Abstract
The scurfy mutant mouse is the genetic and phenotypic equivalent of the single-gene human autoimmune disease immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX). The scurfy mutation disrupts the Foxp3 gene, a putative master switch for T regulatory cell development. Bone marrow transplant without conditioning was previously reported to be ineffective in scurfy mice, yet clinical remission occurs in transplanted human IPEX patients despite limited donor engraftment. In view of this contradiction, we sought to validate scurfy as a model for studying the pathogenesis and treatment of human IPEX, in particular the phenomenon of dominant immune regulation. One half of scurfy mice given bone marrow transplants after sublethal irradiation recovered and survived long-term with donor chimerism ranging from 1·7% to 50%. Early transfer of 2 × 107 normal T cell-enriched splenocytes also prevented or limited disease and permitted long-term survival. Donor T cells in rescued mice made up 3–5% of lymphocytes and became highly enriched for CD25+ T cells over time. Transfer of 106 CD4+ CD25+ sorted T cells showed some beneficial effect, while CD4+ CD25- cells did not. Thus, both partial bone marrow transplant and T-enriched splenocyte transfer are effective treatments for scurfy. These results indicate that scurfy results from a lack of cells with dominant immune regulatory capacity, possibly T regulatory cells. The potency of small numbers of normal cells indicates that IPEX may be a feasible target for gene therapy.
Keywords: inherited autoimmune disease, bone marrow transplantation, T regulatory lymphocytes, immunoregulation, gene therapy
Introduction
Immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) is a human single-gene recessive disorder that results in multiple autoimmune conditions in infancy (reviewed in [1]). Affected males manifest a variable combination of type I diabetes mellitus, severe enteropathy, autoimmune cytopenias, hypothyroidism and eczema and often develop autoantibodies. Long-term immunosuppressive drugs may reduce manifestations and delay an otherwise rapidly fatal outcome, but drug toxicities limit this approach. In cases treated with bone marrow transplant (BMT), the disease remitted, in one case for nearly three years [1,2]. Remarkably, remission was observed despite limited donor engraftment, indicating that the mutant cells mediating the disease were quieted in the presence of a minority of normal bone marrow-derived cells. A similar phenomenon is believed to occur in IPEX carrier females who are clinically unaffected despite nonskewed X-inactivation patterns [3].
The gene responsible for IPEX, FOXP3, was recently shown to be the same as that causing a similar clinical phenotype in a strain of mutant mice, scurfy (Foxp3sf) [4–7]. Scurfy is thus the natural mouse model for IPEX. Like their human counterparts, scurfy mice exhibit hallmarks of cellular autoimmune activity, which manifests as dermatitis, enteritis, anaemia, failure to thrive, splenomegaly and lymphadenopathy, organ lymphocyte infiltration, elevated levels of multiple cytokines, and very early death [8–10]. Scurfy disease is mediated by CD4+ T cells that are hyper-responsive to stimulation via the T cell receptor (TCR) and have a decreased requirement for CD28 costimulation [9,11–13].
Foxp3 encodes scurfin, a putative transcription regulator containing a forkhead-family winged-helix domain [7]. Normal scurfin has been shown to suppress IL-2 transcription and secretion in transfected cultured cells, suggesting its antiautoimmune properties relate to modulation of pro-inflammatory cytokines [14], but the possibility of other functional roles has not been eliminated.
Scurfy shares clinical and immunologic features with mouse strains engineered to lack cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) [15–17], CD25 (IL-2 receptor alpha) [18], IL-2 receptor beta [19], and others. The immune dysregulation in these mice and in induced autoimmune models is blocked by T regulatory cells, a functionally defined subset that appears most frequent among cells with a CD4+ CD25+ phenotype, and much less frequent among CD4+ CD25- cells, in mice [20–23]. Hori et al.[24] recently showed that Foxp3 expression is markedly higher in CD4+ CD25+ cells than in CD4+ CD25- in normal mice and suggested that it may be a master regulatory gene contributing to the differentiation or development of T regulatory cells. They further showed that forced expression of Foxp3 in normal naïve CD4+ CD25- thymocytes conferred T regulatory function in vitro and blocked experimentally induced inflammatory bowel disease in severe-combined immunodeficiency mice (SCID) mice injected with normal BALB/c effector cells. These findings support the hypothesis that small numbers of Foxp3 expressing cells could prove therapeutic in the scurfy mouse, and, by extension, in human IPEX.
Since partial BMT had shown promise in IPEX patients but was previously reported to be ineffective in scurfy mice [12], we re-examined its effectiveness in scurfy. We asked whether the phenomenon of dominant regulation was evident in scurfy mice, and, if so, what was the minimum chimerism required. Lastly, we asked whether T-enriched splenocytes (TES) could mediate this dominant regulation.
We report that partial BMT at birth can rescue scurfy mice long-term, even with low level donor chimerism, and that a single infusion of normal TES has a similar beneficial effect. These results indicate that scurfy effector T cells are susceptible to dominant regulation by a small fraction of cells in vivo.
MATERIALS AND METHODS
Mice
Animals were housed in an accredited facility and their use approved and monitored by the Institutional Animal Use and Care Committee. Scurfy carrier females (Foxp3sf/+) back crossed to C57Bl/6 J at least 7 times were obtained from Drs Mary Brunkow and Mark Appleby (Celltech Chiroscience R & D, Inc., Bothell, WA, USA). These mice lack the closely linked sparse-fur (spf) mutation present in the original source animals. A colony was maintained by continued back-crossing to C57Bl/6 J males (Jackson Laboratory, Bar Harbor, ME, USA). A subcolony of sf mice bearing the alternative CD45·1-Ptprca allele was created by breeding our CD45·2 sf carrier females to C57Bl/6 J congenic males with the CD45·1-Ptprca allele (Jackson Laboratory, Cat. 002014). Newborn mice were genotyped for the scurfy mutation using allele-specific PCR: antisense 5′-GAACTATTGCCATG GCTTCC-3′, wt sense 5′-TGGCCTCAATGGACAAGAGC-3′, and sf sense 5′-GCCTCAATGGACAAAAGAGC-3′. Mice appearing moribund were humanely euthanized.
Bone marrow transplants
Bone marrow was harvested from 6 to 12-week-old normal (Foxp3+/+ or +/Y) or control scurfy (Foxp3sf/Y) donors from the colony or subcolony by flushing tibias and femurs with Hank's balanced salt solution (Gibco BRL, Gaithersburg MD, USA) supplemented with 1% bovine serum albumen (Sigma, St. Louis, MO, USA) (BSS/BSA). Single cell suspensions, minus settled aggregates, were washed once with BSS/BSA. Treated mice received 5 Gy total body irradiation (TBI) using a 137Cs Irradiator (J. L. Shepherd Co. Model I) followed by injection of 5 × 106 cells IP in 25 µl of PBS using a 30-gauge needle. No antibiotics or other supportive care was given to mice before or after transplantation.
T-enriched splenocytes infusions
Donor T cells were obtained by passage of normal mouse splenocytes over a Mouse T Cell Enrichment Column (R & D Systems, Minneapolis, MN, USA) following the manufacturer's procedure. Donors were as described for bone marrow transplants. Yields were 10–20% of total cells applied to column. In a typical experiment, the T-enriched product was 87% CD3e+ and 6% CD19+ by flow cytometry. The cells were washed once with PBS, and 2 × 107 cells in 25 µl PBS were injected IP into scurfy mice.
Flow cytometry and cell sorting
Peripheral blood lymphocytes were stained using fluorochrome- or biotin-conjugated monoclonal antibodies for CD45·1 (clone A20), CD45·2 (clone 104), CD3e (clone 145–2C11), CD19 (clone 1D3), CD4 (clone H129·19), and CD25 (clone 7D4) from BD PharMingen (San Diego, CA, USA). Cell surface phenotypes were determined using the FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ, USA). For cell sorting, nonenriched normal splenocytes in suspension were stained and directly subjected to flow sort under sterile, viable conditions, using the FACSVantage (Becton-Dickinson).
RESULTS
Mice
Affected males in our scurfy colony begin to show external signs of scurfy – scaly tail and paw skin, thickened ears, short palpebral fissures, and decreased weight gain – by 10–12 days of age. They die at a median age of 27 days (Fig. 1a) with failure to thrive, decreased activity, hunched posture, tail and ear necrosis, poor coat and tight body skin, inflammatory reactions at mucocutaneous junctions, and marked lymphadenopathy and splenomegaly (Figs 2a and 4a,e).
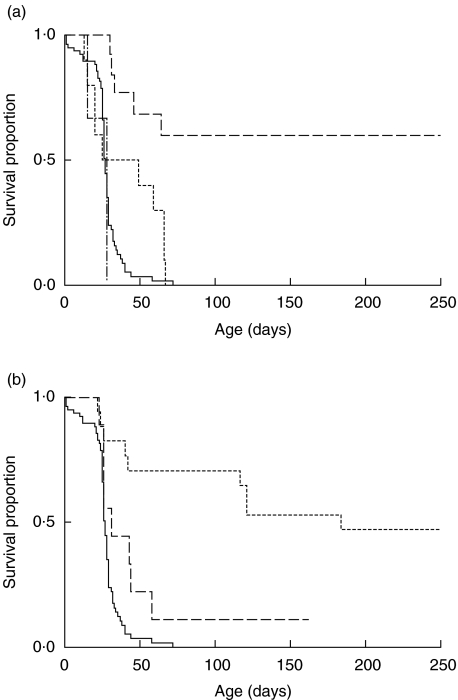
Fig. 1.
Survival of scurfy mice after partial BMT or injection of TES. (a) Partial BMT survival. Scurfy mice receiving no or sham treatment (——n = 77); or 5 Gy irradiation followed by IP injection of normal bone marrow (——n = 12), scurfy bone marrow (–·–·n = 3), or no marrow (------ n = 10) on day 2 of life. P = 0·057 for untreated versus irradiated; P < 0·0001 for untreated versus normal BMT; P = 0·0001 for scurfy BMT versus normal BMT (Logrank tests). (b) Survival after TES injection. Scurfy mice receiving no or sham treatment (——n = 77), or 2 × 107 TES at 3–8 days (---- n = 14), or at 10–16 days (——, n = 9) of life. P < 0·0001 for untreated versus TES at 3–8 days; P < 0·03 for untreated versus TES at 10–16 days (Logrank tests).
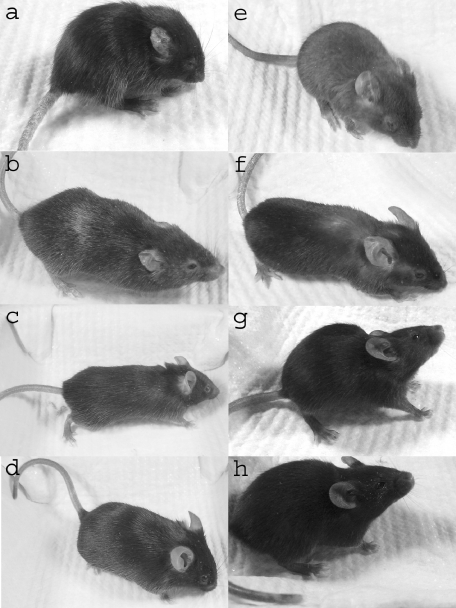
Fig. 2.
Phenotypes of representative scurfy mice after BMT or injection of TES. (a) Untreated scurfy mouse, 31 days old: note blepharitis, scaly tail, thick crumpled ear, and hunched posture; (b) MT, 133 days old; (c) BMT, 79 days old; (d) normal littermate of (c); (e) irradiation only, 31 days old; (f) 14-day TES, 132 days old; (g) 3-day TES, 31 days old; (h) normal littermate of (g).
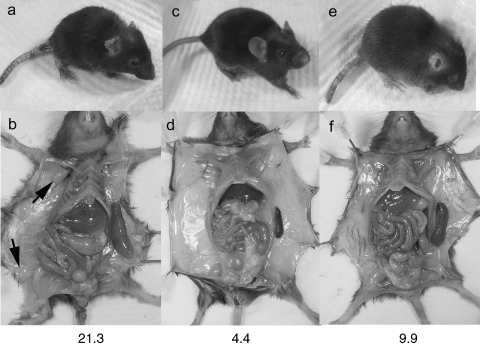
Fig. 4.
TES reduce lymphadenopathy and splenomegaly more than skin disease. Comparison of external (a, c, e) and internal (b, d, f) phenotypes at 37–43 days. Untreated scurfy mouse (a, b); normal littermate (c, d); scurfy mouse after failed TES (e, f). Spleens have been dissected to display size. The ratio of spleen weight (mg) to body weight (gm) is given at the bottom of each set. Lymphadenopathy is present in untreated scurfy mouse (b, arrows) but not in the failed TES recipient (f). These data are representative of other nonrescued TES mice.
Survival was defined as the age, in days, at death or euthanasia. Treated mice that survived at least 61 days, i.e. three weeks longer than the 95th percentile of untreated mice, were designated rescued. Among rescued mice, a few died after 100 days and were categorized as a lost rescue. The remaining rescued mice were designated long-term survivors.
Partial bone marrow transplantation
As we had no basis for predicting the level of chimerism that might permit scurfy to survive, we chose a BMT method that was expected to produce a broad range of chimerism. Partial BMT in 12 two-day-old scurfy males resulted in a delay of scurfy signs and, in half, recovery from disease and long-term rescue (Figs 1a and 2b,c). Survival differed significantly from untreated scurfy mice (P < 0·0001). Rescued mice also lacked scurfy-associated inflammation and lymphadenopathy (Fig. 3b,f). Improvement in survival is thus correlated with a reduction in disease manifestations, implying that increased survival is due to a reversal of the underlying inflammatory process. Mice that were not rescued had typical progressive scurfy features.
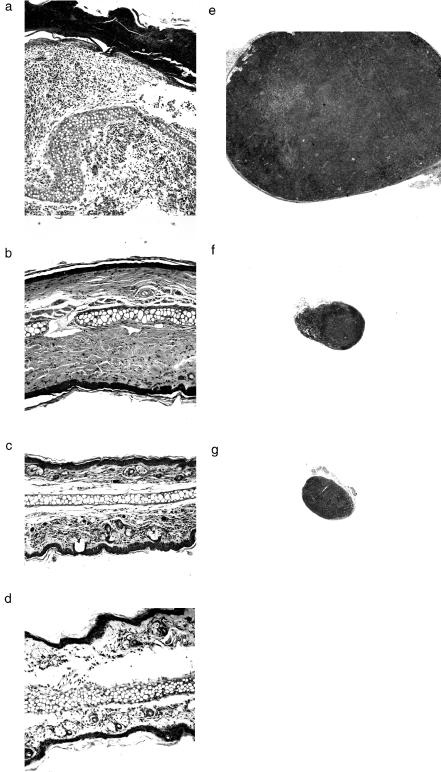
Fig. 3.
Normalization of histology and lymphadenopathy after BMT or injection of TES. Hematoxylin and eosin cross sections of ear (Mag. a–d, ×100) and inguinal lymph node (Mag. e–g, ×25) from an untreated scurfy mouse at 26 days (a, e), a representative BMT rescued mouse at 163 days (b, f), a 14-day TES-rescued mouse at 162 days (c, g), and a wild type mouse at 58 days of age (d). The full thickness of the untreated scurfy ear (a) was too great to be imaged at this magnification. Post-inflammatory fibrosis in the ear (b) reflected recovery from the transient scurfy illness that was generally observed in the BMT treated mice. A wild type lymph node was not collected, but is generally comparable in size to those in (f) and (g).
A delay in disease and death was also observed in control scurfy mice that received total body irradiation (TBI) alone; however, unlike those receiving normal bone marrow, none was rescued (Figs 1a and 2e). Scurfy mice transplanted with scurfy marrow instead of normal marrow died of scurfy disease at the usual age (Fig. 1a). These results indicate that small numbers of normal bone marrow cells transplanted at two days can rescue a substantial portion of scurfy mice from inevitable death.
To assess the degree of donor chimerism associated with rescue, we performed four of the above transplants using congenic donor marrow bearing the alternate allele of the CD45 (Ptprc, Ly-5) polymorphic haematopoietic cell surface marker. The proportions of CD45·1+ (donor-derived) peripheral blood lymphocytes (PBL) measured by flow cytometry at 60–70 days of age were 1·7, 36, 41, and 50% for the four mice. The first mouse died unexpectedly at 329 days without signs of scurfy. The others were sacrificed at approximately one year of age, having PBL chimerism of 51, 62, and 74%, respectively. Spleen chimerism was similar to that in PBL and bone marrow chimerism was slightly lower than in PBL. Thus, the presence of as few as two percent Foxp3+ cells appears sufficient to reverse the scurfy disease process caused by an excess of Foxp3sf cells.
Rescue by normal TES
We next asked whether T cells could be the bone marrow-derived cell type responsible for the rescue. To avoid the possibility that antibodies used in positive selection could alter the nature or effectiveness of the injected cells, we prepared donor TES from normal adult splenocytes by negative selection using a commercial affinity column that removes non-T cells. We injected 2 × 107 TES (80–90% CD3+) IP into scurfy mice once. Injection after the onset of disease signs (at 10–16 days of life) slowed scurfy disease progression and delayed death (Fig. 1b). One mouse injected at 14 days recovered from his disease and lived in good health (Fig. 2f) until he was sacrificed at 162 days. Tissue inflammation and lymphocyte proliferation were absent in this mouse at autopsy (Fig. 3c,g). Even nonrescued TES recipients euthanized due to their severe scurfy appearance showed substantially reduced lymphadenopathy and splenomegaly (Fig. 4e,f). Thus, TES may limit lymphoproliferation and autoimmunity in internal organs more than in the skin.
The rescued proportion was far higher (13/18 versus 1/9, P < 0·005, Fisher exact test) when mice were injected at three to eight days of age, i.e. before external signs of scurfy appeared (Fig. 1b). Furthermore, the severity of subsequent illness was substantially diminished and some mice showed virtually no external signs of disease (Fig. 2g). However, four mice lost rescue; these gradually developed typical signs and died or were euthanized at greater than 100 days of age (Fig. 1b). In retrospect, technical difficulties in at least two of these had resulted in a lower donor cell dose. This corresponds to our observation that four scurfy mice injected at 6–12 days with fewer cells (1 × 107 or 1·5 × 107) showed no benefit (mean survival 25·3 ± 2·4 days).
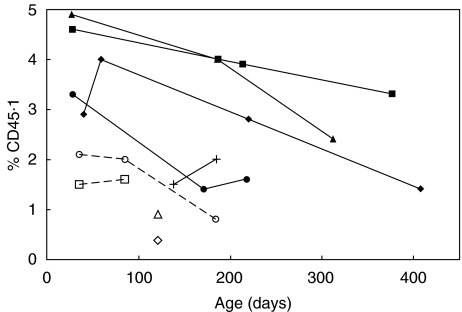
Persistent donor cells (CD45·1+) among PBL of TES-rescued mice were assessed by flow cytometry. As expected, 75–100% were CD3+. Among long-term survivors, early counts indicated donor-derived cells comprised 2·9–4·9% of peripheral blood lymphocytes, while mice that lost rescue had fewer donor cells (Fig. 5, P < 0·03, two-tailed T-test). This suggests that the minimum donor chimerism compatible with long-term survival is approximately three to five percent.
Fig. 5.
Donor chimerism in TES-injected mice over time. Percentage of donor derived cells (CD45·1+) in PBL at different ages among long-term survivors (solid lines and symbols), and among those that lost rescue (broken lines and open symbols). See text for definitions.
The frequency of donor cells present in rescued mice decreased somewhat over time (Fig. 5, P < 0·02, two-tailed T-test). However, the proportion of donor cells bearing CD25 or both CD4 and CD25 markers increased substantially, as did the proportion of CD4+ cells that were also CD25+ (Table 1). Taken together, these trends suggest that the CD25+ subset was enriched by the selective attrition of others. In a healthy heterozygote (sf/wt) control receiving TES at three days of age, no measurable donor cells were present at 110 days. Thus, the persistence of normal donor cells in rescued males is neither an inherent characteristic of the donor cells nor a response to cells expressing mutant scurfin independent of immunodysregulation. Rather, the disease state seems to favour the retention of donor CD4+ CD25+ lymphocytes.
Table 1.
Percent of Donor-derived Cells in Spleen of Rescued T Cell Recipients by CD4 and CD25 Phenotype
| Cell source | CD25+ | CD4+ CD25+ | CD4+ CD25+/CD4+ |
|---|---|---|---|
| Donor T-enriched splenocytes (before injection) | 4·4 | 2·6 | 6 |
| Five long-term rescued scurfy mice (at sacrifice) | |||
| 1 | 22 | 20 | 29 |
| 2 | 29 | 27 | 35 |
| 3 | 93 | 82 | 89 |
| 4 | 21 | 19 | 22 |
| 5 | 37 | 32 | 52 |
CD4+ CD25+ T cells have been implicated as having T regulatory properties in other systems. To assess whether this subset contains cells capable of rescuing scurfy, three mice were injected IP with 106 flow-sorted CD4+ CD25+ cells (85% pure) at 3 or 7 days of age, while two others received CD4+ CD25- sorted cells (95% pure) at 4 or 7 days.
The CD4+ CD25+ recipients died with scurfy at 27, 59, and 104 days. This was significantly better survival than untreated mice (P < 0·02, log rank test) and not different from comparably timed TES recipients. Those receiving CD4+ CD25- cells died at 25 and 29 days, i.e. significantly poorer than TES recipients (P < 0·02) and not significantly different from untreated mice. Thus, scurfy mice benefited from CD4+ CD25+ cells but not from CD4+ CD25- cells. Because of the small sample sizes, direct comparison between the CD4+ CD25+ and CD4+ CD25- recipient groups could not reach statistical significance (P = 0·2, Mann–Whitney test or log Rank test).
DISCUSSION
The usual form of human IPEX is generally fatal [1]. Chronic immunosuppressive medications can prolong life, sometimes for years, but these do not restore health and are associated with undesirable complications when used for long periods. Though more experience is needed, BMT shows promise for inducing remission in IPEX patients.
We found that scurfy mice were rescued by partial BMT with 2% to 50% initial donor chimerism. These results establish the validity of using scurfy as a model for studying cell-based treatments in IPEX and fulfil Patel's prediction that dominant regulation by normal cells in scurfy is possible [25]. The effect appears to be so potent in vivo that gene correction of autologous bone marrow may be within the capabilities of currently popular gene transduction approaches. Gene therapy could be a potential option for IPEX patients who lack an HLA-matched donor.
Our BMT results are at odds with those of Godfrey et al. [12], who concluded that scurfy did not respond to BMT, and with their proposed model for scurfy pathogenesis. We attribute our success to the addition of pretransplant conditioning. We found that sublethal irradiation alone limited aggressive disease and delayed death. In BMT-rescued recipients, irradiation presumably disables autoimmune effector cells for a period, permitting the maturation of normal bone marrow-derived regulatory cells before the natural course of the disease becomes irreversible. The observation of clinical improvement during pre-BMT conditioning in humans with IPEX is consistent with this explanation [1,2].
Our success with BMT led us to reflect on which bone marrow-derived lineage might bring about the dominant regulation and clinical rescue we observed. If a particular cell type were responsible, then modification therapy might be targeted directly to these, decreasing the time to therapeutic effect compared to BMT and limiting the risk of undesirable effects on other lineages. Indeed, we found that normal T-enriched splenocytes had the capacity to prevent, diminish, and occasionally reverse established immune-mediated disease in scurfy mice for long periods. Their potency with respect to disease outcome depended on the age at treatment and appeared dose-dependent. Thus, cellular therapy with differentiated haematopoietic cells may be an alternative to BMT. These results also implicate T cells as the dominant regulatory cell type.
We recognize that that TES-contaminating cell types, including dendritic cells, could have played a role in the observed therapeutic effect. Nonetheless, the parallels between scurfy and other, similar autoimmune mouse models that are susceptible to T regulatory cell dominant regulation are striking. Furthermore, we observed disease-dependent enrichment for CD25+ and CD4+ CD25+ subtypes among circulating donor cells. Since an unaffected mouse failed to preserve these donor cells, their maintenance in scurfy points to a selection for these cells. Further analysis should resolve whether these are T regulatory cells selected to fill an empty niche or effector cells activated by a pro-inflammatory milieu. In addition, the differential ability of positively selected CD4+ CD25+ T cells to rescue scurfy mice compared with CD4+ CD25- cells lends support to the hypothesis that at least one form of regulatory T cell is involved in preventing scurfy disease and, by extension, IPEX in normal individuals.
Since Foxp3 encodes a putative transcription regulator, one explanation for the similarity between scurfy and autoimmune mice lacking CTLA-4, CD25, or IL-2Rβ is that scurfin is necessary for the transcription and expression of these molecules [26–28]. However, untreated scurfy mice have large numbers of peripheral T cells bearing surface CTLA-4 [9], CD25 [29], and IL-2Rβ (RSW and SKS-P, unpublished observation), as well as high levels of numerous cytokines including IL-2 and the regulatory T cell stimulators IL-10 and TGF-β[10,13,29]. The expression of Fas and Fas ligand are likewise permitted in sf mice [29]. Since scurfy mice express the molecules known to be required for T regulatory function, scurfin is not required for their expression; scurfin must therefore act either downstream in the same pathways or in a separate but equally essential pathway.
The winged-helix domain of human Scurfin can repress transcription from IL-2 promoter elements and thereby limit cytokine secretion by activated T cells [14]. However, failure to repress cytokine transcription in Foxp3 deficient cells cannot readily explain the ability of small numbers of normal T cells to dominantly regulate their abnormal reactivity. Another explanation is necessary. Our results are most consistent with the model in which Foxp3 acts as developmental switch for the differentiation and/or maintenance of T regulatory cells [24], without which autoreactive T cells are permitted to mature or to become active.
As such, this model system provides an important, credible link between the science of T regulatory cells in experimental mice and the aetiology of a human autoimmune disease. We believe that manipulating Foxp3/FOXP3 expression in mouse and human bone marrow or in T cells, whether through cellular or molecular approaches, holds potential for correcting diseases caused by immune dysregulation.
Since the original submission of this paper, two important reports addressing the mechanisms of scurfy autoimmunity have appeared [30,31]. These further support the model in which Foxp3 is necessary to generate T regulatory cells. Both confirmed that CD4+ CD25+ cells, the putative T regulatory subset, express far higher quantities of Foxp3 than any other subset and showed that Foxp3- and Foxp3sf mice lack functional regulatory T cells. Fontenot et al. also found that Foxp3 is required for the development of regulatory T cells in vivo, and confirmed that Foxp3 gene-transduction of CD4+ CD25- cells gives them a regulatory phenotype [30]. In experiments similar to those described here, they also showed that sorted CD4+ CD25+ T cells given at birth can rescue scurfy mice while CD4+ CD25- cells cannot, and that the former ‘expand’ in diseased, but not in normal mice.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health (R29-DK47278, R21-DK60207) and the Tartar Family Foundation, Oregon Health & Science University. The authors gratefully thank Profs. David Parker and Antonio Freitas for helpful discussions and technical assistance.
REFERENCES
- 1.Wildin RS, Smyk-Pearson SK, Filipovich AH. Clinical and molecular features of the immuno-dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–5. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baud O, Goulet O, Canioni D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X Linked Syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758–62. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 3.Tommasini A, Ferrari S, Moratto D, et al. X-chromosome inactivation analysis in a female carrier of FOXP3 mutation. Clin Exp Immunol. 2002;130:127–30. doi: 10.1046/j.1365-2249.2002.01940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 5.Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity–allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X–linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379–87. [PMC free article] [PubMed] [Google Scholar]
- 9.Clark LB, Appleby MW, Brunkow ME, et al. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–54. [PubMed] [Google Scholar]
- 10.Kanangat S, Blair P, Reddy R, et al. Disease in the scurfy (sf) mouse is associated with overexpression of cytokine genes. Eur J Immunol. 1996;26:161–5. doi: 10.1002/eji.1830260125. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey VL, Rouse BT, Wilkinson JE. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am J Pathol. 1994;145:281–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey VL, Wilkinson JE, Rinchik EM, et al. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci USA. 1991;88:5528–32. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair PJ, Bultman SJ, Haas JC, et al. CD4+CD8- T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J Immunol. 1994;153:3764–74. [PubMed] [Google Scholar]
- 14.Schubert LA, Jeffery E, Zhang Y, et al. Scurfin (foxp3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672–9. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 15.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–95. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 16.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 17.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 18.Willerford DM, Chen J, Ferry JA, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Zhou YW, Kato M, et al. Normal regulatory alpha/beta T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor beta in vivo. J Exp Med. 1999;190:1561–71. doi: 10.1084/jem.190.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontoux C, Banz A, Papiernik M. Natural CD4 CD25 (+) regulatory T cells control the burst of superantigen-induced cytokine production: the role of IL-10. Int Immunol. 2002;14:233–9. doi: 10.1093/intimm/14.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Malek TR, YuA, Vincek V, et al. Control of autoimmunity by CD4+ CD25+ regulatory T cells depends on thymic IL-2 receptor signalling. In: Bluestone JA, Abbas AK, Goodrow CC, editors. Mechanisms and Applications of Immune Tolerance. 2002. pp. 3–9. Keystone Symposia, Steamboat Springs, CO, USA. April, Abstract 328. [Google Scholar]
- 23.Bachmann MF, Koehler G, Ecabert B, et al. Cutting edge. Lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–31. [PubMed] [Google Scholar]
- 24.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 25.Patel DD. Escape from tolerance in the human X-linked autoimmunity–allergic disregulation syndrome and the Scurfy mouse. J Clin Invest. 2001;107:155–7. doi: 10.1172/JCI11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4 (+) CD25 (+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevach EM, McHugh RS, Piccirillo CA, et al. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells. their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 29.Khattri R, Kasprowicz D, Cox T, et al. The amount of scurfin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167:6312–20. doi: 10.4049/jimmunol.167.11.6312. [DOI] [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]