Abstract
Acute rejection (AR) is the principal risk factor for obliterative bronchiolitis (OB), the major complication of lung transplantation. It is known that activated CD4+ T lymphocytes are involved in the development of AR and that interleukin (IL)-16 can inhibit the activity of CD4+ T lymphocytes. In this study, we evaluated whether the concentration of IL-16 in the airways is altered in AR or OB and, if so, how this IL-16 concentration relates to the number or activity of airway lymphocytes. The concentration of IL-16 protein was measured in bronchoalveolar lavage (BAL) fluid at three time-points in lung allograft recipients with either AR or OB and in matched controls using ELISA. The concentration of soluble IL-2 receptor (R) protein was measured in BAL fluid using ELISA as well, as an indicator of lymphocyte activity. The percentage of airway lymphocytes was evaluated by performing BAL differential cell counts. Lung allograft recipients with AR displayed lower IL-16 concentrations compared with matched control patients and this IL-16 concentration correlated negatively with the sIL-2R concentration, but it did not correlate with the percentage of lymphocytes in BAL fluid. In contrast, in BAL fluid from lung allograft recipients with OB, the IL-16 concentration was not altered compared with matched control patients and it did not correlate with the percentage of lymphocytes or with the sIL-2R concentration. These data are compatible with an increase in IL-16 playing a protective role against AR but not against OB and, hypothetically, this type of protective effect could be exerted via a down-regulation of the activity of T lymphocytes.
Keywords: acute rejection, IL-16, lung allograft, lymphocyte, obliterative bronchiolitis
INTRODUCTION
The long-term functional status and survival of lung allograft recipients is often compromised by development of chronic rejection and obliterative bronchiolitis (OB) [1]. With a prevalence of 30–40% of lung allograft recipients, OB leads to progressive airway obstruction, increases the risk for infection and is the most common cause of late death (>6 months) in this patient group [2]. The principal risk factor for OB is recurrent attacks of acute rejection (AR), the major non-infectious early complication of lung transplantation [2,3].
It is believed currently that AR is controlled by the helper (CD4+) subset of T lymphocytes which, via the T cell receptor (TCR)/CD3 complex, recognize donor allopeptides presented to the CD4+ T lymphocytes by antigen-presenting cells [4]. As a consequence, the activated CD4+ T lymphocytes secrete cytokines that stimulate proliferation and activation of cytotoxic (CD8+) T lymphocytes and alveolar macrophages, inflammatory cells that contribute in turn to graft injury.
It is known that the CD4 surface molecule participates in the antigen presentation process as a co-receptor and that the participation of CD4 is needed for activation of T lymphocytes following antigen presentation to the TCR/CD3 complex [5]. Accordingly, the ligation of the CD4 receptor in this TCR/CD3/CD4 complex by a non-depleting antibody leads to inhibition of the antigen-induced lymphocyte activation [6]. Hypothetically, the mechanisms regulating the CD4 co-receptor activity on T lymphocytes could therefore be involved in AR.
The cytokine interleukin (IL)-16 (previously lymphocyte chemoattractant factor) is produced in the airways mainly by the CD8+ subset of T lymphocytes and by bronchial epithelial cells [7,8]. Being a ligand for the CD4 receptor, IL-16 causes effects restricted exclusively to cells bearing this surface molecule, including subsets of T lymphocytes, macrophages and eosinophils [8,9]. IL-16 was characterized initially as a cytokine recruiting CD4+ T lymphocytes [10] and inducing expression of IL-2 receptors (IL-2R) on these cells, thereby increasing the activity and proliferation of CD4+ T lymphocytes [11]. Interestingly, more recent studies have indicated that IL-16 also exerts anti-inflammatory actions in antigen specific events. Pretreatment of human T lymphocytes with IL-16 inhibits antigen- and anti-CD3-induced activation and proliferation of T lymphocytes [12,13]. This effect is mediated probably through the ligation of CD4 co-receptor activity in the TCR/CD3/CD4 complex, blocking the TCR-mediated activation of T lymphocytes. Accordingly, IL-16 has been shown to inhibit the release of proinflammatory cytokines in an in vivo model of rheumatoid arthritis [14] and the release of pro-allergic cytokines in cultures of CD4+ T lymphocytes from atopic subjects [15]. IL-16 thus constitutes a potentially immunosuppressive cytokine.
IL-16 can also play a role in airway inflammation. It is known that the concentration of IL-16 in the airways is increased in patients with allergic asthma [16] as well as in smokers with and without airway symptoms [17]. In bronchoalveolar lavage (BAL) fluid of allergen-challenged asthmatic patients, IL-16 accounts for the major part of the chemotactic activity for lymphocytes [18], whereas in smokers the increased airway IL-16 concentration is associated with decreased number and increased responsiveness of systemic T lymphocytes [17]. At present, however, the airway concentration and function of IL-16 in lung allograft recipients with AR or OB is not known.
The primary aim of this study was to evaluate whether the concentration of IL-16 protein in the airways is altered in lung allograft recipients with AR or OB. The secondary aim was to determine whether a change in this IL-16 concentration is associated with an altered activity or number of airway lymphocytes.
MATERIALS AND METHODS
Study design
Fourteen matched pairs of lung allograft recipients were identified for this study gathered prospectively from all diagnostic and protocol bronchoscopies performed in 80 patients of the Lung Transplant Program of Sahlgrenska University Hospital between 1996 and 2000. The subjects received both oral and written information and thereafter agreed to samples being stored from their bronchoscopies for later analysis of soluble inflammation markers.
Seven lung allograft recipients with AR and seven recipients with OB were matched as far as possible for the analysis of IL-16, sIL-2R and bronchoalveolar lavage (BAL) fluid cells, in order to minimize the influence of confounding factors. Thus, patients were matched for age, gender, pretransplant diagnosis, specific type of lung transplantation, immunosuppressive therapy, absence of infection and the time after transplant surgery when the control bronchoscopy was undertaken. Also, for the same reason, all samples chosen for the study were free from lymphocytic bronchiolitis.
Three consecutive BAL fluid samples were analysed in each lung allograft recipient. In lung allograft recipients with AR, the BAL fluid harvested during an acute mild rejection (A2) was preceded and followed, respectively, by one normal bronchoscopy (in total three bronchoscopies). The matched control patients had no signs of AR during any of the three bronchoscopies, conducted at matched time-points. In lung allograft recipients with OB, one BAL fluid sample was harvested at the time of OB diagnosis, and it was preceded by two normal bronchoscopies (three bronchoscopies in total). The matched control patients had no signs of OB during any of the three bronchoscopies, conducted at matched time-points. The study protocol was approved by the ethical committee for clinical studies at Göteborg University.
Subjects
The clinical characteristics are shown in Table 1. Organ donors and recipients were matched for cytomegalovirus (CMV) serological status, and all organs were harvested in a similar fashion. Surgical procedures and immunosuppressive therapy were performed as described previously [19].
Table 1.
Presurgical diagnosis (D), gender (G), surgical procedure (P) and age (A) of 28 lung allograft recipients, paired into a group with (a) one episode of mild acute rejection (AR) and a group without acute rejection (controls); and into a group with (b) obliterative bronchiolitis (OB) and a group without OB (controls)
| Pair No. | D | G | P | A | D | G | P | A |
|---|---|---|---|---|---|---|---|---|
| (a) | Controls | AR | ||||||
| 1 | PPH | F | Bilateral lung | 40 | PPH | M | Bilateral lung | 53 |
| 2 | ES | F | Heart–lung | 40 | PPH | F | Bilateral lung | 43 |
| 3 | Alpha-1 AT | M | Single lung | 50 | Alpha-1 AT | M | Single lung | 50 |
| 4 | COPD | F | Single lung | 54 | COPD | F | Single lung | 56 |
| 5 | Alpha-1 AT | F | Single lung | 43 | Alpha-1 AT | M | Single lung | 46 |
| 6 | CF | M | Bilateral lung | 30 | CF | F | Bilateral lung | 23 |
| 7 | COPD | F | Single lung | 51 | COPD | F | Single lung | 52 |
| Ratio | 5F/2 M | Median age | 43 | Ratio | 4F/3M | Median age | 50 | |
| (b) | Controls | OB | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | CF | F | Bilateral lung | 28 | CF | M | Bilateral lung | 25 |
| 2 | PPH | M | Bilateral lung | 29 | PPH | F | Bilateral lung | 26 |
| 3 | Alpha-1 AT | M | Single lung | 51 | Alpha-1 AT | M | Single lung | 51 |
| 4 | COPD | M | Single lung | 64 | COPD | M | Single lung | 56 |
| 5 | Alpha-1 AT | M | Bilateral lung | 47 | Alpha-1 AT | F | Bilateral lung | 51 |
| 6 | COPD | F | Single lung | 53 | COPD | F | Single lung | 62 |
| 7 | COPD | F | Single lung | 46 | COPD | F | Single lung | 46 |
| Ratio | 3F/4M | Median age | 47 | Ratio | 4F/3M | Median age | 51 | |
PPH = primary pulmonary hypertension; ES = Eisenmenger's syndrome; Alpha-1 AT = alpha-1 antitrypsin deficiency; COPD = chronic obstructive pulmonary disease; CF = cystic fibrosis.
In lung allograft recipients developing AR, eight patients underwent single lung transplantation, five bilateral lung transplantation and one combined heart–lung transplantation. In lung allograft recipients developing OB, eight patients underwent single lung transplantation and six bilateral lung transplantation.
Postsurgery follow-up
The follow-up design of the Lung Transplant Program of Sahlgrenska University Hospital was applied as described previously [20]. In short, protocol bronchoscopies with transbronchial biopsies (TBB) and BAL were performed at regular intervals post-transplant surgery, and whenever indicated by a deterioration in the clinical condition of the patient. BAL analysis included direct microscopy for cytomegalovirus (CMV) inclusion bodies, Pneumocystis carinii (PCP), fungi and mycobacteria. In addition, immunocytochemistry techniques for PCP, CMV and Legionella pneumophilia in BAL and/or TBB were applied routinely. Cultures for bacteria including legionella and mycobacteria, fungi and virus were performed, and presence of CMV and respiratory syncytial virus (RSV) genome was investigated by polymerase chain reaction (PCR) amplification. Diagnosis of CMV pneumonitis was based on histopathological changes of alveolitis in TBB together with the presence of inclusion bodies in TBB and BAL samples. Diagnosis of bacterial infection was based on the presence of significant bacterial growth in a BAL fluid sample [≥105 colony-forming units (CFU)/ml]. Diagnosis of PCP was based on demonstration of the organism by silver staining of TBB and BAL fluid samples. All samples analysed included were free from co-existing infection.
The morphological evaluation of AR followed the recommendations of the Lung Rejection Study Group of the International Society of Heart and Lung Transplantation (ISHLT) [21]. OB was defined according to the established grading system [22]. It was defined as an irreversible decline in FEV1 of at least 20% of baseline, that was determined in turn as the average maximum FEV1 value of two consecutive measurements 30 days apart during the first postsurgical year. BAL and TBB analysis for infectious agents followed the protocol as described earlier [20].
Collection of samples
All bronchoscopies were performed before 10·30 a.m. in the morning. BAL fluid was harvested by infusion of 6 × 20 ml warmed sterile, pyrogen-free phosphate buffered saline (PBS) solution into a segmental middle lobe or the lingula bronchus with the bronchoscope in a wedged position. The fluid was aspirated after 60 ml infusion each time; it was then collected in a sterile siliconized container and transported immediately on ice to the laboratory. After filtering, cellular components were sedimented by centrifugation at 4°C, 200 g for 10 min, and the supernatant was removed and frozen at –70°C.
Cytocentrifuge slides (Shandon Southern Products Ltd, Runcorn, UK) were made from 100 µl aliquots of the re-suspended cell pellet. Slides were fixed in 96% alcohol and stained (May–Grünwald–Giemsa) for differential counts of cell types on a morphological basis. Percentages of eosinophil granulocytes, neutrophil granulocytes, macrophages and lymphocytes were calculated by counting 200 cells using a standard light microscopy. All samples were analysed in a blinded manner.
TBB were always conducted after BAL. At least five macroscopically adequate biopsy specimens were taken under fluoroscopic guidance from the lower and middle lobes of one lung using alligator forceps, and were placed immediately in 10% buffered formalin and sent for morphological analysis.
IL-16 protein
Frozen BAL fluid supernatants were thawed and concentrated (10-fold) using Centricon-10 centrifugation filters (10-kDa cut-off; Amicon Co., Bevery, MA, USA). The concentration of IL-16 protein was then determined in a blinded fashion using a commercial IL-16 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Europe, Abingdon, UK) in accordance with the manufacturer's instructions. With this ELISA kit, the lowest detectable concentration of IL-16 protein was 31·2 pg/ml.
SIL-2R protein
To assess the activity of T lymphocytes, the concentration of the soluble (s) alpha chain of the IL-2R protein was measured in concentrated (10-fold as described above) BAL using a commercial sIL-2Rα ELISA (R&D Systems Europe, Abingdon, UK) in accordance with the manufacturer's instructions. With this ELISA kit, the lowest detectable concentration of sIL-2R protein was 78 pg/ml.
Data analysis
Statistics
Descriptive statistics are presented as median values with range, unless otherwise stated, whereas parameters utilized for statistical analysis are presented as mean values with s.e.m. For evaluation of differences between groups, the StatView® 4·01 software (Abacus Concepts, Berkeley, CA, USA) was utilized. The Wilcoxon signed-rank test was used for comparisons between two dependent groups, the Mann–Whitney U-test for comparisons between two independent groups and Spearman's rank correlation for detecting a relationship between two variables. A P-value ≤ 0·05 was considered significant.
Data transformation
In order to evaluate the IL-16 and sIL-2R concentrations during the time-period preceding AR or OB, the area under the curve (AUC) for IL-16 and sIL-2R was calculated for each subject by multiplying the level of IL-16 (pg/ml) by the number of months between different sampling time-points. In order to compensate for eventual differences in time for the matched pairs of patients, the AUC was divided by the length of the observation time (months) (AUC/time). In the OB group, all three time-points were used for the calculations of AUC. In the AR group, however, the primary comparison was conducted on data from the time-point prior to and during rejection only, in order to avoid potential confounding by high doses of immunosuppressive treatment given because of AR (after bronchoscopy 2 but before bronchoscopy 3). In the AR group the final time-point, representing data obtained after immunosuppressive treatment, was analysed separately.
RESULTS
Clinical characteristics
Gender was identical in eight out of 14 pairs of patients and preoperative diagnosis was identical in 13 out of 14 pairs of patients. The specific type of transplantation was identical in 13 matched pairs of patients whereas in one case only, heart–lung transplantation was matched with bilateral lung transplantation. (Table 1). For the collection of BAL fluid, the three time-points post surgery were similar for the AR its matched control group, as well as for the OB and its matched control group (Table 2).
Table 2.
. Median differential cell counts with interquartile range (IQR) in 84 BAL samples from 28 lung allograft recipients grouped as (a) patients with mild (A2) acute rejection (AR) or their matched controls (controls) without AR and (b) patients with obliterative bronchiolitis (OB) or their matched controls (controls) without OB, respectively
| Group | Median sampling time (months) | Lymphocytes (%) | Macrophages (%) | Neutrophils (%) | Eosinophils (%) | |
|---|---|---|---|---|---|---|
| (a) | AR | |||||
| Controls | 4 | A 0 | 3·5 (8) | 87·5 (11) | 3·5 (7·5) | 0 (0) |
| 6 | A 0 | 5 (7·9) | 91 (13·6) | 7 (5·8) | 0 (0) | |
| 12 | A 0 | 8·5 (6·1) | 80 (12) | 5 (6·5) | 0 (0) | |
| AR | 4 | A 0 | 4 (13·6) | 77 (42·9) | 6·5 (4·1) | 0 (0) |
| 7 | A 2 | 15 (28·9)* | 78 (29·4)* | 4·5 (5) | 0 (0) | |
| 12 | A 0 | 15 (17·1) | 82 (12) | 1 (5·1) | 0 (0) |
| (b) | OB | |||||
| Controls | 3 | – | 2·5 (6·0) | 89·0 (25·9) | 3·5 (7·0) | 0 (0) |
| 9 | – | 10·0 (16·3) | 80·0 (26·4) | 5·0 (1·6) | 0 (0) | |
| 18 | – | 7·0 (20·8) | 86·0 (14·5) | 7·0 (3·0) | 0 (0) | |
| OB | 3 | – | 8·0 (17·5) | 80·0 (15·5) | 2·0 (5·5) | 0 (0·3) |
| 9 | – | 51·0 (45·3)* | 47·0 (46·5) | 3·0 (1·3) | 0 (0·3) | |
| 18 | + | 11·5 (30·0) | 64·0 (18·5)* | 22·0 (18·0)* | 0·5 (1·0) |
Statistically significant difference compared to patients without acute rejection (Wilcoxon's signed-rank: P < 0·05).
The lung allograft recipients developing OB had a greater number of AR ≥ A2 than the matched control patients during their first three postsurgical months [mean (s.e.m.): 2·2 (0·5) versus 1·5 (0·3)] as well as during the first postsurgical year [mean (s.e.m.): 3·2 (0·6) versus 2·4 (0·5)]. However, these differences were not statistically significant (Wilcoxon signed-rank: P ≥ 0·05, n = 7).
Immunosuppressive therapy was given as described previously [19]. The lung allograft recipients developing AR and their matched control patients all recieved immunosuppressive treatment consisting of cyclosporin, azathioprine and prednisolone, according to the referred guidelines. One matched pair of patients used inhaled glucocorticoids regularly throughout the study.
The lung allograft recipients developing OB and their matched control patients started out with the standard triple regime for immunosuppression, but when suspicion of OB became apparent, individual changes in this pharmacotherapy were conducted with a switch from azathioprine to mycophenolate mofetil in three cases, and from cyclosporin to tacrolimus in two cases. However, these changes were relevant only for the analysis of the BAL fluid harvested during the third time-point.
IL-16 and sIL-2α in BAL fluid from lung allograft recipients with AR
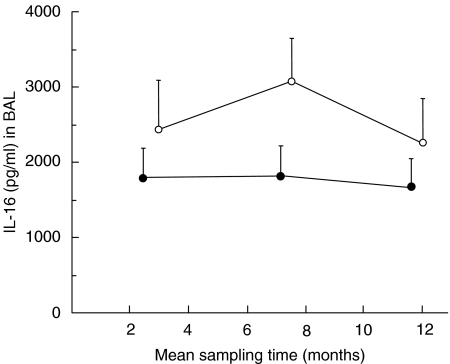
Lung allograft recipients developing AR displayed a lower mean IL-16 concentration in BAL fluid than did their matched control patients at all time-points (Fig. 1). As a consequence, the IL-16 AUC/time value was significantly lower in AR patients than in their matched control patients for the time period preceding AR (Fig. 1). The same was true for the AUC value when compensation for time differences were not made (data not shown). The IL-16 concentration after AR did not demonstrate any statistically significant difference compared with matched control patients (P > 0·05, n = 7).
Fig. 1.
BAL IL-16 protein (concentration in pg/ml, mean values with s.e.m.) prior to, during and after acute lung allograft rejection (AR) (•), compared with matched lung allograft recipients not developing AR (○). The IL-16 concentration preceding AR (calculated as AUC for two first time-points/time) was lower (Wilcoxon's signed-rank test: P = 0·03, n = 7) than in matched control patients.
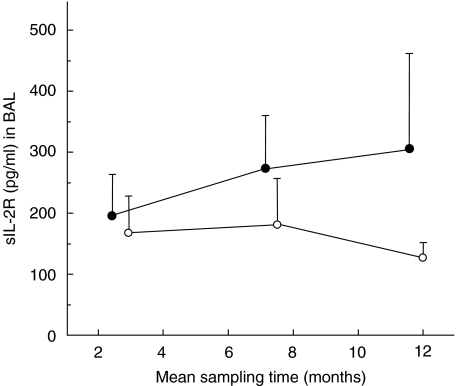
The sIL-2Rα concentration in BAL fluid tended to be higher in AR patients at all time-points, but assessed as AUC/time this difference did not reach statistical significance (Fig. 2). The sIL-2Rα concentration after AR did not demonstrate any statistically significant difference compared with matched control patients (P > 0·05, n = 7).
Fig. 2.
BAL sIL-2Rα protein (concentration in pg/ml, mean values with s.e.m.) prior to, during and after AR (see Fig. 1), (•) compared with matched control patients not developing AR (○). The sIL-2Rα concentration preceding AR (calculated as AUC for two first time-points/time) was not markedly different (Wilcoxon's signed-rank test: P = 0·4, n = 7) from that in matched control patients.
There was a negative and significant correlation between the concentration of IL-16 and sIL-2Rα in BAL fluid for the time period preceding AR (calculated as AUC for time-points before and during rejection/time, Spearman's rank correlation: P = 0·03, r = −0·6, n = 14).
IL-16 and sIL-2α in BAL fluid from lung allograft recipients with OB
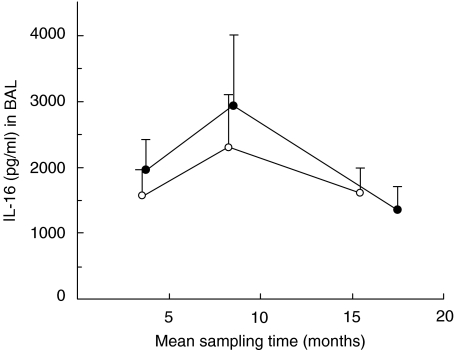
Lung allograft recipients developing OB did not display a substantially different mean IL-16 concentration in BAL fluid compared with their matched control patients at any time-point (Fig. 3). Consequently, the IL-16 AUC/time value in OB patients was not significantly different from matched control patients for the time period preceding OB (Fig. 3).
Fig. 3.
BAL IL-16 protein (concentration in pg/ml, mean values with s.e.m.) prior to and at the time of obliterative bronchiolitis (OB) (•), compared with matched lung allograft recipients not developing OB (○). The IL-16 concentration preceding OB (calculated as AUC/time) was not markedly different (Wilcoxon's signed-rank test: P = 0·6, n = 7) from that in matched control patients.
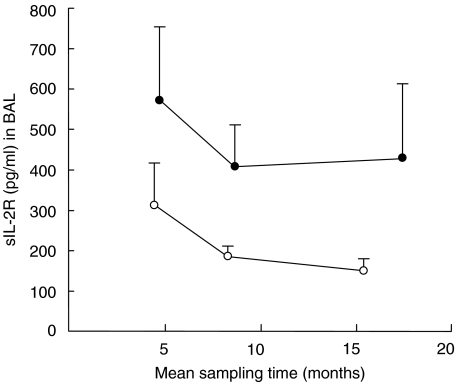
The sIL-2Rα concentration in BAL fluid tended to be higher in OB patients at all time-points, but assessed as AUC/time this difference did not reach statistical significance (Fig. 4).
Fig. 4.
BAL sIL-2Rα (concentration in pg/ml, mean values with s.e.m.) prior to and at the time of obliterative bronchiolitis (OB) (•), compared with matched lung allograft recipients not developing OB (○). The sIL-2Rα concentration preceding OB (calculated as AUC/time) was not markedly different (Wilcoxon's signed-rank test: P = 0·1, n = 7) from that in matched control patients.
There was no substantial correlation between the BAL IL-16 AUC/time value and the BAL sIL-2Rα AUC/time value in OB patients compared with matched control patients (P = 0·7, r = −0·1, n = 14).
Differential cell-count versus IL-16 in lung allograft recipients with AR
The percentage of lymphocytes was significantly higher and the percentage of macrophages was significantly lower in lung allograft recipients developing AR compared with matched control patients (Table 2). However, the concentration of IL-16 did not display any apparent relation to the percentage of T lymphocytes (data not shown).
Differential cell-count versus IL-16 in lung allograft recipients with OB
The percentage of neutrophils was significantly higher and the percentage of macrophages was significantly lower in lung allograft recipients developing OB compared with matched control patients (Table 2). However, the concentration of IL-16 did not display any apparent relation to the percentage of T lymphocytes (data not shown).
DISCUSSION
This study shows that lung allograft recipients developing AR lack the increase in IL-16 protein that is present in the airways of lung allograft recipients not developing AR. In our study, this lack of increase in IL-16 was evident in six out of seven matched pairs of lung allograft recipients, whereas one pair of matched patients displayed an inverse pattern for IL-16. Hypothetically, this discrepancy could be due to this particular pair of patients having a much shorter time postsurgery than the other pairs, a factor that may influence the concentration of IL-16 protein per se[23]. However, although the lung allograft recipients developing AR displayed significantly lower concentrations of IL-16 for the time period preceding AR compared with their matched control patients, there was an overlap for the IL-16 concentrations between the groups if the patients were not matched. Thus, because of several confounding factors, the usefulness of determining the IL-16 concentration in the airways to evaluate the risk for AR in the clinical situation is questionable.
It has been shown that the expression of the lymphocyte activation marker IL-2R is increased in the airways during AR and proposed that it can be used to monitor AR [24,25]. It has also been shown that the antigen-induced expression of IL-2R is inhibited by IL-16 protein in vitro[12] and that this inhibition of lymphocyte activation is truly immunosuppressive in a model of skin transplantation [26]. This type of mechanistic relationship is also supported by our observation that the airway concentration of IL-16 and IL-2R, respectively, correlates negatively in lung allograft recipients developing AR. In line with this, we observed that the concentration of sIL-2R tends to be higher in lung allograft recipients developing AR compared with matched control patients, even though this difference was not statistically significant. Taken together, these findings are compatible with the hypothesis that IL-16 by inhibiting antigen recognition, and the consequent activation of the CD4+ subset of T lymphocytes, does exert an immunosuppressive role preventing from AR.
Although the percentage of BAL lymphocytes was increased during AR, we were unable to demonstrate any correlation between airway IL-16 and the percentage of BAL lymphocytes. New and larger studies are therefore needed to determine whether the concentration of IL-16 in the airways relates only to the activation of lymphocytes without affecting their number or whether it is related to the number of CD4+ T lymphocytes alone, without having an effect on the total number of lymphocytes.
In our study, there was no substantial difference in the concentration of IL-16 in the airways of lung allograft recipients developing OB compared with their matched control patients, thus providing no conclusive evidence for a role of IL-16 in OB. Regarding the corresponding concentration of sIL-2R, although higher at all three time-points, the AUC/time values for sIL-2R did not display any statistically significant difference in lung allograft recipients developing OB compared with their matched control patients; this might have been due to the small sample size. Furthermore, in the airways the concentration of IL-16 was not related either to the concentration of sIL-2R nor to the percentage of airway lymphocytes in lung allograft recipients developing OB, suggesting that factors other than IL-16 determine the number and activity of lymphocytes in these patients.
In conclusion, this study shows that development of AR but not OB is associated with a lack of increase in airway IL-16 protein in lung allograft recipients. This study also points out the possibility that in patients developing AR, IL-16 inhibits lymphocyte activity eventually via down-regulation the CD4 co-receptor activity in the TCR/CD3/CD4 receptor complex. Interventional studies will be required to determine whether administration of exogenous, recombinant IL-16 protein or synthetic analoges of IL-16 can protect against AR in lung allograft recipients.
Acknowledgments
This work was supported by Göteborg University, the Swedish Heart Lung Foundation, the Swedish Medical Research Council (K2002-74X-09048–13A), the Swedish Medical Society and the Vårdal Foundation.
REFERENCES
- 1.Theodore J, Starnes VA, Lewiston NJ. Obliterative bronchiolitis. Clin Chest Med. 1990;11:309–21. [PubMed] [Google Scholar]
- 2.Bando K, Paradis IL, Similo S, et al. Obliterative bronchiolitis after lung and heart–lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110:4–13. doi: 10.1016/S0022-5223(05)80003-0. ; discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 3.Scott JP, Higenbottam TW, Sharples L, et al. Risk factors for obliterative bronchiolitis in heart–lung transplant recipients. Transplantation. 1991;51:813–7. doi: 10.1097/00007890-199104000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Watschinger B. How T cells recognize alloantigen: evidence for two pathways of allorecognition. Nephrol Dial Transplant. 1995;10:1556–8. [PubMed] [Google Scholar]
- 5.Chirmule N, Avots A, LakshmiTamma SM, Pahwa S, Serfling E. CD4-mediated signals induce T cell dysfunction in vivo. J Immunol. 1999;163:644–9. [PubMed] [Google Scholar]
- 6.Waldmann H, Cobbold S. Monoclonal antibodies for the induction of transplantation tolerance. Curr Opin Immunol. 1993;5:753–8. doi: 10.1016/0952-7915(93)90133-d. [DOI] [PubMed] [Google Scholar]
- 7.Van Epps DE, Potter JW, Durant DA. Production of a human T lymphocyte chemotactic factor by T cell subpopulations. J Immunol. 1983;130:2727–31. [PubMed] [Google Scholar]
- 8.Bellini A, Yoshimura H, Vittori E, Marini M, Mattoli S. Bronchial epithelial cells of patients with asthma release chemoattractant factors for T lymphocytes. J Allergy Clin Immunol. 1993;92:412–24. doi: 10.1016/0091-6749(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 9.Rand TH, Cruikshank WW, Center DM, Weller PF. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J Exp Med. 1991;173:1521–8. doi: 10.1084/jem.173.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center DM, Cruikshank W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982;128:2563–8. [PubMed] [Google Scholar]
- 11.Parada NA, Cruikshank WW, Danis HL, Ryan TC, Center DM. IL-16- and other CD4 ligand-induced migration is dependent upon protein kinase C. Cell Immunol. 1996;168:100–6. doi: 10.1006/cimm.1996.0054. [DOI] [PubMed] [Google Scholar]
- 12.Cruikshank WW, Lim K, Theodore AC, et al. IL-16 inhibition of CD3-dependent lymphocyte activation and proliferation. J Immunol. 1996;157:5240–8. [PubMed] [Google Scholar]
- 13.Theodore AC, Center DM, Nicoll J, Fine G, Kornfeld H, Cruikshank WW. CD4 ligand IL-16 inhibits the mixed lymphocyte reaction. J Immunol. 1996;157:1958–64. [PubMed] [Google Scholar]
- 14.Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol. 1999;162(7):4293–9. [PubMed] [Google Scholar]
- 15.Pinsonneault S, El Bassam S, Mazer B, Cruikshank WW, Laberge S. IL-16 inhibits IL-5 production by antigen-stimulated T cells in atopic subjects. J Allergy Clin Immunol. 2001;107:477–82. doi: 10.1067/mai.2001.112373. [DOI] [PubMed] [Google Scholar]
- 16.Laberge S, Ernst P, Ghaffar O, et al. Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am J Respir Cell Mol Biol. 1997;17:193–202. doi: 10.1165/ajrcmb.17.2.2750. [DOI] [PubMed] [Google Scholar]
- 17.Laan M, Qvarfordt I, Riise GC, Andersson BA, Larsson S, Linden A. Increased levels of interleukin-16 in the airways of tobacco smokers: relationship with peripheral blood T lymphocytes. Thorax. 1999;54:911–6. doi: 10.1136/thx.54.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruikshank WW, Long A, Tarpy RE, et al. Early identification of interleukin-16 (lymphocyte chemoattractant factor) and macrophage inflammatory protein 1 alpha (MIP1 alpha) in bronchoalveolar lavage fluid of antigen-challenged asthmatics. Am J Respir Cell Mol Biol. 1995;13:738–47. doi: 10.1165/ajrcmb.13.6.7576712. [DOI] [PubMed] [Google Scholar]
- 19.Riise GC, Schersten H, Nilsson F, Ryd W, Andersson BA. Activation of eosinophils and fibroblasts assessed by eosinophil cationic protein and hyaluronan in BAL. Association with acute rejection in lung transplant recipients. Chest. 1996;110:89–96. doi: 10.1378/chest.110.1.89. [DOI] [PubMed] [Google Scholar]
- 20.Riise GC, Kjellstrom C, Ryd W, et al. Inflammatory cells and activation markers in BAL during acute rejection and infection in lung transplant recipients: a prospective, longitudinal study. Eur Respir J. 1997;10:1742–6. doi: 10.1183/09031936.97.10081742. [DOI] [PubMed] [Google Scholar]
- 21.Yousem SA, Berry GJ, Brunt EM, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection − lung rejection study-group. J Heart Transplant. 1990;9:593–601. [PubMed] [Google Scholar]
- 22.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–6. [PubMed] [Google Scholar]
- 23.Shimada M, Matsumata T, Taketomi A, et al. The role of interleukin-6, interleukin-16, tumor necrosis factor-alpha and endotoxin in hepatic resection. Hepatogastroenterology. 1995;42:691–7. [PubMed] [Google Scholar]
- 24.Ross DJ, Yeh AY, Nathan SD, et al. Differential soluble interleukin-2R levels in bilateral bronchoalveolar lavage after single lung transplantation. J Heart Lung Transplant. 1994;13:972–9. [PubMed] [Google Scholar]
- 25.Lai KN, Leung JC, Lai FM. Soluble interleukin 2 receptor release, interleukin 2 production, and interleukin 2 receptor expression in activated T lymphocytes in vitro. Pathology. 1991;23:224–8. doi: 10.3109/00313029109063570. [DOI] [PubMed] [Google Scholar]
- 26.Fujita T, Matsumoto Y, Hirai I, et al. Immunosuppressive effect on T cell activation by interleukin-16-cDNA-transfected human squamous cell line. Cell Immunol. 2000;202:54–60. doi: 10.1006/cimm.2000.1657. [DOI] [PubMed] [Google Scholar]