Abstract
Complement C5a is aetiologically linked to inflammatory tissue damage in conditions like septicaemia, immune complex diseases and ischaemia-reperfusion injury. We here describe a monoclonal antibody (mAb), 137–26, that binds to the C5a moiety of human C5 and neutralizes the effects of C5a without interfering with C5 cleavage and the subsequent formation of lytic C5b-9 complex. Mouse anti-human C5 mAbs were generated and the reactivity with C5 and C5a was detected by ELISA and surface plasmon resonance. The inhibition of C5a binding to C5a receptor was studied using a radioligand binding assay. The effects of the antibody on C5a functions were examined using isolated neutrophils and a novel human whole blood model of inflammation. Haemolytic assays were used to study the effect on complement-mediated lysis. mAb 137–26 reacted with both solid- and solution-phase C5 and C5a in a dose-dependent manner with high affinity. The antibody competed C5a binding to C5a receptor and inhibited C5a-mediated chemotaxis of neutrophils. Furthermore, the antibody effectively abrogated complement-dependent E. coli-induced CD11b up-regulation and oxidative burst in neutrophils of human whole blood. mAb 137–26 was more potent than a C5a receptor antagonist and a previously described anti-C5a antibody. mAb 137–26 did not inhibit complement-mediated lysis, nor did it activate complement itself. Together, mAb 137–26 binds both the C5a moiety of native C5 and free C5a, thereby effectively neutralizing the biological effects of C5a. The antibody may have therapeutic potential in inflammatory diseases where C5a inhibition combined with an operative lytic pathway of C5b-9 is particularly desired.
Keywords: complement C5, monoclonal antibody, inflammation
INTRODUCTION
The complement system is an important part of a host defense against invading microorganisms. Activation of complement induces a number of biological effects of which many are potent and mediated by the anaphylatoxin C5a. Although these effects are intended to overcome infection, they may be detrimental to the host when the system is activated in an excessive or uncontrolled manner. Activation of complement may contribute to tissue damage and inflammation in a number of clinical conditions, e.g. bacterial sepsis, immune complex diseases, and ischaemia-reperfusion injury [1–3]. Thus, the complement system could act as a double-edged sword in the body. Increasing knowledge about the role of complement in various diseases has underscored its potential as a therapeutic target to reduce tissue injury and inflammation.
Several approaches to inhibit complement have been applied in experimental models and a few are approaching clinics. In principle, specific inhibition of those components in the complement system contributing to tissue damage and inflammation should be targeted to achieve maximal efficacy and minimal, if any, adverse side-effects. A soluble form of complement receptor 1 (sCR1) and a mAb blocking C5 cleavage are examples of different strategies to reduce complement-dependent tissue injury [4,5]. The former blocks activation of C3 and thus the whole complement cascade at an early step, whereas the latter only blocks terminal pathway activation. A further discrimination in inhibition of the terminal pathway is obtained by neutralizing C5a while keeping the lytic C5b-9 pathway open. This may be advantageous in gram-negative septicaemia, in particular Neisseria infection, where systemic release of C5a may contribute to the irreversible septic shock whereas the lytic pathway may help kill the bacteria [6]. Blocking C5a by mAbs and C5a receptor (C5aR) antagonists has proven to be useful in experimental models of septicaemia, immune complex diseases, and ischaemia-reperfusion injury [7–10].
A number of mAbs to C5a have been described, typically binding to neoepitopes exposed in the C5a fragment after C5 cleavage, but not found in the native C5 molecule [11]. These mAbs bind to C5a after C5 is cleaved into C5a and C5b. We here describe a novel approach of neutralizing C5a by an anti-C5 mAb 137–26 which binds to the C5a moiety of native C5 before cleavage without interfering with the lytic C5b-9 pathway. The antibody also binds C5a even after it is formed.
MATERIALS AND METHODS
Generation of anti-C5 mAbs
Male A/J mice, 7–9 weeks old, were injected subcutaneously with 30 µg of purified human C5 (Advanced Research Technologies, San Diego, CA, USA) in complete Freund's adjuvant (Difco Laboratories, Detroit, MI, USA). At two-week intervals the mice were injected twice subcutaneously with 30 µg of C5 in incomplete Freund's adjuvant. Three days before sacrifice, the mice were injected intraperitoneally with 30 µg of C5 in phosphate buffered saline (PBS). For generation of hybridomas, splenocytes were isolated from immunized mice and fused with SP2/0 myeloma cells. Cells were cultured in a selection medium containing hypoxanthine, aminopterin and thymidine, according to our procedure described earlier [12]. After about 10 days, supernatants from the cell culture were tested for antibody reactivity with purified human C5 by ELISA. Positive hybridomas were then single-cell cloned by a limiting-dilution procedure. The positive hybridomas were expanded for purification of mAbs by protein A chromatography for characterization. Three anti-C5 mAbs used in this study were mAb 137–26 (IgG1), mAb 137–30 (IgG1) and mAb 137–76 (IgG1).
C5 and C5a ELISA
Wells of Immulon II (Dynatech Laboratories, Chantilly, VA, USA) microtest plates were coated overnight with either human C5 or C5a (Sigma, St. Louis, MO, USA) at 0·1 µg/ml (50 µl/well). The nonspecific binding sites in the wells were then saturated by incubation with 200 µl of 2% bovine serum albumin in PBS (PBSB). The wells were then washed with PBST buffer (PBS containing 0·05% Tween 20). Fifty microlitres of culture supernatant from each fusion well or serially diluted purified mAbs were added to each coated well together with 50 µl of PBSB for one hour at room temperature. The wells were washed with PBST. The bound antibodies were then detected by reaction with diluted horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (Fc specific) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for one hour at room temperature. The wells were then washed with PBST. Peroxidase substrate solution containing 0·1% 3,3,5,5, tetramethyl benzidine (Sigma) and 0·003% hydrogen peroxide (Sigma) in 0·1 m sodium acetate, pH 6·0, was added to the wells for colour development for 30 min The reaction was terminated by addition of 50 µl of 2 m H2SO4 per well. The optical density (OD) was read at 450 nm with an ELISA reader.
Polyacrylamide gel electrophoresis and immunoblotting
The reactivity of mAb 137–26 with purified human C5 and recombinant C5a was also determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, under nonreducing condition [13]. The proteins in the gel were stained with either Coomassie Blue for visual inspection or transferred to polyvinylidene difluoride membrane for Western blot analysis [14]. The binding of mAb 137–26 at 1 µg/ml to C5 and C5a on the membrane was detected by incubation with horseradish peroxidase conjugated goat anti-mouse IgG (1 : 5000) (Jackson ImmunoResearch Laboratories). The immunoreactive proteins were identified on film, using enhanced chemiluminescence detection (Supersignal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA).
Complement-mediated haemolysis
For the classical pathway haemolysis, chicken red blood cells (RBC) (5 × 107 cells/ml) in gelatin/veronal buffered saline (GVB++) containing 0·5 mm MgCl2 and 0·15 mm CaCl2 were sensitized with purified rabbit anti-chicken RBC immunoglobulins at 8 µg/ml (Inter-Cell Technologies, Hoperwell, NJ, USA) for 15 min at 4°C. The cells were then washed with GVB++. The washed cells were re-suspended in the same buffer at 1·7 × 108 cells/ml. In each well of a round-bottom 96-well microtest plate, 50 µl of normal human serum (5·2%) was mixed with 50 µl of GVB++ of serially diluted mAb 137–26 or a neutralizing anti-C2 mAb 175–62 (IgG1) as a positive control. mAb 175–62 was generated by our laboratory (M.F; unpublished observation). Then 30 µl of the washed sensitized chicken RBCs suspension was added to the wells containing the mixtures. Fifty microlitres of normal human serum (5·2%) were mixed with 80 µl of GVB++ to give the serum colour background. An isotype-matched anti-HIV-1 gp120 mAb G3-519 (IgG1) was used as a negative control [12]. The final human serum concentration used was 2%. The mixture was incubated at 37°C for 30 min The plate was shaken on a microtest plate shaker for 15 s. The plate was then centrifuged at 300 × g for 3 min Supernatants (80 µl) were collected and transferred to wells in a flat-bottom 96-well microtest plates for measurement of OD at 405 nm by an ELISA plate reader. The percent inhibition of haemolysis is defined as:
For alternative pathway haemolysis, unsensitized rabbit RBCs were washed three times with gelatin/veronal-buffered saline (GVB/Mg-EGTA) containing 2 mm MgCl2 and 1·6 mm EGTA. EGTA was used to inhibit the classical complement pathway. The assay procedures are similar to those of the classical pathway haemolytic assay described above. The final concentration of human serum used in the assay was 10%. Anti-factor D mAb 166–32 (IgG1) specifically neutralzing the alternative pathway was used as a positive control [15,16]. The same isotype-matched control mAb G3-519 was used as a negative control.
Surface plasmon resonance for antibody affinity determination
The affinity equilibrium constant and the binding kinetic constants (association and dissociation) of mAb 137–26 with C5 and C5a were determined by a BIAcore instrument (Pharmacia Biosensor AB, Uppsala, Sweden). All the binding measurements were performed in HEPES-buffered saline (HBS) (10 mm HEPES, pH 7·4, 150 mm NaCl, 3·4 mm EDTA, 0·005% Surfactant P20) at 25°C. To measure the binding rate constants of C5 and C5a to mAb 137–26, rabbit anti-mouse IgG(Fc) antibodies were immobilized onto a CM5 sensorchip by amine coupling using N-hydroxysuccinimide and N-ethyl-N′-(3-diethylaminopropyl)cardo-diimide. mAb 137–26 was then captured onto the coated sensorchip before the injection of C5 or C5a at different concentrations. Kinetic parameters were obtained by global fitting of kinetic traces under pseudo-first order kinetics using BIAvaluation version 3·0 (Biacore Inc. Piscataway, NJ, USA).
125I-C5a binding assay with purified human neutrophils
Neutrophils were purified from freshly collected, heparinized human whole blood as described earlier [17]. The procedure consists of Dextran T-500 (Pharmacia, Piscataway, NJ, USA) sedimentation, density centrifugation in Histopaque-1077 (Sigma, St. Louis, MO), and hypotonic lysis of RBCs. In the binding assay, mAb 137–26 was serially diluted with a binding buffer (1% BSA in RPMI1640 medium) to give final concentrations of 0·04–640 nm. Ten microlitres of 4 nm125I-C5a (NEN Life Science Products, Inc., Boston, MA, USA) were added to 40 µl of diluted mAb 137–26 for incubation at room temperature for 15 min Purified recombinant human C5a (Sigma) was used as a positive control, whereas the isotype-matched mAb G3-519 was used as a negative control. For the maximum binding of 125I-C5a, 36 µl of the binding buffer without the antibodies or C5a was used instead. Then 50 µl of neutrophil suspension (2 × 107 cells/ml) was added to the mixture for incubation on ice for 40 min. At the end of the incubation, the mixture from each tube was transferred to the top of 800 µl of a separation buffer (6% BSA in PBS) in another Eppendorf tube. The tubes were centrifuged at 2000× g for 3 min at room temperature. After the supernatant was aspirated, the cell pellet was re-suspended in 0·5 ml of de-ionized water to lyse the cells. The cell lysate was then mixed with 3 ml of Ultima Gold scintillation fluid (Packard Instrument, Meriden, CT) for radioactive counting. The percent inhibition of 125I-C5a binding is defined as:
where Cpmmax= maximum count per minute without competing agents; Cpmbkg= background cpm without addition of 125I-C5a; Cpmca= cpm with competing agents.
C5a-induced chemotaxis of human neutrophils
Human neutrophils were isolated as described above. The assays were performed using the ChemoTx plate system with membrane pore size of 5 µ in diameter (Neuro Probe, Gaithersburg, MD, USA). As a positive control, a cyclic peptidic C5aR antagonist, AcPhe[l-ornithine-Pro-D-cyclohexylanine-Trp-Arg] (abbreviated as AcF-[OPdChaWR]), was synthesized according to the method described earlier [18]. Serially diluted mAb 137–26, the isotype-matched control mAb G3-519, or AcF-[OPdChaWR] (final concentration, 0·3–100 nm) was mixed with 1 nm C5a in the wells of the plate. This concentration of C5a was found to give the maximum chemotaxis of neutrophils in the assay. For controls, PBSB with or without C5a was used to measure the C5a-induced and spontaneous migration of neutrophils, respectively. Fifty microlitres of neutrophil suspension (3·6 × 106 cells/ml) were added to the top of the framed filter for incubation in 5% CO2 at 37°C for 45 min After incubation, the quantity of neutrophils migrated to the lower chambers was measured by a nonradioactive cell proliferation assay using a tetrazolium compound MTS (Promega, Madison, WI, USA). The number of chemotactic neutrophils was represented by the optical density of soluble formazan product measured at 490 nm using an ELISA plate reader.
Human whole blood model of inflammation
The model has been described in detail previously [19]. It was used to study the activation of neutrophils and monocytes by C5a produced as a result of complement activation by E. coli in anticoagulated human whole blood. All equipment (tubes, tips, etc.) and solutions used were endotoxin-free according to information from the manufacturers. Burst-test kit with opsonized E. coli strain LE392 (ATCC 33572), was obtained from ORPEGEN (Pharma, Heidelberg, Germany). The oxidative burst and CD11b expression was examined in whole human blood obtained from voluntary healthy blood donors. The blood was anticoagulated with lepirudin (Refludan®, Hoechst, Frankfurt am Main, Germany). Lepirudin was tested not to interfere with complement activation. All incubations were performed at 37°C. The samples were preincubated for 4 min with antibodies, peptides or PBS until PBS (baseline samles) or E. coli (test samples) was added. mAb anti-C5a antibody 561 (IgG2a), a kind gift from Prof J. Köhl, Hannover [20] and the C5aR antagonist AcF-[OPdChaWR] were used as positive controls. The E. coli concentrations used in the CD11b experiments and oxidative burst experiments were 1 × 107 bacteria/ml blood and 1 × 108 bacteria/ml blood, respectively. The baseline sample (T-0) was processed immediately. Subsequent incubations for CD11b detection and oxidative burst assay were performed for 10 and 20 min, respectively. After incubation, 100 µl of blood was used for the flow cytometric assays. The oxidative burst was measured using the substrate dihydrorhodamine123 and performed as described in the Burst-test procedure. For the measurement of the CD11b expression the whole blood was fixed with paraformaldehyde and then stained with anti-CD11b PE (Becton Dickinson, San Jose, CA, USA) and the nuclear dye LDS-751 (Molecular Probes, Inc., Eugene, OR, USA). Oxidative burst and CD11b expression were measured as median fluorescence intensity (MFI) using a FACSCalibur flow cytometer (Becton Dickinson). All experiments were performed 3–5 times.
Complement activating potency
To examine the potential of complement activation by mAb 137–26 after complexed with C5, the antibody was incubated with 150 µl of pooled, normal human serum obtained from five blood donors in each of two experiments. The isotype-matched antibody mAb G3-519 was used as a negative control and heat aggregated IgG (HAIGG) (Gammaglobulin Kabi, Uppsala, Sweden) as a positive control. All incubations were performed at 37°C for 1 h. EDTA was then added at a final concentration of 10 mm to stop further complement activation and the serum samples were then stored at − 70°C until being analysed. Samples were analysed for complement activation products described below.
Enzyme immunoassays for complement activation products
C1rs-C1inhibitor complexes (C1rs-C1inh), C4bc, C3bc and the terminal sC5b-9 complex (TCC) were quantified by ELISAs principally as described in detail earlier [21–24]. Each sample was analysed in triplicate. The monoclonal antibodies used in the C1rs-C1inh and C4bc assays were a kind gift from Prof C.E. Hack, Amsterdam, the Netherlands.
Data presentation
Data were obtained from 2 to 5 independent experiments for each assay and were presented either as mean ± standard deviation or as one representative of the experiments performed.
RESULTS
mAb 137–26 binds human C5 and C5a with high affinity
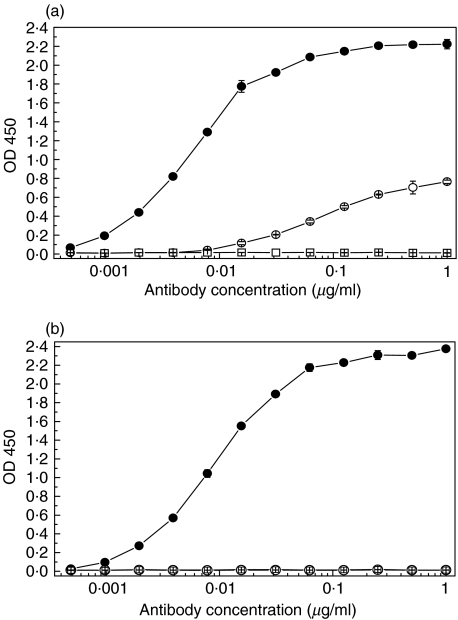
mAb 137–26 was tested for reactivity with purified human C5 and recombinant C5a by ELISA. mAb 137–26 reacted with C5 in a dose-dependent manner with high potency (Fig. 1a). Another anti-C5 mAb 137–76, specific for the β-chain of human C5, also reacted with C5 in the same assay (Fig. 1a). mAb 137–26, but not mAb 137–76, reacted with C5a in a dose-dependent manner with high potency (Fig. 1b). In order to preclude the possibility that mAb 137–26 binds to an induced epitope on C5 as a result of immobilization of the antigen on ELISA plates, we measured the affinity equilibrium constants and the binding kinetic constants (association and dissociation) of the antibody with solution-phase C5a and C5 by surface plasmon resonance using BIAcore. mAb 137–26 has a high binding affinity for both C5 and C5a, with equilibrium dissociation constants KD in subnanomolar range (Table 1). The relatively lower binding affinity of mAb137–26 to C5 than C5a could be attributed to some denatured C5 molecules in the preparation which was purified from pooled human plasma. The antibody also binds effectively to C5 and C5a on Western immunoblots (data not shown). Taken together, the results indicate that mAb 137–26 binds to a common epitope found on C5a and C5.
Fig. 1.
Reactivity of mAb 137–26 with (a) human C5 and (b) human C5a in ELISA. mAb 137–26 (•) reacts with both C5 and C5a, whereas the C5 β-chain specific antibody, mAb 137–76 (○), reacts with C5 only. Isotype-matched control mAb G3-519 (□) does not react with C5 and C5a.
Table 1.
Binding affinity of mAb 137–26 to C5 and C5a
| kon(M−1s−1) | koff(s−1) | KD(M) | |
|---|---|---|---|
| C5 | 1·42 × 105 | 6·97 × 10−5 | 4·92 × 10−10 |
| C5a | 3·70 × 106 | 2·25 × 10−4 | 6·09 × 10−11 |
kon, kinetic association constant; koff, kinetic dissociation constant; KD, equilibrium dissociation constant = koff/kon
mAb 137–26 does not inhibit C5 activation
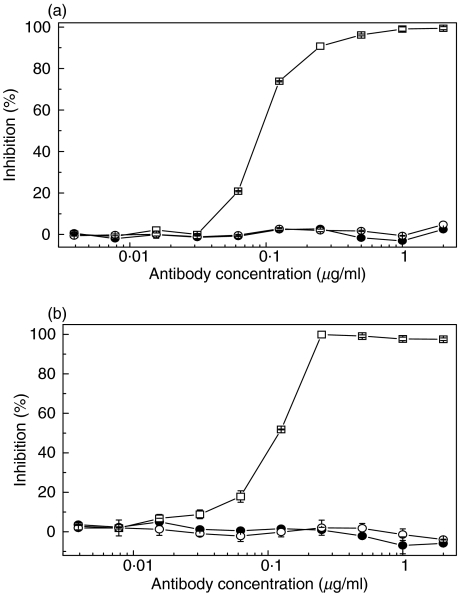
The effect of mAb 137–26 on C5 cleavage was studied by haemolytic assays. mAb 137–26 and the isotype-matched control mAb G3-519 did not inhibit the classical pathway haemolysis of sensitized chicken RBCs, whereas the positive control, anti-C2 mAb 175–62, effectively inhibited the haemolysis (Fig. 2a). mAb 137–26 did not inhibit the alternative pathway haemolysis of unsensitized rabbit RBCs, whereas the positive control, anti-factor D mAb 166–32, effectively inhibited the haemolysis (Fig. 2b).
Fig. 2.
Effects of mAb 137–26 on complement-mediated haemolysis (a) via the classical pathway in sensitized chicken red blood cells and (b) the alternative pathway in unsensitized rabbit red blood cells. mAb 137–26 (•) does not inhibit haemolysis via either the classical or the alternative complement pathway. In contrast, the anti-C2 mAb 175–62 (□, panel a) inhibits haemolysis via the classical pathway and the anti-factor D mAb 166–32 (□, panel b) inhibits haemolysis via the alternative pathway. Isotype-matched control mAb G3-519 (○) has no effect.
mAb 137–26 inhibits the binding of C5a to human neutrophils
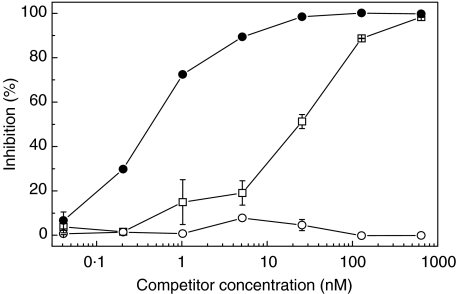
mAb 137–26 was tested for inhibition of C5a binding to C5aR on human neutrophils by a radioligand binding assay. The results show that mAb 137–26 is more potent than unlabelled C5a in inhibiting the binding of 125I-C5a to purified human neutrophils (Fig. 3). For the binding assay with 0·4 nm of 125I-C5a, the concentration for 50% inhibition (IC50) for mAb 137–26 and C5a are 0·45 nm and 30 nm, respectively.
Fig. 3.
Inhibition of C5a binding to C5aR on human neutrophils by mAb 137–26. 125I-human C5a (0·4 nm) were incubated with purified human neutrophils, in the presence or absence of varying concentrations of the inhibitors, mAb 137–26 (•) or recombinant human C5a (□). The concentration for 50% inhibition (IC50) for mAb 137–26 and C5a are 0·45 and 30 nm, respectively. Isotype-matched control mAb G3-519 (○) has no effect.
mAb 137–26 inhibits C5a-induced chemotaxis of human neutrophils
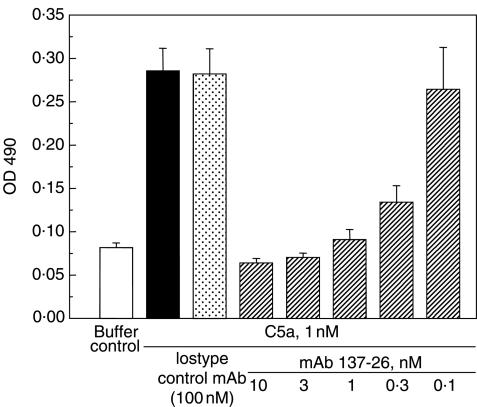
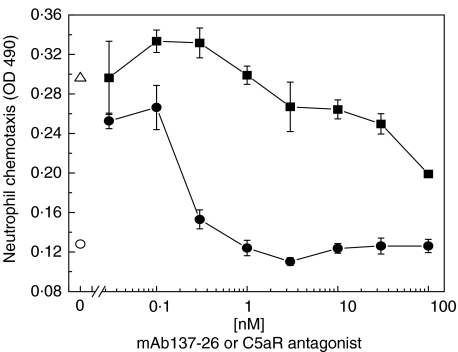
The activity of mAb 137–26 to inhibit C5a-mediated chemotaxis was tested in an assay using human neutrophils. The antibody at a concentration higher than 1 nm completely inhibited the chemotaxis of human neutrophils induced by 1 nm C5a (Fig. 4). Furthermore, mAb 137–26 is more potent than the C5aR antagonist AcF-[OPdChaWR] in inhibiting C5a-induced chemotaxis of neutrophils (Fig. 5). Consistent with the data from the competitive radioligand study described above, the results indicated that mAb 137–26 is a potent neutralizing antibody against C5a.
Fig. 4.
Inhibition of C5a-induced chemotaxis of human neutrophils by mAb 137–26. C5a at 1 nm was used to induce chemotaxis of human neutrophils. Varying concentrations of mAb 137–26 were added for blocking of C5a. The degree of neutrophil migration was represented by the optical density of the formazan product as described in the text.
Fig. 5.
Relative potency of inhibition by mAb 137–26 and C5aR antagonist AcF-[OPdChaWR] in C5a-induced chemotaxis of human neutrophils. C5a at 1 nm was used to induce chemotaxis of human neutrophils. Chemotaxis of neutrophils in the presence (▵) or absence of C5a (○) was used as control. Varying concentrations of mAb 137–26 (•) and AcF-[OPdChaWR] (▪) were added for blocking C5a. The degree of neutrophil migration was represented by the optical density of the formazan product described in the text.
mAb 137–26 inhibits CD11b expression and oxidative burst in whole blood
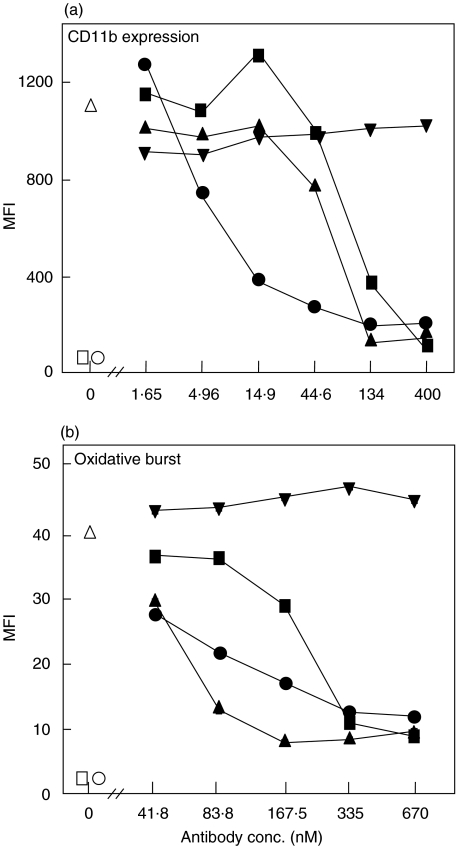
The effect of mAb 137–26 on complement-dependent E. coli-induced up-regulation of CD11b and oxidative burst was examined in a novel whole human blood model of inflammation [19]. We first compared the effect of mAb 137–26 with two other mAbs to C5, one (mAb 137–30) binding to native C5 and blocking the cleavage into C5a and C5b and the other (clone 561) which binds both C5a and C5 [20]. Neutrophil CD11b expression was reduced by more than 90% by all three antibodies, in contrast to the isotype-matched control antibody G3-519 which had no effect (Fig. 6a). Notably, the potency of mAb 137–26 in reducing neutrophil CD11b expression was 10 times higher than the anti-C5 mAb 137–30 and anti-C5a mAb 561. Similarly, all three anti-C5/C5a antibodies efficiently reduced neutrophil oxidative burst by more than 80%, in contrast to the control antibody G3-519 which had no effect (Fig. 6b). In contrast to the effect on CD11b expression, the anti-C5 mAb 137–30 was the most potent in reducing neutrophil oxidative burst. An efficient effect of mAb 137–26 in reducing monocyte CD11b expression and oxidative burst was also observed, although less pronounced than for neutrophils, amounting approximately 50% reduction in both markers, which was also the case for the other anti-C5 antibodies (results not shown).
Fig. 6.
Inhibition of (a) CD11b expression and (b) oxidative burst by anti-C5 mAbs in human whole blood. E. coli was incubated in lepirudin-anticoagulated whole human blood at 1 × 107 bacteria/ml for CD11b expression and 1 × 108 bacteria/ml for oxidative burst. Neutrophils and monocytes were separately gated and examined and the neutrophil results presented. mAb 137–26 (•) abrogated very efficiently CD11b expression with a substantial higher potency than the anti-C5 mAb 137–30 (▴) and the anti-C5a mAb 561 (▪), whereas the isotype-matched control mAb G3-519 (▾) had no effect. The effect of mAb 137–26 on oxidative burst was intermediate between mAbs 137–30 and 561. T-0 = baseline sample (□). T-10 = whole blood incubated only with PBS (○). Pos. ctr. = whole blood incubated with E. coli (open triangle ▵). The experiment was repeated four times with identical results.
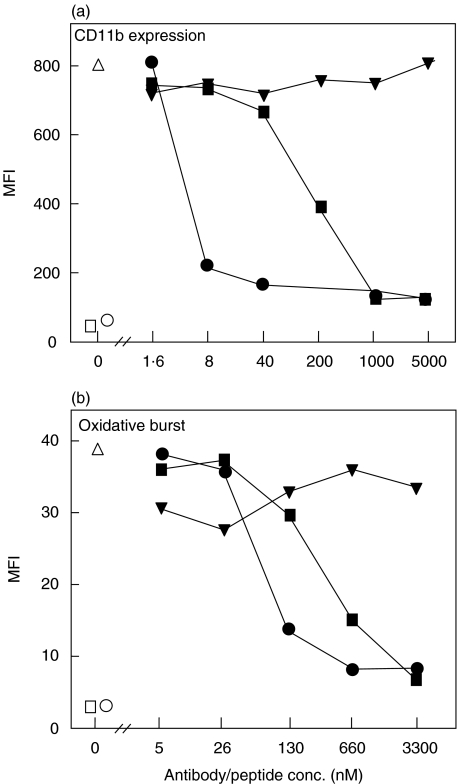
We then compared the effect of mAb 137–26 with that of the C5aR antagonist AcF-[OPdChaWR]. On a molar basis mAb 137–26 was approximately 100 times more potent in reducing the CD11b expression and 5 times more potent in reducing oxidative burst (Fig. 7a,b).
Fig. 7.
Inhibition of (a) CD11b expression and (b) oxidative burst by mAb 137–26 and the C5aR antagonist AcF-[OPdChaWR] in human whole blood. The experiment was performed as described in Fig. 6. Both mAb 137–26 (•) and the C5aR antagonist (▪) inhibited neutrophil CD11b expression and oxidative burst efficiently, but mAb 137–26 was substantially more potent, particularly in inhibition of CD11b expression. Irrelevant control peptide (▾) has no effect. Other symbols are as described in Fig. 6.
mAb 137–26 does not activate complement
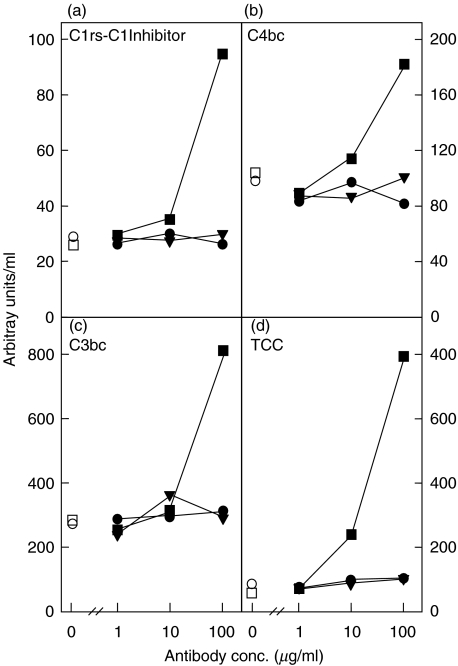
To investigate whether mAb 136–26 would activate complement after forming a complex with C5, we incubated the antibody and controls in human serum and measured complement activation products from the classical pathway (C1rs-C1 inhibitor complexes and C4bc), as well as activation of C3 (C3bc) and the terminal pathway (TCC). In contrast to heat-aggregated immunoglobulins which showed a substantial and dose-dependent activation revealed by all four activation markers, mAb 137–26 had no complement activating capacity (Fig. 8).
Fig. 8.
Complement activation potential of mAb 137–26. A possible complement activation capacity of mAb 137–26 (•) was investigated by incubating with human serum at 37°C for 1 h. The isotype-matched control mAb G3-519 (▾) was used as a negative control and heat-aggregated immunoglobulins HAIGG (▪) as a positive control. Detection of the complement activation products (a) C1rs-C1Inhibitor complexes, (b) C4bc, (c) C3bc and (d) TCC revealed a dose-dependent classical complement activation by HAIGG, whereas mAb 137–26 and mAb G3-519 did not activate complement. T-0, baseline serum sample (□); T-60, serum incubated with PBS for 60 min (○). The experiment was repeated once with identical results.
DISCUSSION
C5a is the most potent proinflammatory mediator of the complement system. It is formed through enzymatic cleavage of C5 by C5 convertases formed after activation of C3 through either the classical, the lecitin or the alternative pathway. C5a is a potent stimulator of neutrophils and monocytes [25,26], induces chemotactic migration of neutrophils [27], eosinophils [28], basophils [29], and monocytes [30], activates endothelial cells to express adhesion molecules essential for sequestration of activated leucocytes, which mediate tissue inflammation and injury [31–33]. C5a also mediates inflammatory reactions by causing smooth muscle contraction, increasing vascular permeability, inducing basophil and mast cell degranulation and inducing release of lysosomal proteases and oxidative free radicals [34]. Furthermore, C5a modulates hepatic acute-phase gene expression and augments the overall immune response by increasing the production of TNFα, IL-1, IL-6, and IL-8 [1–3].
C5a binds to C5aR (CD88), with very high affinity [34]. More recently, an orphan receptor called C5L2 has been reported to bind C5a and C5a desArg [35]. The functions of C5L2 remain to be studied. C5aR belongs to a superfamily of seven-transmembrane-domain, G protein-coupled receptors. It is broadly expressed on neutrophils, monocytes, basophils, eosinophils, hepatocytes, lung smooth muscle and endothelial cells, and renal glomerular tissues [36–41]. Since the biological functions of C5a are mainly receptor-mediated, the alternative strategy of blocking C5a would be to block the C5aR. However, if C5a is blocked before receptor binding, any receptor-mediated effect will be neutralized, even if there are several different C5aRs present. Furthermore, possible receptor-independent effects will be abolished as well. In support of this argument, we found in the present study that at the same molar concentrations mAb 137–26 inhibited chemotaxis, up-regulation of CD11b and oxidative burst of human neutrophils more efficiently than the C5aR inhibitor AcF-[OPdChaWR].
Our data show that mAb 137–26 recognizes a unique epitope shared by C5 and C5a. The antibody binds to the C5a moiety of C5 before its being activated. It also binds to C5a that has already been formed. It neutralizes C5a with high potency by inhibiting its binding to C5aR. This property is very beneficial in view of the fact that C5a, once formed, could bind very rapidly with high affinity to C5aR on various cells, thereby activating signal transduction cascade which results in inflammation and tissue injury. The high neutralizing capacity of mAb 137–26 is further supported by its more potent inhibition of C5a-mediated effects than mAb 561 which also binds both C5 and C5a [20]. mAb 561 did not compete the binding of mAb 137–26 to human C5 in ELISA (unpublished data), indicating that these mAbs recognize distinct epitopes on human C5.
There are two principally different ways of inhibiting C5 functions, namely by mAbs blocking cleavage of C5, like mAb 137–30 and mAb 137–76, and 5G1·1 [42], or by neutralizing C5a, e.g. by mAb 137–26, without inhibiting the cleavage of C5. In the latter case the ability to form lytic C5b-9 is maintained. Our data clearly demonstrate that mAb 137–26 inhibits the pro-inflammatory effects of C5a, but does not inhibit C5b-9-mediated haemolysis. Although most bacteria are cleared by phagocytosis at the level of C3 activation, C5b-9 is an important killing mechanism of some gram-negative bacteria like Neisseriae, and may in these cases be an important defense mechanism against bacterial sepsis [6]. Consistent with the findings of this study, we have recently showed in a whole blood model of meningococcal sepsis with Neisseria meningitides that mAb 137–26 inhibits C5a-mediated oxidative burst of neutrophils and monocytes (Tom Mollnes et al., unpublished observation). Moreover, in that study we showed that the antibody did not inhibit the formation of fluid-phase SC5b-9, as detected by a quantitative ELISA, and preserved C5b-9-mediated bacteriocidal activity. These results are consistent with the current finding that mAb 137–26 does not inhibit complement-mediated haemolysis.
C5a is involved in the systemic inflammatory response syndrome (SIRS) as manifested by acute respiratory distress syndrome (ARDS) and multiorgan failure (MOF) [43–45]. C5a has also been shown to play an important role in the development of tissue injury, and particularly pulmonary injury, in animal models of septic shock [46]. In sepsis models using rats, pigs and nonhuman primates, anti-C5a antibodies administered to the animals before treatment with endotoxin or E. coli resulted in decreased tissue injury, as well as decreased production of IL-6 [46–48]. More importantly, blockade of C5a and C5aR with neutralizing polyclonal antibodies has been shown to significantly improve survival rate [7], prevent MOF [49], and ameliorate dysfunction of the coagulation and fibrinolysis system [50] in a caecal ligation/puncture model of sepsis in rats and mice. Interestingly, the growth of aerobic bacteria, cultured from the spleen and liver of septic animals, was significantly reduced following neutralization of C5a [7]. It is interpreted that the generation of C5a during sepsis suppresses polymorphonuclear function, and this leads to decreased bacterial clearance by mechanisms, such as leukotriene (LT)B4 or IL8, perhaps by heterologous desensitization [51]. Together, inhibition of the C5a/C5aR pathway in sepsis could be an attractive therapeutic approach.
Immunoglobulin G-containing immune complexes (IC) contribute to the pathophysiology in a number of autoimmune diseases, such as systemic lupus erthyematosus and rheumatoid arthritis [52,53]. Elevation of plasma C5a levels correlates with the severity of the disease state [54,55]. Recent studies show that C5aR deficient mice are protected from tissue injury induced by IC [56,57]. The results are consistent with the observation that a small peptidic C5aR antagonist inhibits the inflammatory response caused by IC deposition [58]. Therefore, there is mounting evidence that C5a plays an important aetiological role in IC diseases.
C5a is thought to be a major mediator in myocardial ischaemia-reperfusion injury [59]. Complement inhibition reduced myocardial infarct size in mice [60], and treatment with anti-C5a antibodies reduced injury in a rat model of hindlimb ischaemia-reperfusion [61]. Reperfusion injury during myocardial infarction was also markedly reduced in pigs that were retreated with a monoclonal anti-C5a IgG [9]. A recombinant human C5aR antagonist reduces infarct size in a porcine model of surgical revascularization [62]. Patients undergoing extra-corporeal circulation like cardiopulmonary bypass and haemodialysis, suffer from a systemic inflammation due to biomaterial-induced alternative complement pathway activation [63–65]. C5a causes increased capillary permeability and oedema, bronchoconstriction, pulmonary vasoconstriction, leucocyte and platelet activation and infiltration to tissues, in particular the lung [66]. Administration of an anti-C5a monoclonal antibody was shown to reduce cardiopulmonary bypass and cardioplegia-induced coronary endothelial dysfunction [67].
Complement inhibition is a therapeutic strategy which is being tested clinically, as a result of the increasing knowledge of the role of complement in tissue damage and the promising results from experimental animal models. As in any clinical treatment the primary goal is ‘primare non nocere’ (do no harm). The use of mAbs as pharmaceuticals could lead to in vivo complement activation when the mAbs binds to their antigens [68]. mAbs to soluble proteins, like mAb 137–26, could theoretically generate immune complexes, particularly if they react with a repeating epitope. In the present study we show that mAb 137–26 does not activate complement in human serum after it is complexed with C5. This attentuates the concern that therapeutic application of the antibody would lead to complement activation.
An important principle in complement inhibition is to balance the beneficial effects obtained by the inhibition, with preservation of sufficient functional activity for microbial protection and tissue renovation. Blocking at the level of C5 does not inhibit C3 activation and thereby preserves the crucial defense functions like opsonization of bacteria, immune complex clearance and immune response to foreign antigens. Whether C5 cleavage should be blocked or only C5a should be inhibited, depends on the pathophysiology of the actual condition. Although C5a plays a major role in inflammation and tissue injury of bacterial sepsis and immune complex disease, C5b-9 could be involved in tissue injury in other conditions. C5b-9 stimulates platelets to express CD62P (P-selectin) which is involved in inflammatory reactions found in cardiopulmonary bypass [15,69]. Inhibition of C5b-9 formation reduces renal ischaemia/reperfusion injury in a mouse model [70]. In such conditions, complete inhibition of C5 activation should be more effective than C5a blockade alone. On the other hand, in cases where it is important to leave the lytic pathway open and block the inflammatory effects of C5a, e.g. in Neisserial or other gram-negative septicaemia, mAb 137–26 could be a suitable candidate.
Taken together, this study indicates that the novel anti-C5/C5a mAb 137–26 is a potent inhibitor of C5a functions. This antibody could be useful for treatment of C5a-mediated clinical conditions, such as bacterial sepsis, immune complex diseases and certain ischaemia-reperfusion injuries. Selective inhibition of complement targets for specific clinical conditions is an attractive strategy to maximize clinical benefits and to reduce potential side-effects.
Acknowledgments
Financial supports were kindly provided to T. E. Mollnes by Tanox, Inc., The Norwegian Council on Cardiovascular Disease, The Norwegian Foundation for Health and Rehabilitation, Gythfeldt's legacy and Norsk Revmatikerforbund.
REFERENCES
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends Immunol. 2002;23:61–4. doi: 10.1016/s1471-4906(01)02129-9. [DOI] [PubMed] [Google Scholar]
- 4.Riou P. TP-10 (Avant Immunotherapeutics) Curr Opin Invest Drugs. 2001;2:364–71. [PubMed] [Google Scholar]
- 5.Kaplan M. Eculizumab (Alexion) Curr Opin Invest Drugs. 2002;3:1017–23. [PubMed] [Google Scholar]
- 6.Drogari-Apiranthitou M, Kuijper EJ, Dekker N, Danjert J. Complement activation and formation of the membrane attack complex on serogroup B Neisseria meningitides in the presence or absence of serum bactericidal activity. Infect Immun. 2002;70:3752–8. doi: 10.1128/IAI.70.7.3752-3758.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czermak BJ, Sarma V, Pierson CL, et al. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–92. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 8.Strachan AJ, Woodruff TM, Haaima G, et al. A new small molecule C5a receptor antagonist inhibits the reverse-passive Arthus reaction and endotoxic shock in rats. J Immunol. 2000;164:6560–5. doi: 10.4049/jimmunol.164.12.6560. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Stahl GL, Pan HL, et al. Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am J Physiol. 1995;268:H448–57. doi: 10.1152/ajpheart.1995.268.1.H448. [DOI] [PubMed] [Google Scholar]
- 10.Arumugam TV, Shiels IA, Strachan AJ, et al. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;65:134–42. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- 11.Bergh K, Iversen OJ. Production of monoclonal antibodies against the human anaphylatoxin C5a desArg and their application in the neoepitope-specific sandwitch-ELISA for the quantification of C5a desArg in plasma. J Immunol Meth. 1992;152:79–87. doi: 10.1016/0022-1759(92)90091-7. [DOI] [PubMed] [Google Scholar]
- 12.Sun NC, Ho DD, Sun CR, et al. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989;63:3579–85. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structured proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung M, Loubser PG, Ündar A, et al. Inhibition of complement, neutrophil, and platelet activation by an anti-factor D monoclonal antibody in simulated cardiopulmonary bypass circuits. J Thorac Cardiovasc Surg. 2001;122:113–22. doi: 10.1067/mtc.2001.114777. [DOI] [PubMed] [Google Scholar]
- 16.Ündar A, Eichstaedt HC, Clubb FJ, et al. Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass. Ann Thorac Surg. 2002;74:355–62. doi: 10.1016/s0003-4975(02)03656-1. [DOI] [PubMed] [Google Scholar]
- 17.Grisham MB, Engerson TD, McCord JM, et al. A comparative study of neutrophil purification and function. J Immunol Meth. 1985;82:315–20. doi: 10.1016/0022-1759(85)90363-1. [DOI] [PubMed] [Google Scholar]
- 18.Short A, Wong AK, Finch AM, et al. Effects of a new C5a receptor antagonist on C5a-and endotoxin-induced neutropenia in the rat. Br J Pharmacol. 1999;126:551–4. doi: 10.1038/sj.bjp.0702338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mollnes TE, Brekke OL, Fung M, et al. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–77. [PubMed] [Google Scholar]
- 20.Klos A, Ihrig V, Messner M, et al. Detection of native human complement components C3 and C5 and their primary activation peptides C3a and C5a (anaphylatoxic peptides) by ELISAs with monoclonal antibodies. J Immunol Meth. 1988;111:241–52. doi: 10.1016/0022-1759(88)90133-0. [DOI] [PubMed] [Google Scholar]
- 21.Fure H, Nielsen EW, Hack CE, Mollnes TE. A neoepitope-based enzyme immunoassay for quantification of C1-inhibitor in complex with C1r and C1s. Scand J. 1997;46:553–7. doi: 10.1046/j.1365-3083.1997.d01-168.x. [DOI] [PubMed] [Google Scholar]
- 22.Wolbink GJ, Bollen J, Baars JW, et al. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for the quantification of complement activation via the classical pathway. J Immunol Meth. 1993;163:67–76. doi: 10.1016/0022-1759(93)90240-8. [DOI] [PubMed] [Google Scholar]
- 23.Garred P, Mollnes TE, Lea T. Quantification in enzyme-linked immunosorbent assay of a C3 neoepitope expressed on activated human complement factor C3. Scand J Immunol. 1988;27:329–35. doi: 10.1111/j.1365-3083.1988.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 24.Mollnes TE, Lea T, Froland SS, Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 25.Schindeler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of IL-1 and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–8. [PubMed] [Google Scholar]
- 26.Cavaillon JM, Fitting C, Haeffner-Cavaillon N. Recombinant C5a enhances interleukin 1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocytes and macrophages. Eur J Immunol. 1990;20:253–7. doi: 10.1002/eji.1830200204. [DOI] [PubMed] [Google Scholar]
- 27.Ward PA, Newman LJ. A neutrophil chemotactic factor from human C5. J Immunol. 1969;102:93–9. [PubMed] [Google Scholar]
- 28.Kay AB, Shin HS, Austen KF. Selective attraction of eosinophils and synergism between eosinophil chemotactic factor of anaphylaxis (ECF-A) and a fragment cleaved from the fifth component of complement (C5a) Immunology. 1973;24:969–76. [PMC free article] [PubMed] [Google Scholar]
- 29.Lett-Brown MA, Boetcher DA, Leonard EJ. Chemotactic responses of normal human basophils to C5a and lymphocyte-derived chemotactic factor. J Immunol. 1976;117:246–52. [PubMed] [Google Scholar]
- 30.Snyderman R, Shin HS, Hausman MH. A chemotactor factor for mononuclear leukocytes. Proc Soc Exp Biol Medical. 1971;138:387–90. doi: 10.3181/00379727-138-35903. [DOI] [PubMed] [Google Scholar]
- 31.Foreman KE, Vaporciyan AA, Bonish BK, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–55. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foreman KE, Glovsky MM, Warner RL, et al. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20:1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- 33.Rollins SA, Johnson KK, Li L, et al. Role of porcine P-selectin in complement-dependent adhesion on human leukocytes to porcine endothelial cells. Transplantation. 2000;69:1659–67. doi: 10.1097/00007890-200004270-00023. [DOI] [PubMed] [Google Scholar]
- 34.Gerard C, Gerard NP. C5a anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 35.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg. J Biol Chem. 2002;277:7165–9. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- 36.van Epps DE, Chenoweth DE. Analysis of the binding of fluorescent C5a and C3a to human peripheral blood leukocytes. J Immunol. 1984;132:2862–7. [PubMed] [Google Scholar]
- 37.Haviland DL, McCoy RL, Whitehead WT, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–9. [PubMed] [Google Scholar]
- 38.Wetsel RA. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cell. Immunol Lett. 1995;44:183–7. doi: 10.1016/0165-2478(94)00212-a. [DOI] [PubMed] [Google Scholar]
- 39.Buchner RR, Hugli TE, Ember JA, et al. Expression of functional receptors for human C5a anaphylatoxin (CD88) on the human hepatocellular carcinoma cell line HepG2. Stimulation of acute-phase protein-specific mRNA and protein synthesis by human C5a anaphylatoxin. J Immunol. 1995;155:308–15. [PubMed] [Google Scholar]
- 40.Chenoweth DE, Hugli TE. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci USA. 1978;75:3943–7. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwirner J, Fayyazi A, Gotze O. Expression of the anaphylatoxin C5a receptor in non-myeloid cells. Mol Immunol. 1999;36:877–84. doi: 10.1016/s0161-5890(99)00109-1. [DOI] [PubMed] [Google Scholar]
- 42.Fitch JC, Rollins S, Matis L, et al. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation. 1999;100:2499–506. doi: 10.1161/01.cir.100.25.2499. [DOI] [PubMed] [Google Scholar]
- 43.Hack CE, Nuijens JH, Felt-Bersma RJ, et al. Elevated plasma levels of the anaphylatoxin C3a and C4a are associated with a fatal outcome in sepsis. Am J Med. 1989;86:20–6. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 44.Hammerschmidt DE, Weaver LJ, Hudson LD, et al. Association of complement activation and elevated plasma C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980;1:947–9. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- 45.Heideman M, Hugli TE. Anaphylatoxin generation in multisystem organ failure. J Trauma. 1984;24:1038–43. doi: 10.1097/00005373-198412000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Smedegard G, Cui LX, Hugli TE. Endotoxin-induced shock in the rat. A role of C5a. Am J Pathol. 1989;135:489–97. [PMC free article] [PubMed] [Google Scholar]
- 47.Hopken U, Mohr M, Struber A, et al. Inhibition of interleukin-6 synthesis in an animal model of septic shock by anti-C5a monoclonal antibodies. Eur J Immunol. 1996;26:1103–9. doi: 10.1002/eji.1830260522. [DOI] [PubMed] [Google Scholar]
- 48.Stevens JH, O'Hanley P, Shapiro JM, et al. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic patients. J Clin Invest. 1986;77:1812–6. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber-Lang M, Sarma VJ, Lu KT, et al. Role of C5a in multiorgan failure during sepsis. J Immunol. 2001;166:1193–9. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
- 50.Laudes IJ, Chu JC, Sikranth S, et al. Anti-C5a ameliorates coagulation/fibrinolytic protein changes in a rat model of sepsis. Am J Path. 2002;160:1867–75. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerard NP, Gerard C. Complement in allergy and asthma. Curr Opinion Immunol. 2002;14:705–8. doi: 10.1016/s0952-7915(02)00410-7. [DOI] [PubMed] [Google Scholar]
- 52.Madaio MP. The role of autoantibodies in the pathogenesis of lupus nephritis. Semin Nephrol. 1999;19:48–56. [PubMed] [Google Scholar]
- 53.Korganow ASH, Ji Mangialaio S, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 54.Jose PJ, Moss IK, Maini RN, et al. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. 1990;49:747–52. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porcel JM, Ordi J, Castro-Salomo A, et al. The value of complement activation products in the assessment of systemic lupus erythematosus flares. Clin Immunol Immunopathol. 1995;74:283–8. doi: 10.1006/clin.1995.1040. [DOI] [PubMed] [Google Scholar]
- 56.Kohl J, Gessner JE. On the role of complement and Fc gamma-receptors in the Arthus reaction. Mol Immunol. 1999;36:893–903. doi: 10.1016/s0161-5890(99)00111-x. [DOI] [PubMed] [Google Scholar]
- 57.Baumann U, Kohl J, Tschernig T, et al. A codominant role of Fc gamma RI/III and C5aR in the reverse Arthus reaction. J Immunol. 2000;164:1065–70. doi: 10.4049/jimmunol.164.2.1065. [DOI] [PubMed] [Google Scholar]
- 58.Strachan AJ, Woodruff TM, Haaima G, et al. A new small molecule C5a receptor antagonist inhibits the reverse-passive Arthus reaction and endotoxic shock in rats. J Immunol. 2000;164:6560–5. doi: 10.4049/jimmunol.164.12.6560. [DOI] [PubMed] [Google Scholar]
- 59.Angogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 60.Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 61.Bless NM, Warner RL, Padgaonkar VA, et al. Roles of C-X-C chemokines and C5a in lung injury after hindlimb ischemia-reperfusion. Am J Physiol. 1999;276:L57–63. doi: 10.1152/ajplung.1999.276.1.L57. [DOI] [PubMed] [Google Scholar]
- 62.Riley RD, Sato H, Zhao ZQ, et al. Recombinant human complement C5a receptor antagonist reduces infarct size after surgical revascularization. J Thorac Cardiovasc Surg. 2000;120:350–8. doi: 10.1067/mtc.2000.107281. [DOI] [PubMed] [Google Scholar]
- 63.Howard RJ, Crain C, Franzini D A, et al. Effects of cardiopulmonary bypass on pulmonary leukostasis and complement activation. Arch Surg. 1988;123:1296–501. doi: 10.1001/archsurg.1988.01400360066010. [DOI] [PubMed] [Google Scholar]
- 64.Kirklin JK, Westaby S, Blackstone EH, et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845–57. [PubMed] [Google Scholar]
- 65.Craddock PR, Fehr J, Brigham KL, et al. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1997;296:769–74. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- 66.Czermak BJ, Lentsch AB, Bless NM, et al. Role of complement in in vitro and in vivo lung inflammatory reactions. J Leukoc Biol. 1998;64:40–8. doi: 10.1002/jlb.64.1.40. [DOI] [PubMed] [Google Scholar]
- 67.Tofukuji M, Stahl GL, Agah A, et al. Anti-C5a monoclonal antibody reduces cardiopulmonary bypass and cardioplegia-induced coronary endothelial dysfunction. J Thorac Cardiovasc Surg. 1998;116:1060–8. doi: 10.1016/S0022-5223(98)70059-5. [DOI] [PubMed] [Google Scholar]
- 68.vanderKolk LE, Grillo-Lopez AJ, Baars JW, et al. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115:807–11. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 69.Rinder CS, Rinder HM, Smith MJ, et al. Selective blockade of membrane attack complex formation during simulated extracorporeal circulation inhibits platelet but not leukocyte activation. J Thorac Cardiovasc Surg. 1999;118:460–6. doi: 10.1016/S0022-5223(99)70183-2. [DOI] [PubMed] [Google Scholar]
- 70.Zhou W, Farrar CA, Abe K, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]