Abstract
Different genetic mutations have been described in complement component C7 deficiency, a molecular defect which is clinically associated with an increased susceptibility to neisserial recurrent infections, although some cases remain asymptomatic. In this work we report the genetic bases of C7 deficiency in one Spanish family. Exon-specific PCR and sequencing revealed a novel point mutation at nucleotide 615 (exon 6) leading to a stop codon (UGG to UGA) in the patient, his mother, and sister. This transversion causes the premature truncation of the C7 protein (W183X). Additionally, we detected a missense mutation at position 1135 (exon 9) located in the first nucleotide of the codon GGG (CGG), resulting in an amino acid change (G357R) in the patient, his father, as well as in his sister. This latter mutation had been previously described in individuals from Moroccan Sephardic Jewish ancestry. Since both heterozygous mutations were found in the patient as well as in his asymptomatic sister, we analyse other meningococcal defence mechanisms such as polymorphisms of the opsonin receptors on polymorphonuclear cells. Results showed that the patient and his sister bore identical combinations of FcγRIIA-H/R131 and FcγRIIIB-NA1/2 allotypes. Our results provide further evidence that the molecular pathogenesis of C7 deficiency as well as susceptibility to meningococcal disease are heterogeneous, since different families carry different molecular defects, although many of the C7 defects appear to be homogeneous in individuals from certain geographical areas. The missense mutation G357R would make an interesting topic of analysis with regard to meningococcal disease susceptibility in the Spanish population.
Keywords: C7 deficiency, meningococcal infections, Fcγ receptors, Moroccan sephardic Jews
INTRODUCTION
Host defence against meningococci is provided by mucosal immunity, as well as by serum bactericidal and phagocytic activities. Genetically determined human deficiencies of any of the terminal complement components are associated with increased risk to recurrent systemic infections caused mainly by Neisseria meningitidis at age 10–30 years [1,2], when it is assumed that anti-meningococcal antibodies are present [3]. C7 is one of the five terminal complement proteins that upon activation of either the classical or the alternative pathway interact sequentially to form a large protein-protein complex, called membrane attack complex (MAC). Assembly of the MAC on target cells results in the formation of transmembrane pores that can lead to the killing of the cells [4]. The single polypeptide chain of C7 is composed of 821 amino acid residues and is structurally similar to the other MAC components C6, C8α, C8β, and C9 [5,6]. The gene for C7 has been shown to span about 80 kb of DNA, is encoded by 18 exons [6], and is located on chromosome 5p13, as well as the genes for C6 and C9 [7]. C7 deficiency leads to the loss of lytic function of complement; patients show an increased susceptibility to recurrent meningococcal meningitis and systemic Neisseria gonorrhoeae infection, though many, including siblings sharing the deficiency may be healthy [8].
On the other hand, immunity to meningococcal disease may also be determined by the interaction between IgG and the IgG Fc receptor (FcγR) on polymorphonuclear cells (antibody-mediated phagocytosis). It has been hypothesized that particular combinations of FcγR allotypes may determine the susceptibility to meningococcal disease, especially in persons who lack serum bactericidal activity because of deficiency of a component in the terminal pathway of complement activation [9,10]. Of the three classes of FcγR that have been identified, only the FcγRIIa (CD32) and FcγRIIIb (CD16b) are constitutively expressed on polymorphonuclear cells and mediate phagocytosis [11]. Two different allelic polymorphisms among the isoforms of FcγR have been defined, which affect antibody-mediated processes [9–11]. The FcγRIIa occurs in two allotypic forms, designated FcγRIIa-H131, which binds human IgG2 complexes, and FcγRIIa-R131, which does not bind human IgG2 complexes [12], because of the presence of an Arg or His at position 131 in the extracellular domain of the receptor [13]. Neutrophils from homozygous IIa-R/R131 donors are less effective than IIa-H/H131 neutrophils, and heterozygous neutrophils exhibit intermediate levels of phagocytosis of menigococci [14]. On the other hand, the two allotypic FcγRIIIb forms (NA1 and NA2) have the same affinity for IgG subclasses, but phagocytosis by polymorphonuclear cells of the NA2 allotype is consistently less effective than by cells with the NA1 allotype [9]. The combined FcγRIIa-R/R131 and FcγRIIIb- NA2/2 phenotype has been reported to be associated with meningococcal disease [10].
The investigations reported here focused on the study of a C7 deficient index case and his immediate family. The deficiency was ascertained becaused of repeated meningococcal infections. The determination of C7 DNA sequence of the index case allowed us to proceed to testing and confirming the mutations detected in his family. In addition we analysed FcγRIIA-H/R131 and FcγRIIIB-NA1/2 polymorphisms in all members of the family.
SUBJECTS AND METHODS
Subjects
A Spanish family comprising both parents, a son and daughter, was included in this study. Informed consent was obtained from all members of the family, according to the guidelines of the Hospital Bioethic Committee. The C7 deficient index case, a 15-year-old-boy, had been discovered because of three meningococcal disease episodes. Total haemolytic activity (CH50) was undetectable in the serum of the patient and his sister. The patient's father and mother had total serum haemolytic activities (250 U/ml and 312 U/ml, respectively) within the normal range (200–400 U/ml). Subsequent analysis by radial immunodiffusion assay with antibodies against the main principal complement components revealed no detectable C7 in the serum of both siblings, whereas C7 concentrations in the serum of the father and mother were 1800 U/ml and 1200 U/ml, respectively (normal C7 concentration in human serum is 3200 U/ml). The functional absence of this component was confirmed by a haemolytic assay in which the propositus's serum was unable to recover the haemolytical activity of a previously found C7-deficient patient [15] and by the availability of functional C7 (Cordis, Miami, FL, USA) for recovering the haemolytical activity of patient's serum to levels within the normal range.
DNA preparation
Genomic DNA was isolated from 100 µl of whole peripheral EDTA-treated blood as described previously [16]. DNA was prepared by lysis of white cells with proteinase K (Amersham Pharmacia Biotech AB, Uppsala, Sweden) (100 µg/ml) in 200 µl of a tris buffer containing 0·5% tween 20 (Sigma, St Louis, MO, USA).
Polymerase chain reaction
Primers for exon-specific PCR for exons 1–17 of the C7 gene were described previously [17]. Briefly, PCR was performed by using 5 µl of DNA, 1 µm of each primer, 25 µm dNTPs (Amersham Pharmacia Biotech AB), 0·25 U Taq polymerase (Amersham Pharmacia Biotech AB) and the standard buffer provided by the supplier in a total reaction volume of 50 µl. Cycling conditions were 95°C 1 min for initial denaturation followed by 30 cycles of 95°C 1min, 60°C 1min, 72°C 2min in a Perkin-Elmer Gene Amp PCR System 2400. For each sample three separated reactions were carried out.
Nucleotide sequencing
The PCR products were purified using sephacryl S-400 columns (Amersham Pharmacia Biotech AB) and reamplified using the ABI Prism dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA), using the following conditions: for each reaction we added 5 µl of PCR product, 4 µl of Terminator Ready Reaction Mix and 3 pmol of primer. The cycle sequencing was performed on a Perkin-Elmer Gene Amp PCR System 2400 (Perkin-Elmer, Norwalk, CT, USA) at the following cycling conditions: 94°C 3min for initial denaturation, followed by 25 cycles of 96°C 10 s, 50°C 5 s, 60°C 4min. The extension products were purified by ethanol/sodium acetate precipitation procedure to remove excess dye terminators. Each sample pellet was resuspended in 12·5 µl of Template Supression Reagent and heated at 95°C for 3 min to denature. The electrophoresis was carried out on the ABI Prism 310 sequencer following manufacturer's instructions. For each sample three independent reactions were carried out. Computer analysis of DNA sequences was performed using the University of Wisconsin Genetics Computer Group Sequence Analysis Software Package [18].
Detection of FcγR polymorphisms
Genotyping of FcγRIIa-H/R131 and FcγRIIIb-NA1/2 was performed using allele-specific PCR with previously described primers [19,20]. For each sample two separated reactions were carried out. In the case of FcγRIIA-H/R131, the reaction was performed using 10x PCR buffer (10 mm Tris-HCl pH 9·0, 2·0 mm MgCl2, 50 mm KCl), 200 µm dNTPs (Pharmacia), 5% of glycerol, 100 µg/ml of cresol red, 5 pmoles of each primer, 0·5 µl of DNA, 0·5 U of Taq polymerase (Pharmacia) and ddH2O to a final volume of 10 µl. In the case of FcγRIIIB-NA1/2 PCR was performed using 10x PCR buffer, 200 µm dNTPs, 5 pmoles of each primer, 0·5 µl of DNA, 0·5 U of Taq polymerase and ddH2O to a final volume of 10 µl.
The following thermal profiles were run: In the case of FcγRIIA-H/R131, 5 min at 95°C for initial denaturalization, followed by 10 cycles of 95°C 30 s, 50°C 30 s and 72°C 30 s followed by 20 cycles of 95°C 30 s, 44°C 30 s and 72°C 30 s. PCR products were resolved in 2% agarose gels stained with ethidium bromide. Samples showing the expected 253 bp fragment in only one tube were genotyped as homozygous (H131 or R131), whereas samples showing amplification in both tubes were genotyped as heterozygous (H/R131). Finally, in the case of FcγRIIIB-NA1/2, 30 cycles consisting of 94°C 1 min, 54°C 1 min and 72°C 1 min were run. After a final extension of 10 min 72°C, samples were resolved in 2% agarose gels stained with ethidium bromide after dilution in blue juice buffer. Samples showing the expected band of 118 bp in tube containing NA1 and the expected band of 169 bp in the tube containing NA2 primers were typed as heterozygous NA1/2, whereas samples showing a band only in one of the tubes were typed as homozygous.
RESULTS
Detection of C7 gene mutations and markers
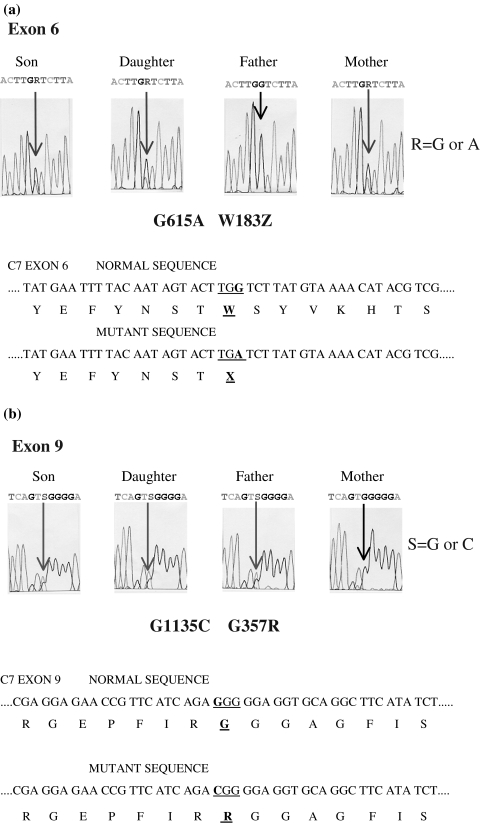
Genomic DNA was isolated from peripheral blood drawn from the C7 deficient patient. Sequence analysis of exons 1–17 revealed a novel point mutation at cDNA nucleotide 615 (exon 6); there was an A instead of G (Fig. 1a). Nucleotide 615 is the third nucleotide of the codon UGG for Trp183 of normal C7. The G to A transversion generates a termination codon, UGA, which leads to the premature truncation of the encoded C7 protein (W183X). We also found in the patient a previously described missense mutation at cDNA nucleotide 1135 located in exon 9 [21]. This position is the first nucleotide of the codon GGG for Gly357 of normal C7 (Fig. 1b). The C to G transversion results in the change of Gly for Arg (G357R) This mutation had been detected in C7 deficient individuals of Moroccan Sephardic Jewish ancestry. Sequencing of PCR-amplified exons 6 and 9 derived from genomic DNA of the family of the patient revealed the presence of these two mutations in his sister, indicating that both siblings were heterozygous for the same abnormalities, whereas his father was heterozygous for the missense mutation G357R, and his mother heterozygous for the nonsense mutation W183X (see again Fig. 1a,b).
Fig. 1.
Definition of the mutations in (a) exon 6 and (b) exon 9. Partial DNA sequence of genomic DNA of the patient and his parents and sister. The normal and defective DNA sequences as well as the deduced amino acid sequences are given.
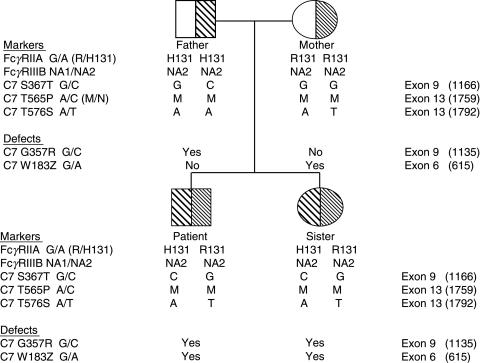
The sequenced PCR products were examined to detect C7 DNA markers in our family. A total of 3 polymorphisms in the C7 gene exons were investigated in order to ascribe a particular DNA marker haplotype to the defective condition, to construct a family tree, as well as to compare them with described haplotypes associated to complement component C7 deficiencies (Fig. 2). Results showed that the proband and his sister inherited from their father the haplotype CMA, corresponding to the G/C polymorphism at position 367 leading to the S/T substitution [21], the base change (A to C) responsible for the M/N protein polymorphism (P565T) [22], and the adjacent A/T point substitution resulting in the T576S polymorphism [23], respectively. This combination is different from the Moroccan Sephardic Jewish G357R mutation-associated C7 deficient haplotype CNA [24]. The C7 DNA markers (GMT) were inherited with the new mutation found in the mother (see again Fig. 2) and, as expected, they had not been described previously in association with C7 deficiency [8,17,23–25].
Fig. 2.
Pedigree of the C7 deficient family. The data on C7 gene markers, C7 gene defects, as well as FcγRIIA-H/R131 and FcγRIIIB-NA1/2 polymorphisms are presented as vertical haplotypes.
FcγR polymorphisms
We next evaluated the distribution of FcγRIIA-H/R131 and FcγRIIIB-NA1/2 polymorphisms in the four members of the family under study. Figure 2 shows the combined FcγRIIA and FcγRIIIB phenotypes observed. Both siblings exhibited identical phenotypes, being heterozygous for FcγRIIA-H/R131, the allelic combination responsible of intermediate levels of meningococcal phagocytosis, and homozygous for FcγRIIIB-NA2/2, the less effective allele reported for phagocytic activity.
DISCUSSION
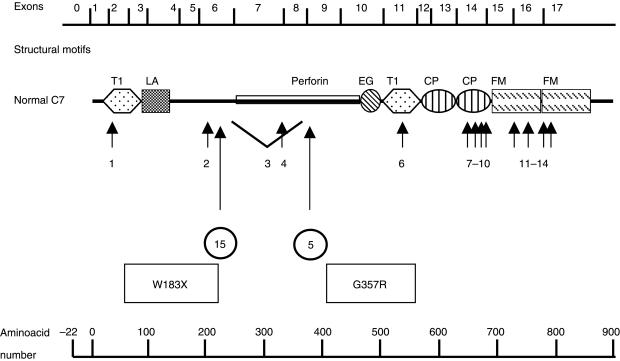
In the present study, we report a novel mutation of the gene in a Spanish family with C7 deficiency, in heterozygosity with a mutation that had been described previously in a number of Israelis of Moroccan Sephardic Jewish ancestry. To date, 14 different molecular defects leading to total or subtotal C7 deficiency defects had been reported [26–28]. All of these mutations, including our newly discovered mutation, are summarized in Fig. 3.
Fig. 3.
Schematic diagram of the molecular structure of normal C7 (adapted from [6]) and the position of mutations described to date (numbered from 1 to 14), and the novel mutation found in our Spanish family (15). Mutations described previously: 1, G> A transition at 3′ acceptor site of intron 1 [8]; 2, R198Q [24]; 3, deletion of around 6·8 kbp including exons 7 and 8 [8–25]; 4, a G> A transversion at 5′ splice donor site of intron 7 [24,26]; 5, G357R [8]; 6, R499S [28]; 7, 1929delC [24]; 8, E631X [27]; 9, E660Q [24]; 10, R665H [24]; 11, 2137delTG or 2138delGT/2139delTG [17]; 12, C728X [17]; 13, 2350delG [24]; 14, T> C transversion at splice donor site of intron 16 [24,26]; 15, W183X (present case). Modules are designated according to the recommendations of a workshop [29], as follows: T1, thrombospondin, type 1; LA, LDL receptor, type A; EG, epidermal growth factor-like; CP, complement control protein; and FM, complement factor I, MAC proteins.
By using exon-specific PCR amplification followed by direct sequencing of the target exons, two different molecular defects were identified in the C7 gene of the patient. The G to A transversion in exon 6 generates a termination codon (TGA). If translated, the mutant C7 would lack the carboxy-terminal 636 amino acid residues, which represent approximately 77·7% of the molecular size of the polypeptide. As this truncated part contains domains that are important for C7 functionality, this heavily truncated C7 (its molecular size is only 12·3% of normal C7) is probably not able to participate in the formation of MAC even if secreted. In this way, it has been reported that deletions in exons 6 and 7 (as well as other mutations located downstream) [8,30] lead to a complete loss of complement lytic function. For these reasons, it seems that null or defective secretion of those severely affected mutant proteins are responsible for the absent functional activity observed. In contrast, Würzner et al. [31] reported a defect in the similar molecule C6 leading to a carboxy-terminally truncated molecule with a molecular size that is 86·5% of normal C6, that allowed secretion of functionally active C6. Family study demonstrated that patient's mother as well as his sister carried one C7 allele with the identical mutation.
Another mutation was found in the patient under study, the C to G transversion in exon 9 leading to G357R. Family study demonstrated that patient's father as well as his sister carry one C7 allele with the identical mutation. As stated above, this missense mutation had been previously found in C7 deficient individuals from the Moroccan Sephardic Israeli population [8]. This change involves the substitution of an Arg for a Gly residue; this replacement would profoundly alter the tertiary structure of the protein, since it implies the replacement of a small nonpolar residue for a large basic one which could result in a turn of the polypeptide backbone. Fernie et al. [8] hypothesized that folding of the C7 gene product could be so disrupted that the functional consequences could also lead to the failure of protein secretion. On the other hand, the sensitivity of the RID method used in this study to measure C7 cannot exclude the possibility that low levels of C7 were present in the serum of the patient and his sister. However, no haemolytically active C7 was detected in either case. Additionally, the presence of any low levels of C7 produced by the genes carrying the defects could be masked due to consumption by circulating C5b6 [26,28]. Although our case appears to have had total C7 deficiency, the occurrence of subtotal C7 deficiency cannot completely being excluded. In this way, a very special case showing a phenotypically complete C7 deficient features was reported [32,33]; the patient was able transiently to produce functional C7, showing an unique feature among C7 deficiencies.
The Sephardi Jews, who were descendants of Jews whose ancestors lived in Spain (Sephardic means ‘from Sepharad’, which is Hebrew for ‘Spain’), were thrown out of Spain in 1492. After the expulsion edict they left and settled mainly in North Africa, the Balkans and Eastern Mediterranean countries, whereas those who stayed in Spain were forced to convert to Christianity [34]. The finding of the G357R mutation in a population closely related to Moroccan Israeli Jews, support the notion that this replacement can be responsible for the C7 deficiency observed in this population, suggesting the existence of a founder effect. Published data indicate that this mutation has not been found elsewhere, indicating that it is not present in sporadic cases of C7 deficiency occurring at widespread locations. In this way, the availability of the large-scale screening method based on the simplified PCR technique using site-directed mutagenesis and designer primers specific for either the wild-type or the mutant allele, developed by Halle et al. [35], makes feasible the investigation of this defect among large cohorts of individuals ethnically related with Sephardic Israeli Jews.
Skewed FcγR distributions have been observed in survivors of meningococcal disease, indicating a role for FcγRIIA-H/R131 and possibly for FcγRIIIB-NA1/2 genotypes as genetic markers for susceptibility to meningococcal disease [10,36,37]. In this work, we could not find any difference between the patient and his asymptomatic sister with respect to the distribution of their FcγRIIA-H/R131 as well as FcγRIIIB-NA1/2 alleles. Although it is not possible to drawn conclusions from this study, since C7 deficiency is not incompatible with apparent normal health [8], it is interesting to point out that in a recent study van der Pool et al. [38] found that the R/R131-NA2/2 genotypic combination failed to reach statistical significance in association with meningococcal disease. These authors suggest that the genetic heterogeneity on the long arm of chromosome 1, which is enriched with immune modulating genes [39], including pentraxin, selectin genes and several complement regulatory proteins, may provide important information with respect to host defense against meningococci.
The results obtained in this study detected a novel point mutation leading to an early truncation of the C7 protein product, supporting the notion that the molecular bases for complement component C7 deficiency are heterogeneous, since different families carry distinct molecular defects. On the other hand, some defects appear to be homogeneous for individuals living in defined geographical areas [23,25,35]. Finally, we suggest that the G357R mutation could be an interesting topic for analysis due to the relationship between Sephardic Jews and Spaniards.
Acknowledgments
We greatly appreciate the collaboration of the family under study. We thank Carmen Guzmán, Lourdes Lagarda, José Manuel Lara and Maribel Magariño for their excellent technical assistance, and to Drs M Francisca González-Escribano and J Raúl García-Lozano for helpful advice. This work was supported by grants from FIS 00/0568 awarded by Fondo de Investigaciones Sanitarias de la Seguridad Social, by grant 28/02 from Consejería de Salud, Junta de Andalucía, by grant REIPI C013 awarded from Instituto de Salud Carlos III, and from Plan Andaluz de Investigación (PAI, grupo CTS-0197), Junta de Andalucía.
REFERENCES
- 1.Sullivan KE, Winkelstein JA. Genetically determined deficiencies of the complement system. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency DiseasesA Molecular and Genetic Approach. New York: Oxford University Press; 1999. pp. 397–418. [Google Scholar]
- 2.Figueroa JE, Densen P. Infectious disease associated with complement deficiencies. Clin Microb Rev. 1991;4:359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldschneider I, Gotslich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–48. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller-Eberhard HJ. The membrane attack complex of complement. Annu Rev Immunol. 1986;4:503–28. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- 5.DiScipio RG, Chakravarti DN, Muller-Eberhard HJ, Fey GH. The structure of human complement component C7 and the C5b-7 complex. J Biol Chem. 1988;263:549–60. [PubMed] [Google Scholar]
- 6.Hobart MJ, Fernie BA, DiScipio RG. Structure of the human C7 gene and comparison with the C6, C8A, C8B, and C9 genes. J Immunol. 1995;154:5188–94. [PubMed] [Google Scholar]
- 7.Abbott C, West L, Povey S, Jeremiah S, Murad Z, DiScipio RG, Fey G. The gene for the human complement component C9 mapped to chromosome 5 by polymerase chain reaction. Genomics. 1989;4:605–9. doi: 10.1016/0888-7543(89)90286-3. [DOI] [PubMed] [Google Scholar]
- 8.Fernie BA, Orren A, Sheehan G, Schlesinger M, Hobart MJ. Molecular basis of C7 deficiency. Three different defects. J Immunol. 1997;159:1019–26. [PubMed] [Google Scholar]
- 9.Salmon JE, Edberg JC, Brogle NL, Kimberly RP. Allelic polymorphism of human Fcγ Receptor IIA and Fcγ Receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992;89:1274–81. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fijen CAP, Bredius RGM, Kuijper EJ, Out TA, de Haas M, de Wit APM. The role of Fcγ receptor polymorphisms and C3 in the immune defence against Neisseria meningitidis in complement-deficient individuals. Clin Exp Immunol. 2000;120:338–45. doi: 10.1046/j.1365-2249.2000.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deo YM, Graziano RF, Repp R, van de Winkel JG. Clinical significance of IgG Fc receptors and Fc gamma R-directed immunotherapies. Immunol Today. 1997;18:127–35. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- 12.van Wamerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fcγ receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–43. [PubMed] [Google Scholar]
- 13.van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity. molecular and clinical implications. Immunol Today. 1993;14:215–21. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 14.Bredius RGM, Derkx BHF, Fijen CAP, de Wit TP, de Haas M, Weening RS, van de Winkel JG, Out TA. Fcγ receptor IIa (CD32) polymorphism in fulminant meningococcal septic shock in children. J Infect Dis. 1994;170:848–53. doi: 10.1093/infdis/170.4.848. [DOI] [PubMed] [Google Scholar]
- 15.Segurado OG, Arnáiz-Villena A, Iglesias-Casarrubios P, Martínez-Laso J, Vicario JL, Fontán G, López-Trascasa M. Combined total deficiency of C7 and C4B with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1992;87:410–4. doi: 10.1111/j.1365-2249.1992.tb03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki E. Sample preparation from blood, cells and other fluids. In: Innis M, Gelfand D, Sinisky J, White T, editors. PCR Protocols. A Guide to Methods and Applications. San Diego: Academic Press; 1990. pp. 146–52. [Google Scholar]
- 17.Nishizaka H, Horiuchi T, Zhu ZB, Fukumori Y, Volanakis JE. Genetic bases of human complement C7 deficiency. J Immunol. 1996;157:4239–43. [PubMed] [Google Scholar]
- 18.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis for the VAX. Nucl Acid Res. 1984;12:387–95. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flesch BK, Bauer F, Neppert J. Rapid typing of the human Fc gamma receptor IIA polymorphism by polymerase chain reaction amplification with allele-specific primers. Transfusion. 1998;38:174–6. doi: 10.1046/j.1537-2995.1998.38298193100.x. [DOI] [PubMed] [Google Scholar]
- 20.Hessner MJ, Curtis BR, Endean DJ, Aster RH. Determination of neutrophil antigen gene frequencies in five ethnic groups by polymerase chain reaction with sequence-specific primers. Transfusion. 1996;36:895–9. doi: 10.1046/j.1537-2995.1996.361097017176.x. [DOI] [PubMed] [Google Scholar]
- 21.Fernie BA, Würzner R, Unsworth DJ, Tuxworth RI, Hobart MJ. Molecular basis of the complement C6 and C7 genes. Ann Hum Genet. 1995;59:163–81. doi: 10.1111/j.1469-1809.1995.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 22.Würzner R, Fernie BA, Jones AM, Lachmann Pj, Hobart MJ. Molecular basis of the complement MN polymorphism-a neutral amino acid substitution outside the epitope of the allospecific monoclonal antibody. J Immunol. 1995;154:4813–9. [PubMed] [Google Scholar]
- 23.Fernie BA, Orren A, Schlesinger M, Würzner R, Platonov AE, Cooper RC, Williams YE, Hobart MJ. DNA haplotypes of the complement C6 and C7 genes associated with deficiencies of the seventh component; and a new polymorphism in C7 exon 13. Ann Hum Genet. 1997;61:287–98. doi: 10.1046/j.1469-1809.1997.6140287.x. [DOI] [PubMed] [Google Scholar]
- 24.Fernie BA, Hobart MJ. Complement C7 deficiency. seven further molecular defects and their associated marker hapolotypes. Hum Genet. 1998;103:513–9. doi: 10.1007/s004390050859. [DOI] [PubMed] [Google Scholar]
- 25.O'Hara AM, Fernie BA, Moran AP, Williams YE, Connaughton JJ, Orren A, Hobart MJ. C7 deficiency in an Irish family: a deletion defect which is predominant in the Irish. Clin Exp Immunol. 1998;114:355–61. doi: 10.1046/j.1365-2249.1998.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Würzner R, Witzel-Schlömp K, Tokunaga K, Fernie BA, Hobart MJ, Orren A. Reference typing report for complement components C6, C7 and C9 including mutations leading to deficiencies. Exp Clin Immunogenet. 1998;15:268–85. doi: 10.1159/000019082. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi T, Ferrer JM, Serra P, Matamoros N, López-Trascasa M, Hashimura C, Niho Y. A novel nonsense mutation at Glu-631 in a Spanish family with complement component 7 deficiency. J Human Genet. 1999;44:215–8. doi: 10.1007/s100380050146. [DOI] [PubMed] [Google Scholar]
- 28.Fernie BA, Würzner R, Orren A, et al. Molecular bases of combined and subtotal deficiencies of C6 and C7 and their effects in combination with other C6 and C7 deficiencies. J Immunol. 1996;157:3648–57. [PubMed] [Google Scholar]
- 29.Doolittle RF. The multiplicity of domains in proteins. Annu Rev Biochem. 1995;64:287–314. doi: 10.1146/annurev.bi.64.070195.001443. [DOI] [PubMed] [Google Scholar]
- 30.Hobart M. In: The Complement Facts Book. Morley BJ, Walport MJ, editors. London: Academic Press; 2000. pp. 117–22. C7. [Google Scholar]
- 31.Würzner R, Hobart MJ, Fernie BA, Mewar D, Potter PC. Molecular basis of subtotal complement C6 deficiency. J Clin Invest. 1995;95:1877–83. doi: 10.1172/JCI117868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platonov AE, Würzner R, Belobodorov B, Jones AM, Trshansky DV, Vershinina IV, Lachmann PJ, Orren A. Paradoxical reconstitution of complement activity following plasma transfusion of an individual with deficiency of the seventh component of complement. Immunology. 1994;81:142–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Würzner R, Platonov AE, Belobodorov VB, Pereverzeb AI, Vershinina IV, Fernie BA, Hobart MJ, Lachmann PJ, Orren A. How partial C7 deficiency with chronic and recurrent bacterial infections can mimic total C7 deficiency: temporary restoration of Host C7 levels following plasma transfusion. Immunology. 1996;88:407–11. doi: 10.1046/j.1365-2567.1996.d01-663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crespí C, Milá J, Martínez-Pomar N, et al. HLA polymorphism in a Majorcan population of Jewish descent: Comparison with Majorca, Minorca, Ibiza (Balearic Islands) and other Jewish communities. Tissue Antigens. 2002;60:282–91. doi: 10.1034/j.1399-0039.2002.600402.x. [DOI] [PubMed] [Google Scholar]
- 35.Halle D, Elstein D, Geudalia D, Sasson A, Shinar E, Schlesinger M, Zinram A. High prevalence of complement C7 deficiency among healthy blood donors of Moroccan Jewish ancestry. Am J Med Genet. 2001;99:325–7. doi: 10.1002/ajmg.1183. [DOI] [PubMed] [Google Scholar]
- 36.Platonov AE, Shipulin GA, Vershinina IV, Dankert J, van de Winkel JGJ, Kuijper EJ. Association of human FcγRIIa (CD32) polymorphism with susceptibility to and severity of meningococcal disease. Clin Infect Dis. 1998;27:746–50. doi: 10.1086/514935. [DOI] [PubMed] [Google Scholar]
- 37.Platonov AE, Kuijper EJ, Vershinina IV, Shipulin GA, Westerdaal N, Fijen CAP, van de Winkel JGJ. Meningococcal disease and polymorphism of FcγRIIa (CD32) in late complement component-deficient individuals. Clin Exp Immunol. 1998;111:97–101. doi: 10.1046/j.1365-2249.1998.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Pol WL, Huizinga TWJ, Vidarsson G, et al. Relevance of Fcγ receptor and interleukin-10 polymorphism for meningococcal disease. J Infect Dis. 2001;184:1548–55. doi: 10.1086/324662. [DOI] [PubMed] [Google Scholar]
- 39.Tsokos GC, Liossis SNC. Immune cell signaling defects in lupus. activation, anergy and death. Immunol Today. 1999;20:119–24. doi: 10.1016/s0167-5699(98)01395-4. [DOI] [PubMed] [Google Scholar]