects neonatal mice from lethal herpes simplex virus infection: a macrophage-mediated, gamma interferon-induced mechanism. J Infect Dis. 1989;159:239–47. doi: 10.1093/infdis/159.2.239. [DOI] [PubMed] [Google Scholar]
- 37.Yu Z, Manickan E, Rouse BT. Role of interferon-gamma in immunity to herpes simplex virus. J Leukoc Biol. 1996;60:528–32. doi: 10.1002/jlb.60.4.528. [DOI] [PubMed] [Google Scholar]
- 38.Burchett SK, Corey L, Mohan KM, Westall J, Ashley R, Wilson CB. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J Infect Dis. 1992;165:813–8. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 39.Kohl S. The neonatal human's immune response to herpes simplex virus infection: a critical review. Pediatr Infect Dis J. 1989;8:67–74. [PubMed] [Google Scholar]
- 40.Mikloska Z, Kesson AM, Penfold ME, Cunningham AL. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 41.Baskin H, Ellermann-Eriksen S, Lovmand J, Mogensen SC. Herpes simplex virus type 2 synergizes with interferon-gamma in the induction of nitric oxide production in mouse macrophages through autocrine secretion of tumour necrosis factor-alpha. J Gen Virol. 1997;78:195–203. doi: 10.1099/0022-1317-78-1-195. [DOI] [PubMed] [Google Scholar]
- 42.Croen KD. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–52. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]