Abstract
Normal human immunoglobulin G (IgG) has anti-inflammatory and immuno-regulatory properties, which are exploited in the therapy of selected diseases. A putative mechanisms of action is the direct regulation of endothelial cell function by natural antiendothelial cell antibodies. Endothelium activation is a critical event in atherosclerosis. We have verified the ability of normal human IgG to modulate endothelial responses to the atherogenic stimuli tumour necrosis factor-α (TNFα) and oxidized low-density lipoproteins (oxLDL) in vitro. Confocal microscopy was used to visualize vascular cell adhesion molecule-1 (CD106) expression on endothelial cells, cytoplasmic free calcium ([Ca++]i) modifications and fluorescein-coupled oxLDL internalization. Cytokine secretion was measured by ELISA on cell supernatants. IgG prevented TNFα induced CD106 membrane expression and an increase in [Ca++]i, and inhibited the secretion of interleukin-6 (IL-6) and macrophage-colony-stimulating factor (M-CSF). IgG also inhibited CD106 expression induced by oxLDL and one pathway of their internalization, but were ineffective on oxLDL induced [Ca++]i rise and apoptosis. F(ab)′2 fragments from IgG, but not monoclonal IgG, reproduce IgG effects. These findings point to a regulatory role for specific antibodies included in circulating normal IgG towards proinflammatory responses of endothelial cells in atherogenesis and suggest possible development of new therapeutic strategies.
Keywords: atherosclerosis, immunoglobulin, endothelial cells, oxidized LDL, TNFα
INTRODUCTION
Normal human IgG has anti-inflammatory and immunomodulatory properties well demonstrated in vivo and in vitro. We have previously reported that normal IgG pools and single healthy donor IgG contains natural antiendothelial cell antibodies (AECA) [1] that interact with living endothelial cell (EC) membrane, are internalized by a process similar to ligand-receptor endocytosis [2,3] and modulate EC basal secretion of thromboxane and endothelin-1 with an anti-inflammatory effect [4]. We have also shown that IgG inhibits EC response to TNFα in terms of secretion of metalloprotease 9 [5], an effect potentially relevant to atherosclerosis, as metalloproteases are involved in plaque matrix remodelling. We have thus addressed the question of whether IgG modulates specific endothelial functions involved in atherogenesis. Dyslipidaemia, vascular inflammation and infections are known to be involved in the development of atherosclerosis through complex processes in which endothelial cells, rather than merely targets, are active elements [6]. Exposed to lipoproteins, shear stress, glycosylated proteins, hypertension, smoke components, and microorganisms, EC become activated and contribute to the selective recruitment of monocytes and T lymphocytes. This events, together with the subendothelial deposition of oxidized low-density lipoproteins (oxLDL), constitute an early step in atheroma formation [7,8]. Autoimmune reactions triggered by infectious or endogenous antigens are involved in the development of atherosclerotic lesions [9]. However induced, activation of endothelial cells entails the expression of adhesion molecules and the secretion of cytokines, chemokines and growth factors. Intercellular adhesion molecule-1 (CD54) and CD106, which interact with leucocyte receptors leucocyte function antigen-1 (CD11a/CD18) and very late antigen-4 (CD49D), respectively, are present in human atherosclerotic plaques [10,11] and in animal models of accelerated atherosclerosis [12]. In particular CD106 has been detected in lipidic striae, before the accumulation of monocytes [13]. M-CSF and IL-6 produced by EC are present in atherosclerotic plaques and are involved in monocyte accumulation and activation.
The role of oxLDL is widely recognized in the atherosclerotic process, and various mechanisms of action have been demonstrated: induction of CD106 expression on EC membrane [14]; stimulation of M-CSF and monocyte chemoattractant protein-1 (MCP-1) secretion by EC [15]; induction of apoptosis in EC [16]; activation of macrophages to secrete cytokines [17]; overloading of macrophages with subsequent foam cell formation [18]; and release of enzymes and oxidant molecules [19].
TNFα, secreted by activated macrophages, increases procoagulant activity of EC, induces EC expression of CD106 and secretion of M-CSF and IL-6 by vascular cells, stimulates smooth muscle cell proliferation, thus amplifying the inflammatory-atherogenic process.
In this study we investigated whether normal human IgG interferes with EC responses to TNFα and oxLDL by analysing intracellular free calcium ([Ca++]i) modifications, CD106 expression, IL-6 and M-CSF secretion, and oxLDL internalization in living endothelial cells. We utilized an IgG concentration in the low range of normal serum, previously demonstrated to induce a marked, although not maximal, regulation of endothelial cell function, in studies evaluating dose-dependent effects of IgG [4,5]. We also studied the effect of F(ab)′2 fragments from normal IgG, and IgG with a predominant monoclonal component from a patient with multiple myeloma.
MATERIALS AND METHODS
Endothelial cell culture
EC were obtained from umbilical cord by collagenase digestion and cultured in DMEM with 20% fetal bovine serum (FBS) [1].
Sources of normal IgG
Normal human IgG were pools from thousands of blood donors commercially available for intravenous therapy (IVIg) (Sandoglobulin, Sandoz, SA, Basel, Switzerland). IVIg was extensively dialysed in the same DMEM aliquot used to grow EC, and sterile filtered. IgG concentration was measured by radial immunodiffusion (Nor Partigen Plates, Scoppito, L’Aquila, Italy) and by spectrophotometry. It was adjusted to the desired value with sterile medium. In all experiments incubation of EC with IVIg was performed in the presence of 20% FCS.
F(ab)′2 fragments preparation
F(ab)′2 fragments were prepared from IVIg by pepsin digestion (2% wt/wt) (Sigma Aldrich, Milan, Italy) as described [1]. After confirming the absence of IgG contamination by means of polyacrylamide gel electrophoresis, F(ab)′2 fragments were dialysed in the same DMEM aliquot used to grow EC. F(ab)′2 concentration was measured by spectrophotometry and adjusted to the desired value with sterile medium. In all experiments incubation of EC with F(ab)′2 fragments was performed in the presence of 20% FCS.
IgG from a multiple myeloma patient
IgG was purified from the serum of a patient affected by multiple myeloma (MIgG) by affinity chromatography on a protein G-Sepharose column as described [2]. Aliquots of IgG at the desired concentration to incubate with EC were prepared as described for IVIg. Total serum IgG in this patient was 46 mg/ml; the serum electrophoresis pattern and the strong inhibition of IgA and IgM production (serum concentrations of 0·99 and 0·1 mg/ml, respectively) indicate that the monoclonal component was predominant in circulating IgG. In all experiments incubation of EC with MIgG was performed in the presence of 20% FCS.
TNFα and oxLDL preparation
Recombinant TNFα (Sigma Aldrich) was solubilized in DMEM and sterile filtered. LDL were obtained from fresh plasma of healthy donors by sequential centrifugation [20]. LDL concentration was measured according to Lowry's method and adjusted to 200 µg/ml in PBS with 20 µmol/l CuSO4. After 24 h of incubation at 37°C, the oxidative reaction was blocked by adding 40 µmol/l butylhydroxytoluene in ethanol, dialysed against culture medium and sterile filtered. The oxidative modification was verified by the change in the electrophoretic mobility as compared to normal LDL [21,22].
EC surface IgG detection
EC were grown on coverslides, incubated with 0·05 mmol/l (corresponding to 8 mg/ml) IVIg for 24 h. Coverslides were mounted on a microincubator fitted to the stage of a Multiprobe 2001 confocal microscope (Molecular Dynamics, Sunnyvale, CA, USA). This device allows time lapse, long-term observation of a preselected microscopic field in living cells and ad libitum changes of the culture medium [3]. Cells were then washed with culture medium and incubated with an antihuman IgG fluoresceinated antibody (Dakopatt, Milan, Italy) and observed to verify whether IgG was present on the cell membrane.
Immunohistochemistry for CD106 detection
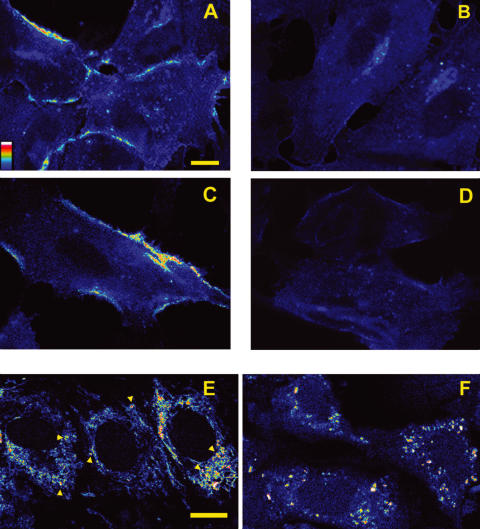
EC grown on slides were incubated with 1 nmol/l TNFα for 4 h, washed and fixed for 2 min with methanol at 4°C. After washing, cells were incubated with a mouse monoclonal antihuman CD106 (Serotec Ltd, Oxford, UK), washed and the reaction revealed with an antimouse antibody coupled to fluorescein. Slides were observed under the confocal laser scanning microscope (CLSM). When testing the effect of IgG on TNFα induced CD106 expression, cells were pre-incubated for 24 h with 0·05 mmol/l IVIg and washed before TNFα addition. Color images were rendered in discrete pseudocolor scale (range 0–255) and the relevant palette is included in Fig. 1a(ImageSpace, Molecular Dynamics, Sunnyvale, CA, USA).
Fig. 1.
(A–D) Effect of IgG on TNFα and oxLDL-dependent CD106 expression by EC. (A) EC incubated with 1 nmol/l TNFα for 4 h exposed CD106 as shown by direct immunofluorescence with a monoclonal antihuman CD106. (B) expression of CD106 was entirely inhibited in EC pre-incubated with 0·05 mmol/l IVIg for 24 h and washed before TNFα incubation. (C) immunofluorescence membrane labelling of CD106 after 4 h incubation with oxLDL 40 µg/ml. (D) pre-incubation with 0·05 mmol/l IVIg for 24 h completely prevented CD106 expression. Scale bar = 5 µm. (E–F) Effect of IgG on oxLDL internalization by EC. Intracellular signal was detectable by CLSM after about 10 minutes incubation of EC with 40 µg/ml oxLDL-FITC. (E) the fluorescence pattern after 40 min incubation includes filaments and granules (arrowheads: granules). (F) pretreatment with 0·05 mmol/l IVIg for 24 h strongly reduces internalization of oxLDL, in particular filaments are not detectable. This image was acquired after 40 min incubation with oxLDL. Scale bar = 10 µm. The colour scale shown in panel A applies to all colour images.
Monocyte adhesion assay
THP-1, a human monocyte cell line, was obtained from the American Type Culture Collection (TIB202) and cultured in RPMI 1640 with 10% FBS. THP-1 were diluted at 4 × 104 cells/ml in DMEM and added to EC grown on multiwell slides: (1) non treated EC; (2) EC incubated with 0·05 mmol/l IVIg for 24 h and washed with DMEM; (3) EC treated with TNFα for 4 h and washed with DMEM; (4) EC incubated with 0·05 mmol/l IVIg, washed with culture medium and treated with TNFα for 4 h and finally washed with DMEM. After 20 min incubation with THP-1, EC were extensively washed with PBS at 37°C and fixed with methanol at 4°C for 2 min Slides were processed according to May–Grunwald Giemsa staining. THP-1 and EC were counted and results expressed as percentage THP-1/EC. Counting was carried out on five microscopic fields for each EC treatment in a series of 4 separate experiments.
Measurements of IL-6 and M-CSF in EC supernatant
EC were grown to confluence on 24-well culture plates. After incubating half of the cells with IVIg, or F(ab)′2 fragments of IVIg, or MIgG at 0·05 mmol/l, for 24 h, all EC were washed with culture medium and incubated with TNFα at concentrations of 0·04, 0·2, 1, and 5 nmol/l or with culture medium only. Supernatants were collected after 24 h, centrifuged and tested for cytokine concentration with commercially available ELISA kits (Amersham Pharmacia Biotech Italia, Milano, Italy). Aliquots of the solutions containing IVIg, F(ab)′2 and MIgG were also tested for IL-6 and M-CSF concentrations. Cell supernatants were tested for IgG concentration.
Free cytoplasmic calcium modifications
Coverslides with subconfluent living EC were mounted on the microincubator fitted on the stage of the confocal microscope as described above. EC were loaded with 15 µmol/l Fluo-3 (Molecular Probes, Eugene OR, USA) until a stable intracellular signal was recorded (usually 20 min). In this set of experiments we employed EC both in standard culture conditions and upon pre-incubation for 24 h with 0·05 mmol/l IVIg. IVIg was washed away before the application of medium containing 1 nmol/l TNFα or 40 µg/ml oxLDL. In the case of oxLDL, experiments were also carried out in the presence of IVIg during the stimulation. TNFα and oxLDL were washed after 10 min with standard culture medium. Images were acquired at fixed intervals. Intracellular fluorescence was measured by the software implemented with the confocal microscope data system (ImageSpace, Molecular Dynamics, Sunnyvale, CA, USA) which assigns to each cell-pertaining pixel a discrete value of intensity from zero to 255.
Visualization of EC apoptosis
EC were loaded with the vital fluorescent probe calcein-AM, which allows both the visualization of morphological signs of apoptosis and assessment of the preservation of plasma membrane integrity throughout the process [23]. For qualitative evaluation a preselected microscopic field was tracked in time-lapse confocal microscopy every 30 min up to 12 h. For quantitative purposes cells were seeded on a four-chamber slide in four separate experiments. In each one the percentage of apoptotic elements was counted in 16 microscopic fields (4 fields per well) by two observers after 4, 6, 8 and 12 h from treatment with LDLox (40 µg/ml). During observation cells were kept in controlled microenvironmental conditions as described above.
Visualization of oxLDL interaction with living EC
OxLDL were coupled to fluorescein isothiocyanate (oxLDL-FITC) as described [2]. Coverslides with subconfluent EC were placed in the above described CLSM flow chamber and incubated for 20 min in standard culture medium with 40 µg/ml oxLDL-FITC and then washed. Images were acquired every 5 min in the first 30 min and every 30 min thereafter. Images were rendered in a discrete scale of 256 colours. We also tested the effect of 24 h pre-incubation with 0·05 mmol/l IVIg in DMEM.
Statistical analysis
Student's t-test for unpaired data was adopted to assess the statistical significance of differences in cytokine production by EC and one way anova and Bonferroni's test for THP-1 adhesion to EC.
RESULTS
IVIg effects on EC viability
The incubation of EC with IVIg, at the physiological concentrations used, did not induce any modification in morphology, as assessed by confocal microscopy, cell count and LDH measurement on lysed cells (data not shown).
Clearing of IVIg from EC membrane
As reported in our previous papers IVIg partly and specifically bind to EC membrane and this IgG subset is rapidly internalized [2,3,5]. In this study we excluded the presence of residual membrane bound IgG after incubation with IgG and washing: surface immunoglobulins were not detectable by immunostaining with a FITC conjugated antihuman IgG antibody (data not shown).
Effects of IVIg on endothelial responses to TNFα
CD106 expression
1 nmol/l TNFα induced CD106 expression on endothelial cell membrane after 1 h. Longer incubations up to 4 h resulted in a more intense labelling, although the immunofluorescence pattern did not change (Fig. 1a). Control cells, incubated with culture medium only, did not expose CD106 on the cell suface (not shown).
Pretreatment of cells with IVIg completely inhibited CD106 expression elicited by TNFα incubation, performed after removal of IgG (Fig. 1b).
Monocytes adhesion assay
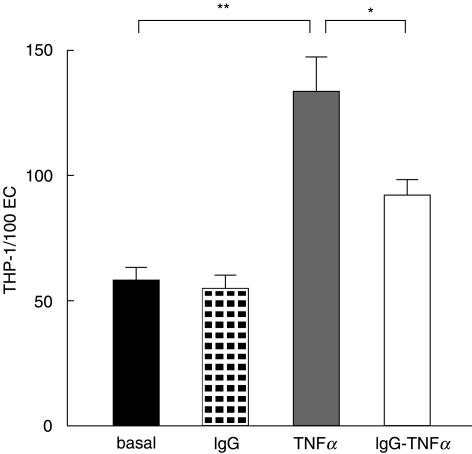
THP-1 adhesion to EC was significantly enhanced by pretreatment of EC with 1 nmol/l TNFα for 4 h. This effect was partially but significantly prevented by pre-incubation of EC with 0·05 mmol/l IVIg (Fig. 2).
Fig. 2.
Pretreament with IVIg significantly inhibited TNFα induced increased monocyte adhesion to EC. (Mean ± SD; n= 4; *P < 0·05; **P < 0·001)
Cytokine secretion
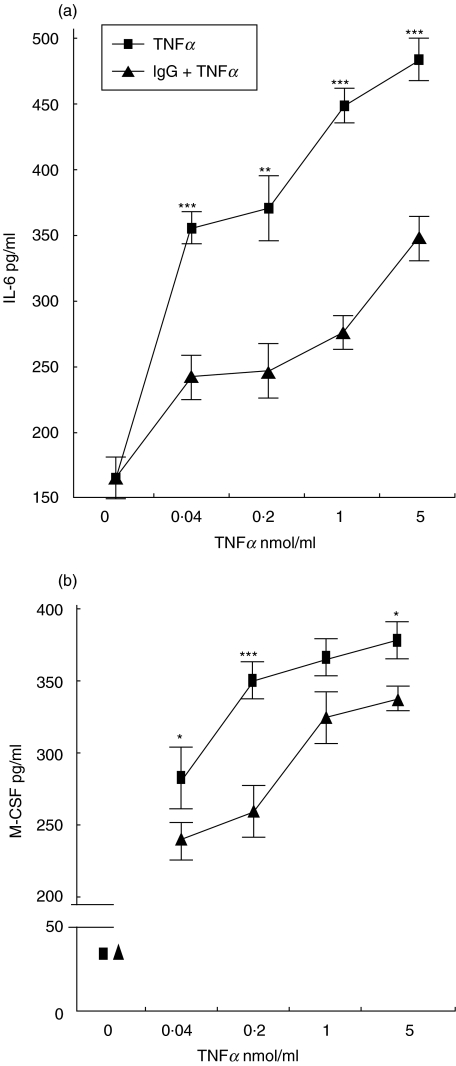
Basal IL-6 secretion of confluent EC incubated for 12 h with standard culture medium was 165 ± 15 pg/ml (mean ± SD). The treatment of cells with 0·04, 0·2, 1, and 5 nmol/l TNFα induced a two to three fold dose-dependent increase in IL-6 secretion (Fig. 3a). Pretreatment of cells with IVIg before TNFα stimulation, performed in absence of IgG, significantly inhibited IL-6 production (Fig. 3a). Similarly a dose-dependent increase in M-CSF secretion was obtained by TNFα stimulation. The increase ranged between seven- and nine-fold for the lowest and the highest dose tested, respectively. Pre-incubation with IVIg had a slight, but statistically significant inhibitory effect on TNFα action (Fig. 3b). The solutions containing IVIg, F(ab)′2 fragments of IVIg and MIgG were found negative for contaminating IL-6 and M-CSF. Cell supernatants obtained for cytokines measurement were tested for IgG concentration in the hypothesis that IgG could be released by EC during the experiment. IgG were not detectable.
Fig. 3.
(a) effect of IgG on TNFα-dependent increase in IL-6 secretion by EC. TNFα induced a dose-dependent increase in EC IL-6 secretion (▪), that was partially inhibited in cells exposed to 0·05 mmol/l IVIg for 24 h and washed before TNFα stimulation (▴). Lower panel: effect of IgG on TNFα-dependent increase in M-CSF secretion by EC. TNFα induced a dose-dependent increase in EC M-CSF secretion (▪), that was partially inhibited by 0·05 mmol/l IVIg for 24 h (▴). (Mean ± SD; n= 6; *P < 0·01, **P < 0·001, ***P < 0·0001).
Free calcium modifications
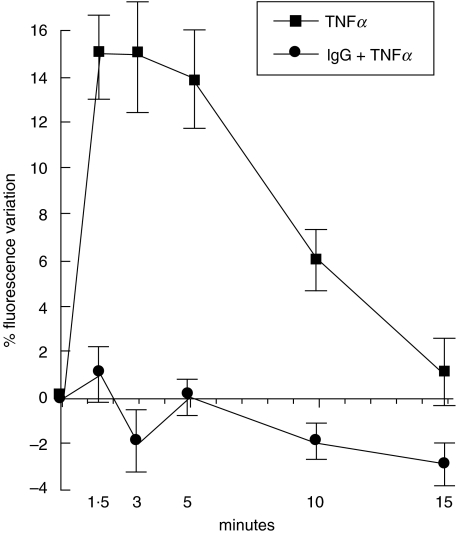
EC loaded with 15 µmol/l Fluo-3 showed a stable fluorescent signal after about 20 min, without signs of cell distress. Immediately after addition of 1 nmol/l TNFα to the culture medium we recorded an increase in cell fluorescence that was maximal within 1·5 min (Fig. 4). The signal was stable for the following 3 min and then began to decrease, reaching intensities close to baseline at about 15 min from time zero. IVIg pre-incubation of EC completely prevented TNFα-induced [Ca++]i increase (Fig. 4).
Fig. 4.
Effect of IgG on TNFα-dependent [Ca++]i modification in EC. 1 nmol/l TNFα induced a sharp and immediate intracellular signal rise in EC loaded with the fluorescent calcium sensitive nonratiometric probe Fluo-3 (15 µmol/l) (▪). Return to baseline occurred in about 15 min. Values are expressed as percent variation in fluorescence (mean ± SD; n= 4). A complete inhibition of free cytoplasmic calcium response to TNFα was observed following 0·05 mmol/l IVIg pre-incubation for 24 h (•).
Effects of IVIg on endothelial responses to oxLDL
Internalization of oxLDL
40 µg/ml oxLDL-FITC was internalized by EC within about 10 min, although intracellular fluorescent signal was maximal after 1 h (Fig. 1E). The fluorescence pattern consisted mostly of filaments, but small granules were also evident. When cells were pre-incubated with IVIg the fluorescence pattern differed markedly. In particular after 1 h fluorescent filaments were almost absent, although granules were still detectable (Fig. 1F). In both cases the described patterns remained unchanged during the following hour.
CD106 expression
CD106 expression was detectable by immunofluorescence as membrane labelling after 4 h of incubation with 40 µg/ml oxLDL (Fig. 1C), but not in cells pre-incubated with IVIg (Fig. 1D). At this time no signs of morphological distress were detectable in the culture.
Cytokine secretion
The concentration of IL-6 and M-CSF in the supernatant of cells incubated with 0·5, 2·5, and 12·5 µg/ml oxLDL did not differ from that of supernatant of control cells or of cells pre-incubated with IVIg (data not shown). Higher concentrations of oxLDL (from 25 mg/ml and above) induced EC disruption of cell monolayer (see below) which started after about eight hours of incubation, thus preventing a reliable measurement of secreted cytokines.
Free calcium modifications
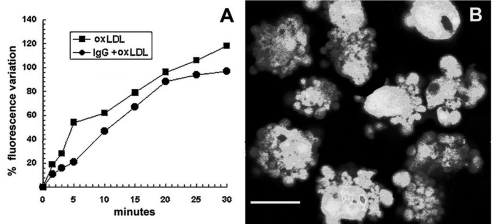
EC were loaded with the calcium sensitive non ratiometric probe Fluo-3 (15 µmol/l) until a stable intracellular signal was recorded (typically 20 min). The addition of oxLDL at 40 µg/ml caused an immediate increase in fluorescence signal, that was progressive and continuous (Fig. 5A). The process was not stopped by washing the cells after 10 min of incubation with oxLDL. After 4–6 h cells showed morphological modifications suggesting the beginning of an apoptotic process (see below). Pre-incubation or coincubation of cells with IVIg did not prevent [Ca++]i increase (Fig. 5A).
Fig. 5.
Effect of IgG on oxLDL-dependent free intracellular calcium modification in EC. (A) The Fluo-3 signal increase (▪) induced by oxLDL was progressive and continuous. High [Ca++]i lasted for several hours until cells started apoptosis. Neither EC pre-incubation nor coincubation with 0·05 mmol/l IVIg changed the effect of oxLDL on [Ca++]i (•). (B) CLSM image of calcein loaded EC in advanced apoptosis, 10 h after 10 min exposure to 40 µg/ml oxLDL. Note chromatin condensation and prominent blebbing. Neither pre-incubation nor coincubation with IVIg prevented oxLDL-induced apoptosis. Scale bar = 5 µm.
Apoptosis induction
In order to verify the actual onset of apoptosis upon stimulation with oxLDL (40 µg/ml, 10 min), EC were loaded with the vital fluorescent probe calcein-AM, which allows both the visualization of morphological signs of apoptosis and assessment of the preservation of plasma membrane integrity throughout the process [23]. In particular, the first few cellular elements undergoing the process were detected after 4–6 h by chromatin condensation and initial bleb formation with preserved membrane integrity. In advanced apoptosis the typical nuclear fragmentation and apoptotic bodies were detectable (Fig. 5B). After 12 h almost 100% of cells were found at different steps of the process. A quantitative evaluation yield the following results. After 4, 6 and 8 h the percentage of apoptotic cells was 14·8 ± 3·6, 36·2 ± 6·7, and 51·9 ± 9·4, respectively. Apoptosis could not be prevented by pre-incubation nor coincubation with IVIg.
Effects of F(ab)′2 fragments of IVIg and MIgG on TNFα-induced IL-6 secretion by EC
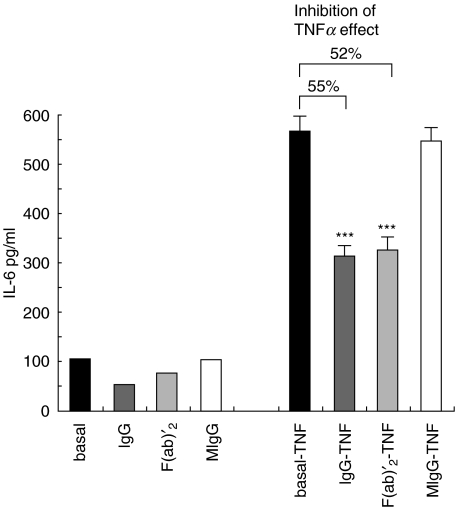
The ability of F(ab)′2 fragments of IVIg and MIgG to reproduce the effect of intact pooled IgG was investigated on TNFα-induced EC secretion of IL-6. Incubation of EC with 0·05 mmol/l F(ab)′2 fragments for 24 h before TNFα treatment resulted in a significant inhibition of IL-6 secretion increase (52%), similar to that induced by IVIg (55%). On the contrary, no inhibition of TNFα response was observed in EC pretreated with MIgG obtained from the patient affected by multiple myeloma (Fig. 6).
Fig. 6.
Effects of F(ab)′2 fragments of IVIg and MIgG on TNFα-induced IL-6 secretion by EC. The graph shows IL-6 concentration in the supernatants of control EC and EC treated for 24 h with 0·05 mmol/l IVIg, F(ab)′2 fragments of IVIg and MIgG (bars on the left), and in the supernatant of the same cells after stimulation with 1 nmol/l TNFα for 16 h (bars on the right). F(ab)′2 of IVIg, but not MIgG, reproduced the effect of intact IgG (Mean ± SD; n= 4; *P < 0·01, **P < 0·001, ***P < 0·0001).
DISCUSSION
The role of endothelial cell activation and damage in early phases of atherosclerosis and in vasculitis is well recognized [6,8]. We have previously demonstrated that normal human IgG directly modulates some functions of resting endothelial cells in vitro at physiological concentrations, thus reducing proinflammatory responses [4,5]. Similar findings have been reported on EC using concentrations of IgG that are reached in the serum of autoimmune patients treated with high doses of IVIg [24].
In the present study we have investigated the ability of normal human IgG to inhibit EC activation induced by TNFα and oxLDL, two factors critically involved in early phases of atherosclerosis. The experimental design allowed us to exclude extracellular direct interaction of IVIg with TNFα or oxLDL, as EC were pre-incubated with IVIg, F(ab)′2 fragments of IVIg or MIgG, and then washed and finally exposed to the atherogenic stimuli. At the physiological concentration employed in this study IgG did not modify cell morphology, viability or number while higher doses have been reported to induce apoptosis [24].
OxLDL and TNFα induced the expression of CD106 on EC plasma membrane within 4 h of incubation. IVIg pretreatment resulted in a complete inhibition of CD106 translocation on the cell membrane. The finding of a direct modulation of endothelial cell CD106 expression by IgG is relevant to atherosclerosis because CD106, absent in resting endothelium, is expressed early in lipidic striae [13] and in advanced lesions [11] in humans. It binds to the receptor very late antigen-4 (CD49D) of monocytes and T lymphocytes. It is noteworthy that neutrophils, which do not express CD49D, are not present in atherosclerotic lesions [13].
Indeed the reduced monocytes adhesion to EC incubated with IVIg first and then with TNFα, as compared to cells treated with TNFα only, that we have shown, is an additional evidence of the relevance of CD106 inhibition and anti-inflammatory effect of IVIg on EC.
EC stimulated with TNFα responded with a dose-dependent increase in IL-6 and M-CSF production that was significantly inhibited in cells pre-incubated with IVIg. The intereference of contaminating cytokines in stimuli solutions and of surface-bound or released IgG were excluded. IL-6 and M-CSF have been demonstrated in atherosclerotic plaques in humans [25] and in lipidic striae in animal models of atherosclerosis [26]. M-CSF is chemotactic for leucocytes, activates macrophages and increases their lipid uptake [27]. IL-6 activates platelets and the clotting cascade [28] and its administration to animals accelerates the atherosclerotic process [29]. Moreover IL-6 can react with EC in an autocrine loop, amplifying and sustaining EC activation. Thus IgG modulation of EC IL-6 and M-CSF production in response to TNFα may also be an important antiatherogenic and anti-inflammatory factor.
TNFα induced a rapid and transient [Ca++]i increase, consistent with that reported by others [30] and with the notion that free cytoplasmic Ca++ is a second messenger mediating for instance IL-6 and CD106 expression [31,32]. Therefore, the complete prevention of [Ca++]i increase induced by IgG pre-incubation may contribute to explain the observed inhibition of CD106 expression and IL-6 production. TNFα did not induce apoptosis on EC, whether pretreated or not with IVIg. Our finding is only in apparent contrast with a recent paper [33], reporting that apoptosis occurs in EC after a two-step stimulation with TNFα first and then with IVIg, both at much higher concentrations than those we used. In that study, anyway, TNFα or IVIg alone did not induce apoptosis, and a double step stimulation with IVIg first and TNFα second was not performed.
Stimulation of EC with oxLDL induced both an immediate and constant rise in [Ca++]i and apoptosis. A link between [Ca++]i and apoptosis induced by oxLDL has already been reported [16]. IVIg pretreatment of cells did not prevent either [Ca++]i increase nor apoptosis, even when IgG was present in culture media throughout the entire experiment. However IgG prevented oxLDL induction of CD106 expression in the first 4 h, when apoptosis was not yet detectable. The apparent discrepancy with the lack of inhibition of [Ca++]i increase may reflect the fact that transduction mechanisms mediating CD106 expression other than [Ca++]i may be activated upon oxLDL stimulation. OxLDL is a complex mixture of molecules, including one form of apolipoprotein B, cholesteryl esters, triglycerides and phospholipids. Many molecular alterations to LDL have been reported following oxidation, e.g. increased lysophosphatidylcholine/phosphatidylcholine ratio, with increased length of acyl groups, peroxidation and fragmentation of the lipid component and modification of apolipoprotein B, the presence of oxysterols, isoprostanes, and the formation of a PAF-like substance [34,35]. The mechanisms of action of oxLDL on EC may be the result of both oxidation of cell membrane components and a specific interaction with the endothelial receptor LOX-1 or other receptors [35,36]. We have shown here that oxLDL internalization by EC is rapid and in the form of two main morphological patterns, filaments and granules. IgG pre-incubation of EC entirely inhibits filaments appearance unaffecting internalization of oxLDL as granules. The new data on oxLDL internalization by EC and its partial inhibition by IgG that we present here suggest that oxLDL interaction with EC is a complex event including more than one pathway.
Addressing the important question of the specificity of the observed effects of IgG on EC, we have shown that F(ab)′2 fragments of IVIg entirely reproduce the action of intact IgG. Moreover our observation that IgG purified from a multiple myeloma patient is ineffective provides a direct demonstration not only that IgG action is specific, but also Fc-independent. Our present observation that IgG modulates EC function independently of Fc region well correlates with our previous results indicating that internalization of natural antibody subpopulation reacting with EC (AECA) is also a Fc-independent process [2].
In summary, in the present study we have shown that normal human IgG specifically reduces EC proinflammatory functional responses to TNFα in terms of CD106 expression and cytokine secretion, and completely prevents the associated rise of [Ca++]i. IgG also inhibits oxLDL-dependent CD106 expression, and at least one of the observed internalization pathways for oxLDL, but does not affect [Ca++]i increase and apoptosis induced by oxLDL. These findings, together with our previous studies regarding IgG modulation of endothelial functions, point to a physiological homeostatic role for normal human IgG and contribute to an explanation of the in vivo anti-inflammatory properties of IVIg in humans. A detailed knowledge of molecular mechanisms of endothelial function regulation by normal IgG may lead to novel therapeutic strategies for the control of vascular inflammation and atherosclerosis.
Acknowledgments
The authors wish to thank Dr Angela Pirillo of the Department of Pharmacological Sciences, University of Milan, for providing oxLDL. This research was supported by FIL-2000. CLSM is a facility of Centro Interfacoltà Misure – University of Parma.
REFERENCES
- 1.Ronda N, Haury M, Nobrega A, et al. Analysis of natural and disease-associated autoantibody repertoires: anti-endothelial cell IgG autoantibody reactivity in the serum of healthy individuals and patients with systemic lupus erythematosus. Int Immunol. 1994;6:1651–62. doi: 10.1093/intimm/6.11.1651. [DOI] [PubMed] [Google Scholar]
- 2.Ronda N, Gatti R, Orlandini G, et al. Binding and internalization of human IgG by living cultured endothelial cells. Clin Exp Immunol. 1997;109:211–20. doi: 10.1046/j.1365-2249.1997.4051305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlandini G, Ronda N, Gatti R, et al. Receptor-ligand internalization. Meth Enzymol. 1998;307:340–8. doi: 10.1016/s0076-6879(99)07022-6. [DOI] [PubMed] [Google Scholar]
- 4.Oravec S, Ronda NMI, Iliez J, et al. Normal human polyspecific immunoglobulin G (intravenous immunoglobulin) modulates endothelial cell function in vitro. Nephrol Dial Transplant. 1995;10:796–801. [PubMed] [Google Scholar]
- 5.Ronda N, Leonardi S, Orlandini G, et al. Natural anti-endothelial cell antibodies (AECA) J Autoimm. 1999;13:121–9. doi: 10.1016/s0896-8411(99)99999-7. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis-An inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. The patogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 8.Rubanyi GM. The role of endothelium in cardiovasular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22:S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 9.Mayr M, Kiechl S, Willeit J, et al. Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000;102:833–9. doi: 10.1161/01.cir.102.8.833. [DOI] [PubMed] [Google Scholar]
- 10.Poston R, Haskard D, Coucher J, et al. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Patohol. 1992;140:665–73. [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien KD, Allen MD, McDonald TO, et al. Vascular cell adhesion molecule-1 is expressed in human coronary artery atherosclerotic plaques. implication for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cybulsky MI, Gimbrone MA. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 13.Saxena U, Golberg IJ. Endothelial cells and atherosclerosis: lipoprotein metabolism, matrix interactions, and monocyte recruitment. Current Opinion Lipidol. 1994;5:316–22. doi: 10.1097/00041433-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–44. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakura E, Tojo N, Tanabe T. Monocyte proliferation induced by modified serum is associated with endogenous M-CSF production. evidence for involvement of a signalling pathway via scavenger receptors. Cell Prolif. 1999;32:185–94. doi: 10.1046/j.1365-2184.1999.3240185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escargueil BI, Meilhac O, Pieraggi MT, et al. Oxidized LDLs induce massive apoptosis of cultured human endothelial cells through a calcium-dependent pathway. Prevention by aurintricarboxylic acid. Arterioscler Thromb Vasc Biol. 1997;17:331–9. doi: 10.1161/01.atv.17.2.331. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Virella MF, Virella G. Atherosclerosis and autoimmunity. Clin Immunol Immunopathol. 1994;73:155–67. doi: 10.1006/clin.1994.1184. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg D. Arterial metabolism of lipoproteins in relation to atherogenesis. Ann NY Acad Sci. 1990;598:125–35. doi: 10.1111/j.1749-6632.1990.tb42284.x. [DOI] [PubMed] [Google Scholar]
- 19.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–92. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havel RJ, Eder HA, Bradgon JH. The distribution and the chemical composition of iltracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roma P, Catapano AL, Bertulli SM, et al. Oxidized LDL increase free cholesterol and fail to stimulate cholesterol esterification in murine macrophages. Biochem Biophys Res Commun. 1990;171:123–31. doi: 10.1016/0006-291x(90)91365-y. [DOI] [PubMed] [Google Scholar]
- 22.Pirillo A, Norata GD, Zanelli T, et al. Overexpression of inducible heat shock protein 70 in COS-1 cells fails to protect from cytotoxicity of oxidized LDLs. Arteriosclerosis Thrombosis Vascular Biol. 2001;21:348–54. doi: 10.1161/01.atv.21.3.348. [DOI] [PubMed] [Google Scholar]
- 23.Gatti R, Belletti S, Orlandini G, et al. Comparison of annexin V and calcein-AM as early vital markers of apoptosis in adherent cells by confocal laser microscopy. J Histochem Cytochem. 1998;46:895–900. doi: 10.1177/002215549804600804. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Poirier B, Duong Van Huyen JP, et al. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins. Am J Pathol. 1998;153:1257–66. doi: 10.1016/S0002-9440(10)65670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenfeld ME, Yla-Hertuala S, Lipton BA, et al. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Hansson GK. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand J Immunol. 1999;50:25–30. doi: 10.1046/j.1365-3083.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 27.Clinton SK, Underwood R, Hayes L, et al. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–16. [PMC free article] [PubMed] [Google Scholar]
- 28.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 1999;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 29.Huber SA, Sakkinen P, Conze D, et al. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–7. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 30.Kuebler WM, Parthasarathi K, Wang PM, et al. A novel signaling mechamism between gas and blood compartments of the lung. J Clin Invest. 2000;105:857–8. doi: 10.1172/JCI8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi L, Li Y, Stehno-Bittel L, et al. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2001;21:231–40. doi: 10.1089/107999001750169871. [DOI] [PubMed] [Google Scholar]
- 32.Allen S, Khan S, Al-Mohanna F, et al. Native low density lipoprotein-induced calcium transients trigger VCAM-1 and E-selectin expression in cultured human vascular endothelial cells. J Clin Invest. 1998;101:1064–75. doi: 10.1172/JCI445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakatani K, Takeshita S, Tsujimoto H, et al. Intravenous immunoglobulin (IVIG) preparations induce apoptosis in TNF-alpha-stimulated endothelial cells via a mitochondria-dependent pathway. Clin Exp Immunol. 2002;127:445–54. doi: 10.1046/j.1365-2249.2002.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colles SM, Maxon JM, Carlson SG, et al. Oxidized LDL-induced injury and apoptosis in atherosclerosis. Potential Roles for Oxysterols Cardiovascular Med. 2001;11:131–8. doi: 10.1016/s1050-1738(01)00106-2. [DOI] [PubMed] [Google Scholar]
- 35.Rader DJ, Dugi KA. The endothelium and lipoproteins: insights from recent cell biology and animal studies. Seminars Thrombosis Hemostasis. 2000;26:521–8. doi: 10.1055/s-2000-13208. [DOI] [PubMed] [Google Scholar]
- 36.Kume N, Kita T. Roles of lectin-like oxidized LDL receptor-1 and its soluble forms in atherogenesis. Current Opinion Lipidol. 2001;12:419–23. doi: 10.1097/00041433-200108000-00008. [DOI] [PubMed] [Google Scholar]