Abstract
Advances in understanding pathogenesis and developing new therapies are hastened by the use of effective animal models of disease. In inflammatory bowel disease, such as Crohn's disease, a variety of models have been used, including the IL-10 knockout mouse. However, in order to be truly valuable, the models need to respond to existing therapy in a way which resembles the human disease. In the light of recent developments, in which refractory Crohn's disease responds well to anti-TNF antibody therapy, we set out to validate the IL-10 knockout model of Crohn's disease by examining its response to anti-TNF therapy. We developed a new scoring system for IL-10 knockout mice, similar to the Crohn's Disease Activity Index in humans, analysed stool samples for cytokines and compared the findings with histology. We found that anti-TNF antibody therapy starting at 4 weeks markedly ameliorated the disease, as judged by the clinical score or by histological analysis of the gut. Furthermore, analysis of stool samples for cytokines revealed a marked diminution of inflammatory cytokines, adding a further accurate measure of the improvement. This model may thus be useful for evaluating other therapeutic modalities of relevance to Crohn's disease.
Keywords: IBD, interleukin-10 knockout mice, anti-TNF antibodies, clinical score
INTRODUCTION
Crohn, Ginzburg and Oppenheimer described in 1932 [1] a disease manifesting chiefly as an inflammation of the terminal ileum, a disease now known as Crohn's disease (CD). It has since been shown that CD can affect discontinuous portions of the entire gastrointestinal tract as well as bone, skin, muscle or synovial tissues. The prevalence of CD ranges from 10 to 70 cases per 100 000 population and has been increasing during the last decades [2]. The aetiology of CD is unknown although there might be a genetic background as susceptibility to CD is reported to be associated with certain families, alleles of the MHC region and known hereditary disorders [3–6]. The immune system plays an important role in the development of CD, as immunological disturbances have been reported in CD patients and immunosuppressive medication is effective in the treatment of CD [7,8]. Of special interest is the finding that IBD patients tend to have a low interleukin-10 (IL-10) producer genotype more often than normal controls [9].
The development of new treatments for CD has been hampered by the lack of a convenient experimental model for the disease. While an intestinal inflammation may be induced by immunological or noxious insults, neither method gives a very good model of inflammatory bowel disease (IBD) [10]. More recently a variety of models have been described [11]. For example the transfer of CD45RBhigh T cells from normal to scid mice produces a colitis resembling IBD in humans [12]. Multiple gene-knockout mice, i.e. mice lacking specific components of the immune response such as IL-2, IL-10 or T-cell receptor chains, develop bowel inflammation spontaneously, and are therefore possible models for IBD. Of these models the IL-10 knockout (ko) mice develop a disease closest to CD while the other resemble more closely ulcerative colitis [10,13].
Of special interest in the treatment of CD are monoclonal antibodies recognizing different proinflammatory cytokines. The synthesis of high amounts of tumour necrosis factor (TNF)α has been linked with granulomatous inflammation and TNFα was thought to play an important part as a mediator in the development of Crohn's disease [14,15]. With the success of anti-TNFα antibody in rheumatoid arthritis (RA) [16], TNFα became a key target for antibody treatment [17]. The treatment of CD with antibodies against TNFα (Remicade™, Centocor Inc., Malvern, PA, USA) has been shown to be most promising [18,19] and has become an approved therapy in US and Europe, while other anti-inflammatory treatments such as IL-10 or IL-11 have been investigated, but are less successful and not approved so far [20,21]. Both collagen induced arthritis and trinitrobenzene sulphonic acid (TNBS) colitis in murine models can be treated by systemic administration of anti-TNFα, while anti-TNFα only ameliorated the disease seen in scid mice transferred with CD45RBhigh T cells from normal mice [22]. However, surprisingly a similar beneficial effect was not seen when IL-10 ko mice with colitis were treated with anti-TNFα[23].
In the present study we have assessed the role of anti-TNFα antibody in the treatment of the chronic colitis that develops spontaneously in IL-10 ko mice kept in specific pathogen free (SPF) conditions. The purpose of this study was also to develop an animal model for assessing the effect of possible future treatments for CD. In order to monitor the effects of the treatment on bowel inflammation we, in addition to examining histological findings, developed a novel clinical score based on the human Crohn's Disease Activity Index, as well as a method for measuring different cytokines in the stool of IL-10 ko mice. Cytokines have previously been measured in human stool and have been shown to correlate with the activity of bowel inflammation [24].
MATERIALS AND METHODS
Mice
IL-10 knockout (ko) mice housed and bred in SPF conditions were used. The mice were purchased from Harlan UK on a C57BL/6 background and were then back-crossed onto a single generation of DBA/1 mice in order to increase breeding vigour and initiate genetic susceptibility to collagen induced arthritis. These mice were interbred to generate mice homozygous for the IL-10 gene deletion. The newly bred mice were then screened for homozygote and wild type littermates. Tail samples were digested in 250 µl PCR lysis buffer (120 µg/ml proteinase K, 50 mm KCl, 10 mm Tris-HCl pH 8·8, 0·1 mg/ml gelatin, 714 µm MgCl2, 0·45% NP40, 0·45% Tween) at 56°C overnight. The DNA encoding the IL-10 and neomycin regions were amplified by PCR using the following synthetic oligonucleotide murine primer pairs: 5′-TAGGCGAATGTTCTTCC-3′ (IL-10 sense) and 5′-CAGGCAGCATAGCAGTG-3′ (IL-10 antisense), IL-10 sense and 5′-CCTGCGTGCAATCCATCTTG-3′ (neomycin). The PCR was subjected to 35 temperature cycles as follows: 95°C for 1 minute to denature, annealing at 60°C for 1 minute and extension at 72°C for 2 min. The final extension step was carried out for 5 min. The products were separated on 1% agarose gels and visualized by ethidium bromimde staining. Bands were observed at 1·5Kb (neomycin) and 1Kb (IL-10. Both IL-10 ko mice and wild type (WT) littermates were used. Two separate long-term experiments were performed, and as the results were similar the data was pooled. The study was approved by the Home Office.
In vivo treatment with antibodies
Antibody treatment commenced when the mice were 4 weeks of age and was administered three times a week until the mice were 20 weeks old. The mice were injected intraperitoneally with equal volumes of either 500 µg anti-TNF antibody (clone cV1q antimurine TNF monoclonal antibody, chimeric rat/murine IgG2a/kappa isotype, no detectable cross-reactivity to lymphotoxin, Centocor Inc., Malvern, PA, USA) in phosphate buffered saline (PBS) or PBS. The dose chosen was that previously shown to have maximum effect in collagen induced mouse arthritis [25].
Clinical score
In order to obtain a clinical score in the mice with resemblances to the Crohn's Disease Activity Index (CDAI) used in Crohn's patients [26], the animals were assessed weekly from the age of four weeks onwards for the clinical score (CS). Each of the following parameters were given a value of either 0 or 1: excreted perianal mucus, rectal prolapse, diarrhoea or weight loss> 5%. A five grade (0–4) CS was thus obtained.
Stool sampling
Stool samples were collected weekly, at the same time as the clinical score was assessed, and weighed. The samples were emulsified in 500 µl per 100 µg of stool in PBS containing soy trypsin inhibitor (1 mg/ml, Sigma Chemical Co, St Louis, USA) and phenyl-methylsulphonyl fluoride (1 mg/ml, Sigma), and centrifuged at 10000 g for 15 min. Supernatants were collected and kept frozen at −20°C until use [27].
Measuring cytokines and -receptors
All cytokines (TNF, IL-1β, MCP-1 and IL-12) and TNF-R p75, were measured by a modified ELISA method described in detail previously [28]. Briefly, microtiter plates (Maxisorb, Nunc, Roskilde, Denmark) were coated with 50 µl/well of 1–6 µg/ml of capture antibodies and incubated overnight at + 4°C. They were blocked with 200 µl/well of 2% bovine serum albumin (BSA) in PBS for 2 h at + 25°C and washed with 0·5% Tween in PBS after this and all subsequent steps. Samples and standards, 50 µl/well, were incubated overnight at + 4°C. Biotinylated detect antibodies were diluted to a concentration of 1 µg/ml in 0·5% BSA in PBS and 50 µl was added per well and incubated for 2 h at 25°C followed by 50 µl of streptavidin conjugated with biotinylated horseradish peroxidase (Amersham Life Sciences, Little Chalfont, UK) for 1 h at + 25°C. Samples were developed with the peroxidase substrate system TMB, the reaction was stopped with 1 m H2SO4 and absorbance read at 450 nm.
Histological score
The terminal ileum and the entire colon were taken after sacrificing the animal by cervical dislocation at the age of 20 weeks. Separate samples were taken from each segment of the colon (caecum, ascending, transverse, and descending colon and rectum) and fixed in 10% neutral buffered formalin. The samples were routinely processed, sectioned at 5 µm and stained with haematoxylin and eosin for light microscopic examination. The samples from the colon were given a histological score (HS) from 0 to 4 as described by Berg et al. giving a grade from 0 (no change in any segment) to 20 (grade 4 lesions in all five segments [29]). Mice with a HS ≤ 5 were considered to be normal, 5 < HS ≤ 10 was considered to correspond with mild, 10 < HS ≤ 15 with moderate and 15 < HS ≤ 20 with severe colitis.
Statistical analysis
The Mann–Whitney U-test was used to compare the nonparametric data for statistical significance. For comparison of more than two means a two-way analysis of the variance (anova) was performed. The significance of correlation was calculated using Fisher's r to z-test. Mean ± SEM is given.
RESULTS
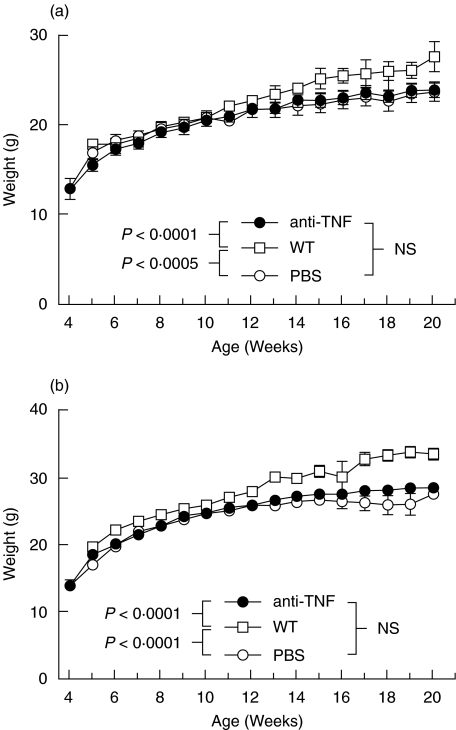
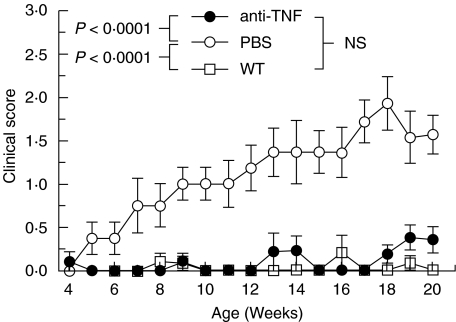
There was no difference in the mean weights between the anti-TNF or PBS treated IL-10 ko female or male mice (Fig. 1). Both female and male WT mice were heavier than the corresponding IL-10 ko mice (Fig. 1). There was also a significant difference in the clinical scores. At the time of sacrificing the PBS treated IL-10 ko mice had significantly higher CS's than the anti-TNF treated mice (1·7 ± 0·3 versus 0·4 ± 0·2, P < 0·0005). The mean CS's of IL-10 ko mice during the treatment with either PBS or anti-TNF and those of WT mice are shown in Fig. 2. The PBS treated IL-10 ko mice had significantly higher CS's than either the anti-TNF treated IL-10 ko mice (P < 0·0001) or the WT mice (P < 0·0001). The latter two groups did not differ significantly from each other (Fig. 2). None of the 21 anti-TNF treated mice but two of the 18 PBS treated IL-10 ko mice died due to colitis during the 20 weeks of follow-up. One of the mice died on week 18 (CS 1) and the other on week 19 (CS 4).
Fig. 1.
Mean weights during follow-up in (a) female and (b) male IL-10 knockout mice treated with either anti-TNF (•) or PBS (○) and wild type (WT) (□) mice.
Fig. 2.
Clinical score during follow-up in IL-10 knockout mice treated with either anti-TNF (•) or PBS (○) and wild type (WT) (□) mice.
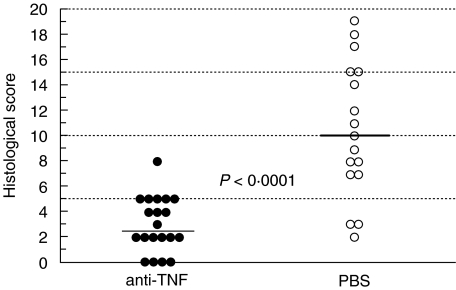
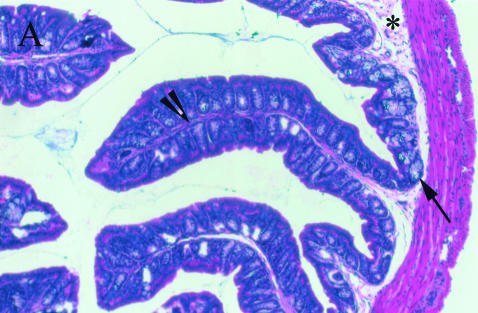
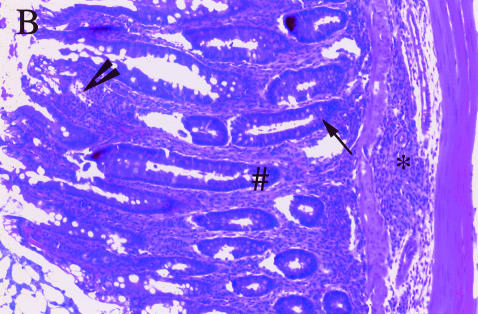
The mean HS was significantly higher in the PBS treated than the anti-TNF treated IL-10 ko mice (10·5 ± 1·3 versus 3·0 ± 0·5, P < 0·0001). Nineteen out of 20 anti-TNF treated IL-10 ko mice had normal histology and 1 had mild colitis. Out of 17 PBS treated Il-10 ko mice only 3 had normal histology, 6 had mild, 5 moderate and 3 severe colitis (Fig. 3). The histopathological findings in our colony of IL-10 ko mice were as described by others [12,29]. The observed inflammatory cell infiltration was seldom transmural and the typical finding in the affected mice was increased lamina propria lymphocytes, inflammatory cells in the submucosa, epithelial hyperplasia, and a reduction in mucin producing goblet cells (Fig. 4b). Lesions were seen in all segments of the colon in mice with severe disease, while skip lesions were found in animals with milder disease. None of the mice had carcinomas at the age of 20 weeks.
Fig. 3.
Histological scores in anti-TNF and PBS treated IL-10 knockout mice at 20 weeks of age.
Fig. 4.
Histological findings in (a)normal and (b)diseased mouse colon: an increased amount of lamina propria lymphocytes (arrowhead), inflammatory cells in the submucosa (*), epithelial hyperplasia (#) and a decreased number of goblet cells (arrow) can be seen in colitis affected gut. Magnification × 100.
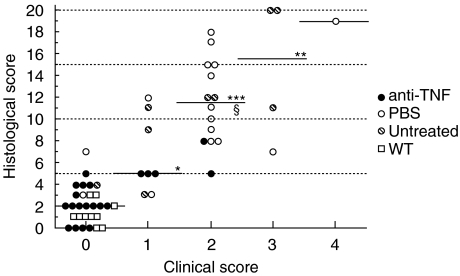
There was a significant association between the clinical and histological score (Fig. 5). With increasing severity of the clinical findings, the histological changes also increased. The mean HS was 2·1 ± 0·3 in mice with no clinical findings (CS 0) and 6·6 ± 1·3, 11·6 ± 1·0, 14·5 ± 3·3 and 19·0 in mice with a CS of 1, 2, 3 or 4, respectively. There were significant differences between the groups, given in Fig. 5.
Fig. 5.
Association between clinical and histological scores in anti-TNF (•, n = 20) or PBS (○, n = 17) treated or untreated ( ), n = 9) IL-10 knockout mice and wild type (□, WT) mice (n = 10). The median is given for each group. *P < 0·005, **P < 0·002, ***P < 0·0001 when compared with those with a clinical score (CS) of 0 and §P < 0·02 when compared with those with a CS of 1. Correlation: r = 0·852, P < 0·0001.
), n = 9) IL-10 knockout mice and wild type (□, WT) mice (n = 10). The median is given for each group. *P < 0·005, **P < 0·002, ***P < 0·0001 when compared with those with a clinical score (CS) of 0 and §P < 0·02 when compared with those with a CS of 1. Correlation: r = 0·852, P < 0·0001.
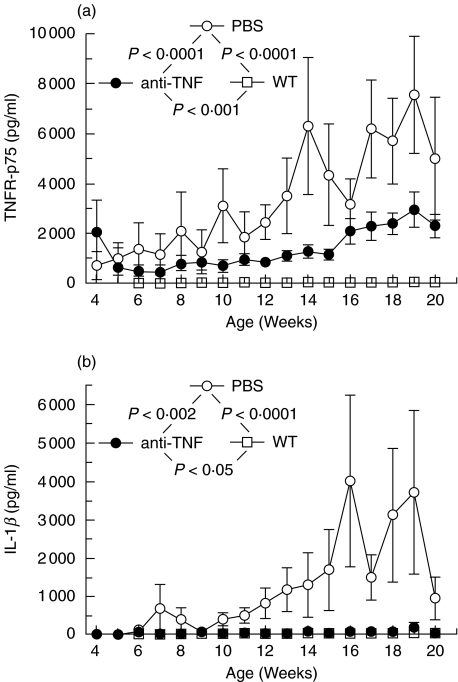
The amounts of TNF-R p75 and IL-1β measured in stool differed clearly between the two groups of IL-10 ko mice. Thus, the amounts of both TNF-R p75 and IL-1β were significantly higher (P < 0·0001 and P < 0·002, respectively) in the PBS treated IL-10 ko mice as compared to the anti-TNF treated IL-10 ko mice (Fig. 6). The PBS treated IL-10 ko mice had highly significantly (P < 0·0001) and the anti-TNF significantly (P < 0·001 and P < 0·05, respectively) more TNF-R p75 and IL-1β in stool than the WT mice had (Fig. 6). The amounts of IL-12 and MCP-1 did not, however, differ significantly between the three groups of mice (data not shown).
Fig. 6.
Comparison between amounts of (a) TNF-R p75 and (b) IL-1β in stool of IL-10 knockout (ko) mice treated with anti-TNF (•) or PBS (○) and wild type (WT) (□) mice.
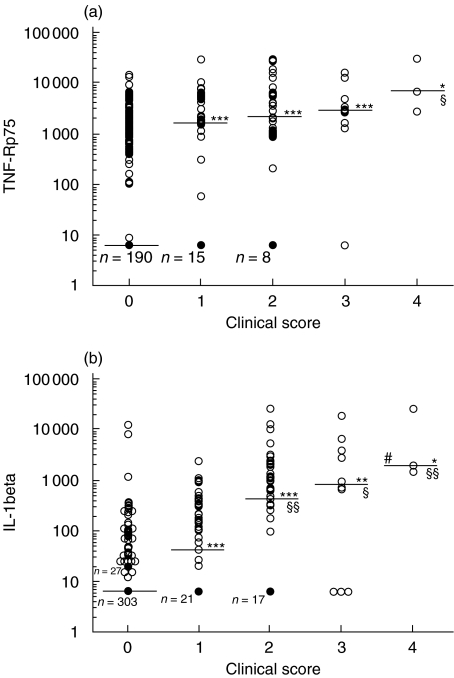
There was a significant association between the clinical score and amounts of TNF-R p75 in stool. Thus, with increasing severity of the disease, the measured amounts of TNF-R p75 in stool samples also increased. The mean amount of TNF-R p75 in stool of mice with a CS of 0 was 970·8 ± 84·9 pg/ml (median 6·2 pg/ml) and 2919·6 ± 675·9 pg/ml (1675·0 pg/ml), 4998·6 ± 1034·1 pg/ml (2179·5 pg/ml), 4929·6 ± 1703·4 pg/ml (2899·0 pg/ml) and 12939·0 ± 8292·6 pg/ml (6682·2 pg/ml) in mice with a CS of 1, 2, 3 or 4, respectively. The significances of differences between the groups are given in Fig. 7a. A similar association was observed between the clinical signs of the colitis and the amounts of IL-1β in stool (Fig. 7b). The mean amount of IL-1β in stool of mice with a CS of 0 was 80·9 ± 43·6 pg/ml (median 6·2 pg/ml) and 234·0 ± 63·2 pg/ml (42·8 pg/ml), 2015·7 ± 719·7 pg/ml (455·4 pg/ml), 3889·5 ± 2100·7 pg/ml (911·3 pg/ml), and 11054·4 ± 9159·2 pg/ml (2177·4 pg/ml) in mice with a CS of 1, 2, 3 or 4, respectively. The amounts of stool IL-12 or MCP-1 did not, however, correlate with the clinical or histological scores (data not shown).
Fig. 7.
Associations between clinical score (CS) and amounts of TNF-R p75 and IL-1β in stool of IL-10 knockout (ko) and wild type (WT) mice. The median is given for each group. A dark cirkle represents several samples giving the same value. A. Amount of TNF-R p75: the number of samples from mice with a CS of 0 is 368, from mice with a CS of 1 n= 48, CS 2 n= 47, CS 3 n= 10, and CS 4 n= 3. *P < 0·005, ***P < 0·0001 when compared with those with a CS of 0 and §P < 0·05 when compared with those with a CS of 1. Correlation: r = 0·433, P < 0·0001, n= 476. B. Amount of IL-1β: the number of samples from mice with a CS of 0 is 369, from mice with a CS of 1 n= 49, CS 2 n= 47, CS 3 n= 10, and CS 4 n= 3. *P < 0·005, **P < 0·001, ***P < 0·0001 when compared with those with a CS of 0. When compared with those with a CS of 1: §P < 0·05, §§P < 0·01, and #P < 0·05 when compared with those with a CS of 2. Correlation: r = 0·393, P < 0·0001, n= 478.
DISCUSSION
The marked clinical benefit of anti-TNF antibodies in patients with rheumatoid arthritis (RA) was predicted by experiments using human synovial tissue in vitro, and the collagen type II model of arthritis prior to their use in the treatment of patients with RA [30]. This in contrast to the case in CD, where clinical treatment preceded successful animal experiments blocking TNF. In some of the previous animal models of CD treatment with anti-TNF has either failed or only marginally improved the colitis [23]. This might either be due to the lack of a sufficiently sensitive method for monitoring the progression of colitis, or the use of relatively ineffective anti-TNF antibodies of low affinity or an inappropriate dose regime. The previously described lack of response to anti-TNF treatment in other animal models also stands in contrast to the clinical success of anti-TNFα antibody (Infliximab) in CD [31], which is now an approved therapy for resistant CD in both the United States and Europe. We thus set out to develop an animal model which might be more predictive of the human disease, and to do so developed new animal clinical scoring criteria, as well as cytokine analyses related to the disease site to validate the clinical findings.
The observed association of the clinical findings with the histological score indicates that the new clinical score described here is valid for the evaluation of disease progression in IL-10 knockout mice. This suggests that the changes in clinical score could be useful in the comparative analysis of other therapies of colitis in this mouse model of IBD, and we are currently using it to evaluate anti-IL-12 antibody as well as anticytokine drug therapy, which should block TNF production or signalling.
The correlation of reductions in TNF-R p75 and IL-1β levels, as detected by ELISA in stool samples, with the clinical findings indicates that these stool cytokine and receptor measurements are useful in the follow-up of the efficacy of different treatments in this animal model, and might also be used in patients with IBD to help evaluate the therapeutic result. The use of noninvasive methods such as clinical score and stool samples permits the follow up of the developing colitis in these mice, without bias and does not interfere with their comfort or health as, for example, blood letting to serially monitor inflammatory markers does.
The present study extends to this animal model the findings of many clinical trials in which anti-TNF treatment has been successful in the treatment of CD [17,18,32]. Treatment with anti-TNF was effective in treating the spontaneously developing colitis of IL-10 deficient mice. The correlation with human clinical results as found in this study infers that this model might be of value in developing and evaluating new treatments for CD, and suggests that this may be the animal model of choice for this type of experiment. In the study here, we did not use isotype matched control antibodies, however, in similar therapeutic experiments isotype control antibodies have been repeatedly used as controls on multiple occasions [25, 28, 33]. They were without therapeutic benefit. It is not clear why the results here are much more definitive than those of Rennick et al. [23]. There are differences in the IL-10 knockout mice with the major ones being the genetics (ours are on a DBA/1 background) and their housing. The actual therapeutic anti-TNF antibody used and its dose of administration may be the most critical feature, as small reductions in dose or neutralizing capacity are important in vivo, as demonstrated by early trials of anti-TNF antibodies in rheumatoid arthritis. For example CDP571 needs to be used at an approximate fold higher concentrations to mimic the effect of infliximab [16,34].
Whether the observed slight increase in the clinical score and the amounts of TNF-R p75 in the stool samples of the anti-TNF treated IL-10 ko mice before sacrifice is due to autoantibodies developing against the anti-TNF given as treatment, thus reducing the effect of the injected anti-TNF, is not known. The antibody used here has a rat Fc, and so is potentially immunogenic. The way it is administered (i.p.) is also potentially more immunogenic than the i.v. route used in humans which is more tolerogenic [35].
The model described here using IL-10 ko mice bred and housed in SPF conditions, followed-up with a clinical score and measuring amounts of TNF-R p75 and IL-1β in stool as well as with histological samples after sacrifice seems to be useful for the evaluation of new possible treatments for CD. The correlation found in this model between stool cytokines and receptors and the clinical findings indicate that stool cytokines might be of value in CD patients for evaluating the effect of the given treatment. Whether stool cytokines might be used in the future to select CD patients likely to benefit most from anti-TNF treatment remains to be evaluated.
Acknowledgments
The authors would like to thank Mr Paul Warden, and the staff of our Biological Services Unit for technical assistance, Mr Philip Connolly for the preparation of the histological sections, and Dr Tom Böhling for kindly helping with photographing the histological slides. Centocor Inc. provided generously anti-TNF for the study. The financial support of Finska Läkaresällskapet, the Wilhelm and Else Stockmann Foundation, the Finnish Academy, Centocor Inc. and the Arthritis and Rheumatism Council of Great Britain are gratefully acknowledged.
REFERENCES
- 1.Crohn B, Ginzburg L, Oppenheimer G. Regional ileitis, a pathological and clinical entity. J Am Med Assoc. 1932;99:1323–9. [Google Scholar]
- 2.Niv Y, Abuksis G, Fraser GM. Epidemiology of Crohn's disease in Israel: a survey of Israeli kibbutz settlements. Am J Gastroenterol. 1999;94:2961–5. doi: 10.1111/j.1572-0241.1999.01371.x. [DOI] [PubMed] [Google Scholar]
- 3.Danze PM, Colombel JF, Jacquot S, et al. Association of HLA class II genes with susceptibility to Crohn's disease. Gut. 1996;39:69–72. doi: 10.1136/gut.39.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cariappa A, Sands B, Forcione D, et al. Analysis of MHC class II DP, DQ and DR alleles in Crohn's disease. Gut. 1998;43:210–5. doi: 10.1136/gut.43.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Plevy SE, Taylor K, et al. Linkage of Crohn's disease to the major histocompatibility complex region is detected by multiple non-parametric analyses. Gut. 1999;44:519–26. doi: 10.1136/gut.44.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trachtenberg EA, Yang H, Hayes E, et al. HLA class II haplotype associations with inflammatory bowel disease in Jewish (Ashkenazi) and non-Jewish caucasian populations. Hum Immunol. 2000;61:326–33. doi: 10.1016/s0198-8859(99)00134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brynskov J, Tvede N. Plasma interleukin-2 and a soluble/shed interleukin-2 receptor in serum of patients with Crohn's disease. Gut. 1990;31:795–9. doi: 10.1136/gut.31.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 9.Tagore A, Gonsalkorale WM, Pravica V, et al. Interleukin-10 (IL-10) genotypes in inflammatory bowel disease. Tissue Antigens. 1999;54:386–90. doi: 10.1034/j.1399-0039.1999.540408.x. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald TT. Inflammatory bowel disease in knockout mice. Current Biol. 1994;4:261–3. doi: 10.1016/s0960-9822(00)00060-9. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation ant their relation to human inflammatory bowel disease. Curr Op Immunol. 1999;11:648–56. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 12.Leach MW, Bean AGD, Mauze S, et al. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am J Pathol. 1996;148:1503–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10 -/- mice: an overview. J Leukoc Biol. 1997;61:389–96. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 14.Braegger CP, Nicholls S, Murch SH, et al. Tumor necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 15.Lukacs NW, Chensue SW, Strieter R, et al. Inflammatory granuloma formation is mediated by TNF-alpha inducible intercellular adhesion molecule-1. J Immunol. 1994;152:5883–9. [PubMed] [Google Scholar]
- 16.Elliott MJ, Maini RN, Feldmann M, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to TNFα. Arthritis Rheum. 1993;36:1681–90. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 17.van Dullemen HM, van Deventer SJH, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 18.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 19.Present DH, Rutgeeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 20.van Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997;113:383–9. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 21.Sands BE, Bank S, Sninsky CA, et al. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn's disease. Gastroenterology. 1999;117:58–64. doi: 10.1016/s0016-5085(99)70550-0. [DOI] [PubMed] [Google Scholar]
- 22.Powrie F, Leach MW, Mauze S, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhigh CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 23.Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice. a model of chronic inflammation. Clin Immunol Immunopathol. 1995;76:174–8. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 24.Saiki T, Mitsuyama K, Toyonaga A, et al. Detection of pro- and anti-inflammatory cytokines in stools of patients with IBD. Scand J Gastroenterol. 1998;33:616–22. doi: 10.1080/00365529850171891. [DOI] [PubMed] [Google Scholar]
- 25.Butler DM, Malfait A-M, Maini RN, et al. Anti-IL-12 and anti-TNF antibodies synergistically suppress the progression of murine collagen-induced arthritis. Eur J Immunol. 1999;29:2205–12. doi: 10.1002/(SICI)1521-4141(199907)29:07<2205::AID-IMMU2205>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Winship DH, Summers RW, Singleton JW, et al. National cooperative Crohn's disease study. study design and conduct of the study. Gastroenterology. 1979;77:829–42. [PubMed] [Google Scholar]
- 27.Raqib R, Lindberg AA, Björk L, et al. Down-regulation of gamma interferon, tumor necrosis factor type I, interleukin 1 (IL-1) type I, IL-3, IL-4, and transforming growth factor β type I receptors at the local site during the acute phase of shigella infection. Infect Immun. 1995;63:3079–87. doi: 10.1128/iai.63.8.3079-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad U S A. 1992;89:9784–8. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg DJ, Davidson N, Kühn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldmann M, Elliott MJ, Woody JN, et al. Anti-tumor necrosis factor-alpha therapy of rheumatoid arthritis. Adv Immunol. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- 31.van Deventer SJH. Immunotherapy of Crohn's disease. Scand J Immunol. 2000;51:18–22. doi: 10.1046/j.1365-3083.2000.00657.x. [DOI] [PubMed] [Google Scholar]
- 32.Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (Infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117:761–9. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 33.Williams RO, Mason LJ, Feldmann M, et al. Synergy between anti-CD4 and antiTNF in the amelioration of established collagen-induced arthritis. Proc Natl Acad Sci USA. 1994;91:2762–6. doi: 10.1073/pnas.91.7.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rankin EC, Choy EHS, Kassimos D, et al. A double blind, placebo-controlled, ascending dose trial of the recombinant humanised anti-TNFα antibody CDP571 in patients with rheumatoid arthritis (RA): a preliminary report. Arth Rheum. 1994;37:S295. [Google Scholar]
- 35.Chiller JM, Habicht GS, Weigle WO. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci USA. 1970;65:551–6. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]